Abstract
New automated hematology analyzers have led to the availability of novel hematological parameters, including the immature platelet fraction (IPF) and the immature reticulocyte fraction (IRF), both of potential interest in patients with myeloproliferative neoplasms (MPNs). We performed a prospective analysis of 217 patients with MPN, including 32 (15%) with essential thrombocythemia (ET), 43 (20%) with polycythemia vera (PV), and 142 (65%) with myelofibrosis (MF); the IPF and IRF were measured by the Sysmex XN analyzer. As compared to patients with ET, both a higher IPF and IRF were observed among patients with PV and MF. Factors associated with high IPF among patients with PV/ET were male sex, thrombocytopenia, and diagnosis of PV; among patients with MF, they were elevated peripheral blasts, low platelet count, JAK2 V617F mutation, and previous therapy. Factors associated with high IRF among patients with PV/ET were low hemoglobin, high reticulocyte count, and PV diagnosis; among patients with MF, they were peripheral blasts and elevated reticulocytes. The IPF and IRF represent novel parameters in patients with MPN with potential relevant clinical implications. Comparison with healthy subjects and those with secondary polycythemia is needed to confirm our preliminary findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, new automated hematology analyzers have led to the development of novel and more precise hematological parameters, including the immature platelet fraction (IPF) and the immature reticulocyte fraction (IRF) [1].
Immature platelets are reticulated platelets, still containing residual RNA, newly released from the bone marrow [2]. Their fractions reflect the rate of thrombopoiesis [3] and they exhibit a greater mass and higher prothrombotic potential compared to smaller and older platelets [4]. As a consequence, the IPF has shown clinical significance in idiopathic thrombocytopenia (differentiating decreased production from accelerated destruction) [5], in myelosuppression after chemotherapy or stem cell transplantation (predicting platelet recovery) [6, 7], and in thrombosis risk assessment in patients with acute coronary syndrome or sickle cell disease [8, 9].
Immature reticulocytes are distinguished from conventional reticulocytes based on their RNA content and represent an early and sensitive index of erythropoiesis [10]. The IRF may be of only limited utility in anemias secondary to hemolysis, bleeding, or bone marrow suppression, as in these situations it parallels the conventional reticulocyte count; however, it acquires significant utility in anemia secondary to acute infections or myelodysplastic syndromes (MDS), in which the total reticulocyte count is reduced or normal, whereas the IRF is increased [11–14].
The fractions of immature platelets and reticulocytes are of obvious potential interest in clonal disorders of hematopoietic stem cells, such as the myeloproliferative neoplasms (MPNs). The elevated IPF has been shown to associate with JAK2 V617F mutation, hydroxyurea treatment, and a higher thrombotic risk in patients with polycythemia vera (PV) and essential thrombocythemia (ET) [15, 16]. However, its role in myelofibrosis (MF) has never been explored. In addition, the clinical significance of IRF has not yet been investigated in any of the MPNs.
We present here a large prospective analysis of the clinical significance of the IPF and IRF in patients with MPN, including PV/ET and MF.
Methods
Patients and samples
We performed a prospective study of 217 unselected patients with a confirmed diagnosis of PV/ET or MF (including both primary MF and post-ET/post-PV MF) in whom IPF/IRF were measured between March 2014 and February 2015, during their routine visit to our center. Samples were collected once per patient, randomly during the course of their disease. PV/ET and MF were diagnosed according to the World Health Organization criteria [17]. For patients with MF, the Dynamic International Prognostic Scoring System (DIPSS) score was assigned to each patient as previously described [18]. Demographic and clinical information, including medical history, results of physical examination, complete blood count and blood chemistries, and assessment of bone marrow aspiration/biopsy, was collected at the time of IPF and IRF measurement. In particular, blast count was measured on the same sample used for IPF and IRF assessment. JAK2 mutation was assessed as previously described [19]. Patients were subsequently followed per physician’s preference.
