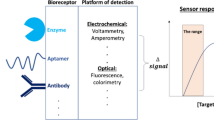
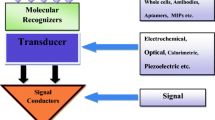
Abstract
One serious environmental problem in the world is food contamination. Many of the environmental pollutants result from agricultural activities. Particularly pesticides, which are commonly used to protect seeds and crops, have serious negative implications for human health. Therefore, biosensors were developed and used to detect pesticides in food and environmental samples in the last few decades. Indeed, utilization of enzyme inhibition method in pesticide detection has received the greatest interest. Therefore, this study screens previous researches on enzyme inhibition biosensors for determining pesticides and highlights different biological sources of these enzymes. The review acts as a guide for the detection of organophosphorus and carbamate pesticides in vegetable crops.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agriculture is the backbone of the economy of agrarian country. Ensuring food safety is an arduous task with declining cultivation land resources and increasing productivity of crops. This necessitates the adoption of high-yielding varieties, balanced fertilization and often the indiscriminate use of pesticides. Pesticides are largely used in vegetables such as brinjal, ladies’ finger, tomato, cabbage, cauliflower, cluster bean, chilli, and green leafy vegetables, since they are highly susceptible to pests and disease (Pandey et al. 2017). To control in-borne pests and obtain a high market value, pesticides are sometimes administered to the vegetables even after they have been harvested (Bhavadharani et al. 2019). Organophosphorus (OP) pesticides are commonly used for pest control, because of their expeditious breakdown and low persistence in the environment. However, OP pesticides cause many adverse health effects like headache, fatigue, breathing problems, abdominal cramps, tingling in extremities, and depression of cholinesterase activity (Nguyen et al. 2019).
The term “enzyme inhibition” means inhibition or decrease of enzymes, processes related to its production, or enzyme activity (Alsanosi et al. 2014). Inhibition by small molecules is often regarded as a control mechanism for various biological systems whose mechanisms are exploited in drug discovery programs (Mazzei et al. 2016). Biosensors have been developed and used to detect insecticides in food and environmental substances during the last few decades. Due to health issues, the food and environmental sectors demand a sensitive, precise, and rapid approach to monitor the release of hazardous compounds from natural or deliberate pollution (Ghosh et al. 2021).
Traditional methods and adequate for detecting pesticides, but they are time-consuming, costly, require chemicals and specialized personnel, and are not suited for real-time analysis (Huertas-Pérez and García-Campaña 2008; Nguyen et al. 2019).
The response of an enzyme inhibition-based biosensor is dependent on the enzyme concentration on the biosensor interface and its interaction with the substrate. Several analytical methods are available to monitor pesticide residues, and include liquid chromatography-mass spectrometry, gas chromatography equipped with a mass spectrometer, high-performance liquid chromatography, and bio-sensors. However, these methods are time-consuming and require a substantial initial investment (Ramachandran et al. 2015), which is reflected in the cost of per sample analysis. Consequently, a number of enzymes inhibition-based biosensors with biological receptors have been developed as an alternative because of their low reagent usage, low cost of production, quick analysis, and low powered requirements (Duford et al. 2013). The logical basis of the application of enzyme inhibition method in pesticide detection depends on incubation of a known amount of active enzyme with the sample (e.g. a vegetable crop species), hence the remaining activity is inversely proportional to the amount of inhibitor (i.e. the pesticide) in the sample (Rajangam et al. 2018; Zhai et al. 2021). The substrate used in the construction of a biosensor is an important consideration since the choice of transducing agent and detection method is dependent on the substrate chosen. For example, when using Acetylcholine and butyrylcholine salts as substrates for cholinesterase in both acetyl and butyryl forms, potentiometric detection is favoured due to the formation of of H + ions during the process. But, amperometric and piezoelectric transducers are favoured when utilizing thiocholine and acetate salts as substrates (Rajangam et al. 2018). Indeed, other factors affect the biosensor efficiency like the type and concentration of the chosen enzyme, enzyme purity and loading, PH range, type of organic solvents, and substrate concentration (Rajangam et al. 2018). Therefore, the goals of this study are to (1) describe enzyme based-biosensors, (2) discuss different strategies of pesticide detection, (3) clarify the application of nanotechnology in biosensor manufacturing, (4) highlight different biological sources of enzymes utilized in pesticide detection, and (5) screen for progress in research on enzymes utilized in pesticide detection.
Toxicity of Organophosphorus and Carbamate Pesticide Residues in Vegetable Crops
Organophosphorus and carbamate compounds are extensively used in agriculture due to their lower toxicity and cheaper cost compared to other pesticides (Chowdhury et al. 2012). Nonetheless, these pesticides pose a serious health danger to humans through the consumption of vegetable crops in daily meals. Several of these compounds are genotoxic and carcinogenic, which can induce mild to severe respiratory and neurological damage. Chlorpyrifos has been linked to neurological diseases such as attention deficit hyperactivity disorder (ADHD) and a developmental disorder in both foetuses and children. Carbofuran, a carbamate, has been linked to major reproductive issues, whereas occupational exposure to carbaryl has been linked to nausea, vomiting, blurred vision, unconsciousness, and breathing difficulties (Chowdhury et al. 2012).