This study was approved by the Institutional Review Board of MDACC and conducted in accordance with our institutional guidelines and the principles of the Declaration of Helsinki.
IPF and IRF measurement
The IPF and IRF were measured by a fully automated hematology analyzer, Sysmex XN (Sysmex Corporation, Kobe, Japan). At our institution, internal quality controls on Sysmex XN are run three times daily, through the commercial control XN Check (Sysmex America, Inc., Lincolnshire, IL, USA). The storage temperature of the sample was room temperature and the time elapsed between collection and analysis was 30 min. The measured value was expressed as the percentage of the total concentration of platelets and erythrocytes, respectively. Using this method, the reference range for IPF in healthy individuals was 1–7.3% [20] and the reference range for IRF in healthy individuals was 2.3–18% in males and 2.6–16.7% in females [10]; values were considered low, normal, or high if below, within, or above these reference ranges, respectively.
Statistical analysis
Categorical variables were compared using the χ2 or Fisher exact tests. Logistic regression was used to determine factors associated with high IPF and IRF. Only factors that were significant on univariate analysis were included in multivariable analyses. Time-to-event outcomes were analyzed using the method of Kaplan and Meier, and comparisons were made using the log-rank test. A p value of <0.05 (two-tailed) was considered statistically significant. Statistical analyses were carried out using IBM SPSS Statistics 22 software for Windows (SPSS Inc., Chicago, IL).
Results
Baseline characteristics
Two-hundred and seventeen patients were included in the study: 32 (15%) with ET, 43 (20%) with PV, and 142 (65%) with MF. Baseline characteristics are shown in Table 1.
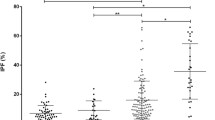
Among all patients, the median IPF was 5% (range, 0.9–28.9%) and was high in 79 (36%) patients. As compared to patients with ET (median 2.3%, range 1–5.3%), a higher IPF was observed among patients with PV (median 3.4%, range 0.9–17.3%; p = 0.02) and MF (median 6.2%, range 1.3–28.6%; p < 0.001) (Fig. 1a). Specifically, all patients with ET had a low or normal IPF, whereas 9 (21%) patients with PV and 70 (49%) with MF had a high IPF.
Among all patients, the median IRF was 20.7% (range 1.2–47%) and was high in 132 (61%) patients. As compared to patients with ET (median 9.8%, range 3.8–33.7%), a higher IRF was observed among patients with PV (median 17.1%, range 1.2–34.4%; p = 0.01) and MF (median 24.9%, range 7.2–47%; p < 0.001) (Fig. 1b). Specifically, a high IRF was observed in 8 (25%) patients with ET, 23 (53%) with PV, and 111 (78%) with MF.
Factors associated with high IPF
Among patients with PV/ET, factors associated with a high IPF on univariate analysis were male sex (78 vs 35%, p = 0.03), low platelet count (median 180 vs 499 × 109/uL, p = 0.005), and a diagnosis of PV as compared to ET (100 vs 52%, p = 0.008). No associations were found for the following factors: age, race, constitutional symptoms, hepatomegaly, splenomegaly, white blood cell count, peripheral blasts, hemoglobin, hematocrit, reticulocyte percentage, IRF, peripheral CD34+ cells, bone marrow blasts, fibrosis grade, JAK2 V617F positivity, previous therapy, previous use of JAK inhibitor, and history of thrombosis. Because of the small number of events (only nine patients had high IPF), no multivariable analysis could be performed.