Pesticide detection by enzyme inhibition is intended for pesticides that inhibit enzymes, particularly phosphoric and thiophosphoric acid esters, as well as organophosphorus and carbamate pesticide residues (Maghsoudi et al. 2021a, 2021b). The pesticide residues inhibit the enzyme by forming an enzyme-inhibitor complex via a chemical process in which the serine hydroxyl moiety in the enzyme active site is phosphorylated or carbamylated (Fukuto 1990).
Recent research revealed that organophosphorus and carbamate pesticide residues adhered more to vegetables than fruits and cereals. This could be because vegetables have a larger surface area to size ratio than fruits and cereals, thus allowing for more contact with pesticides (Fatunsin et al. 2020). Recently, Ramadan et al. (2020) determined pesticide concentrations in 211 vegetable samples. Except for carrot, cauliflower and lettuce, all of the examined vegetables had a significant percentage of identified residues (Fig. 1).
Principle of Enzyme-Based Biosensors
Food contaminants and environmental pollutants have serious repercussions for human being health (Duford et al. 2013; Rajangam et al. 2018; Salam et al. 2016). Many environmental pollutants result from agricultural activities (Mushtaq et al. 2020), particularly pesticides that are commonly used to protect seeds and crops. Thus, their frequent use contributes to human health issues. Arduini et al. (2010) illustrated that organophosphate and carbamate pesticides inhibit acetylcholine esterase (AChE), which is an enzyme involved with the transmission of nerve impulses. Accordingly, corresponding health concerns brought stricter limitations and mounting pressure on analysis laboratories for the proper monitoring of samples. The advantages of using enzymes, such as the high specificity of enzyme–substrate interactions and the high turnover rates of biocatalysts, have made enzyme-based biosensors one of the most extensively studied areas. In addition, they are attractive in food safety and clinical research than the other non-enzymatic sensors due to their high sensitivity and specificity, portability, cost-effectiveness, and the possibilities for miniaturization and point-of-care diagnostic testing (Nguyen et al. 2019).
Pesticide detection in food and environmental samples using biosensors focuses on measuring the activity of an enzyme (pesticide detection process by biosensors is illustrated in Fig. 2). The enzyme is immobilized to transduce elements either directly or indirectly and the immobilized enzyme activity is measured in terms of current, voltage or conductivity (total ions present in the solution), or changes in optical characteristics (Xuejiang et al. 2006). The inhibition percentage is calculated as the ratio of the differences between enzyme activity before and after inhibition. The enzyme reaction could be controlled through kinetics (Clarisse et al. 2010). Most biosensors are based on kinetically controlled reactions. The percentage of inhibition is calculated by the ratio of difference between activity before and after inhibition to immobilized enzyme activity (Guodong and Yuehe 2006). The selection of the immobilization methods depends on the cost of the enzyme, the reagents, and the efficacy of immobilized enzyme in commercial applications. Newer factors such as the buffering capacity of solutions and the membranes used to entrap the biosensors are currently considered. These factors lead to enhanced sensitivity and low limits of detection for organophosphorus compounds (Zhang et al. 2009; Rajangam et al. 2018). Also, AChE immobilization on membranes and the screen-printed electrodes are advantageous as the miniaturization reduces material cost (Zhang et al. 2009). For instance, the adsorption of Creatinine deiminase in a conductometric enzyme biosensor utilizing Creatinine as a substrate (Nguyen et al. 2019). In addition, detection of organophosphorus pesticides remaining in the potted vegetables was applied successfully using potentiometric enzymatic membrane biosensor based on Methylcellulose immobilization (Zhang et al. 2009).
The AChE and butyrylcholinesterases are immobilized using glutaraldehyde and bovine serum albumin (BSA) using a cross-linking technique to detect the paraoxon ethyl, diisopropyl fluorophosphates, paraoxon‐methyl and trichlorfon (Salam et al. 2016; Duford et al. 2013). Both enzymes have different sensitivities towards pesticides, which encourages the development of multi-biosensors.