Among patients with MF, factors associated with a high IPF on univariate analysis were elevated white blood cell count (median 10 vs 7.7 × 109/uL, p = 0.04), elevated peripheral blasts (median 1 vs 0%, p = 0.04), low platelet count (median 109 vs 234 × 109/uL, p < 0.001), a diagnosis of post-PV MF (30 vs 11%, p = 0.004), positive JAK2 V617F mutation (80 vs 54%, p = 0.002), and previous treatment (67 vs 44%, p = 0.007); no associations were found for the following variables: age, race, sex, constitutional symptoms, hepatomegaly, splenomegaly, white blood cell count, peripheral blasts, hemoglobin, hematocrit, reticulocyte percentage, IRF, platelet count, peripheral CD34+ cells, bone marrow blasts, fibrosis grade, DIPSS score, and previous use of JAK inhibitor.
On multivariable analysis, the factors that remained associated with high IPF were elevated peripheral blasts (odd ratio [OR] 3.3, 95% confidence interval [CI] 1.4–8.1; p = 0.009), low platelet count (OR 3.7, 95% CI 1.6–8.8; p = 0.003), JAK2 V617F mutation (OR 4.3, 95% CI 1.7–10.8; p = 0.002), and previous therapy (OR 2.6, 95% CI 1.2–5.8; p = 0.02) (Table 2).
Factors associated with high IRF
Among patients with PV/ET, factors associated with a high IRF on univariate analysis were the following: presence of constitutional symptoms (50 vs 23%, p = 0.02), lower hemoglobin (13 vs 14.1 g/dL, p = 0.04), high reticulocyte percentage (65 vs 39%, p = 0.04), and PV diagnosis (74 vs 45%, p = 0.02); no associations were found for the following variables: age, race, sex, hepatomegaly, splenomegaly, white blood cell count, peripheral blasts, hematocrit, platelet count, IRP, peripheral CD34+ cells, bone marrow blasts, fibrosis grade, JAK2 V617F positivity, previous therapy, previous use of JAK inhibitor, and history of thrombosis.
On multivariable analysis, the factors which remained associated with high IRF were lower hemoglobin levels (OR 4.9, 95% CI 1.4–16.8; p = 0.01), high reticulocyte count (OR 6, 95% CI 1.8–19.9; p = 0.003), and PV diagnosis (OR 4.6, 95% CI 1.4–15.3; p = 0.01) (Table 2).
Among patient with MF, factors associated with a high IRF on univariate analysis were the following: constitutional symptoms (84 vs 68%, p = 0.04), splenomegaly (median 5 vs 0 cm, p = 0.04), elevated peripheral blasts (median 1 vs 0%, p < 0.001), elevated reticulocyte counts (median 87 vs 58%, p = 0.01), and high DIPSS score (63 vs 35%, p = 0.02); no associations were found for the following variables: age, race, sex, hepatomegaly, white blood cell count, hemoglobin, hematocrit, platelet count, IPF, peripheral CD34+ cells, bone marrow blasts, fibrosis grade, type of MF (primary vs post-ET/PV), JAK2 V617F positivity, previous treatment, and previous use of JAK inhibitor.
On multivariable analysis, the factors which remained associated with high IRF were elevated peripheral blasts (OR 7.8, 95% CI 2.1–29.5; p = 0.002) and elevated reticulocyte counts (OR 3.7, 95% CI 1.3–10.3; p = 0.01) (Table 2).
Association with thrombosis, transformation, and survival
After a median follow-up, 22 (1–26) months from IRP/IRF measurement, among patients with PV/ET, no thrombotic events or MF/AML transformations occurred, and only 1 patient died; among patients with MF, no thrombotic events were reported, 11 patients developed secondary AML, and 25 patients died.
No significant association between IPF or IRF and outcomes was observed.
Discussion
The clinical significance of the immature platelet and reticulocyte fraction is of potential interest in patients affected by myeloid malignancies, such as MPN. Here, we provide the first published analysis of the clinical and biological characteristics associated with IPF in patients with MF and of those associated with IRF in both MF and PV/ET.
Our study included 217 patients with PV/ET or MF for whom these two parameters were tested and who were prospectively followed; of interest, the levels of both IPF and IRF were high in patients with MF, intermediate in patients with PV, and low-normal in patients with ET. Given the inverse relation of the IPF with platelet count [3] and of the IRF with hemoglobin levels [10], such findings may not be surprising for patients with MF, in whom anemia and thrombocytopenia are common [21], or in patients with ET, typically presenting with normal to high hemoglobin and thrombocytosis [22]. A high-intermediate IPF and especially a high-intermediate IRF are, however, less intuitive in patients with PV, in whom lower levels would be expected; of particular interest, among patients with PV/ET, in addition to the expected parameters (such as anemia and high reticulocytes), a diagnosis of PV was the only factor associated with high IRF on multivariable analysis. Our data suggest that high IRF in individuals presenting with elevated hemoglobin may suggest a diagnosis of PV rather than secondary polycythemia; its analysis in patients with secondary polycythemia and its validation as a potential diagnostic criterion for PV deserve further investigation.
Among patients with PV/ET, factors associated with high IPF were male sex, thrombocytopenia, and a diagnosis of PV; higher IPF levels are more common in males [23], and an inverse relation with platelet count, as outlined above, is expected [3]. In addition, a higher frequency of high IPF in PV as compared with ET, where thrombocytosis predominates, is not surprising. A previous study had observed an association between high IPF, JAK2 V617F mutation, and absence of previous treatment with hydroxyurea [15], which was not confirmed in our analysis. While both cohorts had similar sizes (about 75 patients), the previous study was enriched with patients with ET (as opposed to PV in our cohort); in addition, the previous study compared untreated and JAK2 V617F-positive patients to healthy individuals, as opposed to previously treated and JAK2-unmutated patients in our analysis.
Among patients with MF, factors associated with high IPF were elevated peripheral blasts, low platelets, previous therapy, and JAK2 V617F mutation; all of these may represent markers of aggressive disease [18, 24–26], and the lack of association with DIPSS score may encourage its investigation as an independent prognostic marker in MF. By contrast, the association between high IRF and adverse prognostic factors in patients with MF was less evident.
The biological significance of IPF and IRF is supported by their increased levels in MF as compared to PV/ET and by their association with unfavorable prognostic factors in these conditions; in fact, the fraction of immature platelet and reticulocyte may indirectly identify pathological myeloid progenitors, responsible for disease progression and aggressive biological behavior; however, correlative studies are needed to confirm these speculations.
While a previous study had reported a higher incidence of thrombotic events in patients with MPN and elevated IPF [16], the limited follow-up of our analysis did not allow appreciation of any association between the IPF/IRF and outcomes.
In conclusion, the IPF and IRF represent novel parameters in patients with MPN; in particular, the IRF may support the diagnosis of PV in individuals presenting with elevated hemoglobin, and the IPF may have an independent prognostic role in patients with MF. Comparison with healthy subjects, those with secondary polycythemia and longer follow-up are needed to confirm our preliminary findings.
References
Lecompte TP, Bernimoulin MP (2015) Novel parameters in blood cell counters. Clin Lab Med 35(1):209–224
Briggs C, Harrison P, Machin SJ (2007) Continuing developments with the automated platelet count. Int J Lab Hematol 29(2):77–91
Dusse LM, Freitas LG (2015) Clinical applicability of reticulated platelets. Clin Chim Acta 439:143–147
Hoffmann JJ (2014) Reticulated platelets: analytical aspects and clinical utility. Clin Chem Lab Med 52(8):1107–1117
Thomas-Kaskel AK, Mattern D, Kohler G, Finke J, Behringer D (2007) Reticulated platelet counts correlate with treatment response in patients with idiopathic thrombocytopenic purpura and help identify the complex causes of thrombocytopenia in patients after allogeneic hematopoietic stem cell transplantation. Cytometry B Clin Cytom 72(4):241–248
Michur H, Maslanka K, Szczepinski A, Marianska B (2008) Reticulated platelets as a marker of platelet recovery after allogeneic stem cell transplantation. Int J Lab Hematol 30(6):519–525
Briggs C, Hart D, Kunka S, Oguni S, Machin SJ (2006) Immature platelet fraction measurement: a future guide to platelet transfusion requirement after haematopoietic stem cell transplantation. Transfus Med 16(2):101–109
Perl L, Lerman-Shivek H, Rechavia E et al (2014) Response to prasugrel and levels of circulating reticulated platelets in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 63(6):513–517
Noronha JF, Costa FF, Saad ST, Lorand-Metze IG, Grotto HZ (2007) Evaluation of reticulated platelets in patients with sickle cell diseases. Thromb Res 121(2):259–267
Buttarello M (2016) Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol 38(Suppl 1):123–132
Tsuda I, Tatsumi N (1989) Maturity of reticulocytes in various hematological disorders. Eur J Haematol 43(3):252–254
Davis BH, Bigelow NC (1994) Automated reticulocyte analysis. Clinical practice and associated new parameters. Hematol Oncol Clin North Am 8(4):617–630
Chang CC, Kass L (1997) Clinical significance of immature reticulocyte fraction determined by automated reticulocyte counting. Am J Clin Pathol 108(1):69–73
Torres Gomez A, Casano J, Sanchez J, Madrigal E, Blanco F, Alvarez MA (2003) Utility of reticulocyte maturation parameters in the differential diagnosis of macrocytic anemias. Clin Lab Haematol 25(5):283–288
Panova-Noeva M, Marchetti M, Buoro S et al (2011) JAK2V617F mutation and hydroxyurea treatment as determinants of immature platelet parameters in essential thrombocythemia and polycythemia vera patients. Blood 118(9):2599–2601
Kissova J, Bulikova A, Ovesna P, Bourkova L, Penka M (2014) Increased mean platelet volume and immature platelet fraction as potential predictors of thrombotic complications in BCR/ABL-negative myeloproliferative neoplasms. Int J Hematol 100(5):429–436
Vardiman JW, Thiele J, Arber DA et al (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114(5):937–951
Passamonti F, Cervantes F, Vannucchi AM et al (2010) A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115(9):1703–1708
Nussenzveig RH, Swierczek SI, Jelinek J et al (2007) Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol 35(1):32–38
Ko YJ, Hur M, Kim H, Choi SG, Moon HW, Yun YM (2015) Reference interval for immature platelet fraction on Sysmex XN hematology analyzer: a comparison study with Sysmex XE-2100. Clin Chem Lab Med 53(7):1091–1097
Tefferi A (2014) Primary myelofibrosis: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol 89(9):915–925
Tefferi A, Barbui T (2015) Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. Am J Hematol 90(2):162–173
Hoffmann JJ, van den Broek NM, Curvers J (2013) Reference intervals of reticulated platelets and other platelet parameters and their associations. Arch Pathol Lab Med 137(11):1635–1640
Gangat N, Caramazza D, Vaidya R et al (2011) DIPSS plus: a refined dynamic international prognostic scoring system for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 29(4):392–397
Tefferi A, Lasho TL, Finke CM et al (2014) CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 28(7):1472–1477
Rumi E, Pietra D, Pascutto C et al (2014) Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 124(7):1062–1069
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board of MDACC and conducted in accordance with our institutional guidelines and the principles of the Declaration of Helsinki.
Authorship
SV designed the study, analyzed the data, provided clinical care to patients, and wrote the paper; PS designed the study, analyzed data, and wrote the paper; PB and JHL provided clinical care to patients and coauthored the paper; CBR, CFHG and DEBM measured and provided laboratory data and coauthored the paper; and KJ, LZ, and SP collected and analyzed the data and coauthored the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Strati, P., Bose, P., Lyle, L. et al. Novel hematological parameters for the evaluation of patients with myeloproliferative neoplasms: the immature platelet and reticulocyte fractions. Ann Hematol 96, 733–738 (2017). https://doi.org/10.1007/s00277-017-2956-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2956-3