Diehl-Faxon et al. (1996) detected trichlorfon with potentiometric biosensors using enzyme immobilization. A trienzyme electrode made of highly teflonized carbon black was used. The procedure included adsorption of peroxidases and immobilization of choline oxidase and choline esterase using glutaraldehyde as a binding agent. Even after 1 month of being stored at 4 °C, the electrode retained 95% of its activity. This work exhibited the potential of using potentiometric enzyme electrodes, which included electrocatalysis of the enzymes, for fast and sensitive organophosphate assays (Rajangam et al. 2018). The AChE is immobilized around the pH electrode with membranes made of gelatine and chitosan. Improved stability is researched by changing the parameters affecting the responses of the biosensors. In addition, cholinesterase from several sources was immobilized using antimony electrode surfaces with a commercially available membrane (nylon and cellulose nitrates), glutaraldehyde vapours and aqueous solutions (Ivanov et al. 2000). Silica gel is also used as supporting material to immobilize acetylcholinesterase, which decreases the volume of the sample needed (Suwansa-ard et al. 2005). The use of a simple solution allowed operating biosensors at a low cost.
Biosensors based on 7,7,8,8‐tetracyanoquino dimethane modified screen-printed electrodes containing immobilized AChE were generated (Ivanov et al. 2003). This solved the problem of pesticide detection by activating phosphorothioate with the chloroperoxidase in the sample. The activity of the biosensor was evaluated before and after incubation with the activated specimen. This innovative enzymatic activation and detection of phosphorothioate were applied to food samples without using time-consuming and costly pre-treatment techniques. Chlorpyrifos, parathion methyl, methidathion, fenitrothion and triazophos were all tested using this method. Triazophos and chlorpyrifos were completely oxidized, but the rest were converted 54 to 61 percent of the time (Ivanov et al. 2003). Chlorpyrifos had a detection limit of 5 g/L. Pesticide analysis took two hours using this procedure.
Enzyme Inhibitor Systems
The information about the inhibition kinetics of the free and the immobilized enzymes are essential in developing inhibitory biosensors (Rajangam et al. 2018; Bucur et al. 2018; Kaur and Singh 2020). The enzyme inhibitory systems are complex, with reversible and irreversible mechanisms. The dissociation constant describing the binding affinity between the inhibitor and the enzyme (Ki value) is needed to determine the lowest detection limit. Kaur and Singh (2020) explained the non-competitive suppression of a pesticide by preincubating the enzyme with the pesticide. Both substrate and inhibitor doses affected inhibition. The degree of inhibition is determined by the incubation time. The Aldridge equation log (100/ percent I) = K2 [I] t describes the relationship between inhibition and preincubation time, where K2 is a biomolecular rate constant (Rajangam et al. 2018). A biosensor was preincubated with pesticide for different durations and the substrate was added to determine the suppression of enzyme activity (Rajangam et al. 2018), the inhibition of which was non-competitive. The choice of substrate concentration [S] is critical when conducting inhibition research and calculating the percentage of inhibition. Higher inhibition was reported at high [S], but pesticide detection was not possible at low substrate concentrations. Kuusk and Rinken (2004) explored the seismic behavior of a biosensor using tyrosinase for the determination of carbaryl (see Fig. 3). They constructed a model with two independent parameters to identify carbaryl concerning an acceptable value, which was reached within 10 min. Pesticide detection was simple and quick with this method, compared to a time-consuming steady-state analysis. Kesik et al. (2014) developed an acetylcholinesterase biosensor for serine detection. Serine was discovered to be a reversible competitive inhibitor with a detection range of 1.0–100 M in inhibition kinetic studies. Enzymes used in biosensor pesticide detection can be extracted from different natural sources such as plants (Bedair 2020; Bedair et al. 2020), algae, insects, animals or microorganisms (Table 1).
Pesticide Detection Strategies
Optical Methods
Optical Biosensors
Optical biosensors are based on enzyme inhibition (Table 2). Optical biosensors are devices that use biological molecules to detect pesticides in samples. These are effectively used to identify organophosphorus pesticide deposits in environmental samples and food. For this situation, the information is assembled from the assessment of photons (than electrons in the case of terminals). The production of optical biosensors relies upon acetylcholinesterase that returns as a bio-recognizer of the separate organophosphorus compound’s pesticides (enzymatic limitation as depicted already). The progressions in the optical limits like chemiluminescence, absorbance, and fluorescence (FL) are assessed by using optoelectronic transducers. Figure 4 shows the schematic portrayal of various optical detecting systems dependent on the hindrance of ACHE by organophosphorus compounds. Figure 5 gives a classification of enzyme inhibition-based applications for organophosphorus compounds and carbamates (Cao et al. 2020).
Optical Colorimetric Assay
The optical colorimetric assay directly tests for ACHE hindrance-based organophosphorus compounds. By utilizing the colorimetric technique, Ellman et al. (1961) proposed a strategy to ensure ACHE action in organic tissues (Cao et al. 2020). The strategy was to gauge the reaction rate of thiocholine, which is dependent on acetylthiocholine hydrolysis. Thiocholine reacts with 5, 5'- dithio-bis (2-nitrobenzoic) corrosive (DTNB) to form a yellow product. This technique is commonly used to test for pesticide accumulation in different environments. It is irrefutable that Ellman's strategy played a vital role in pesticide detection, but has inadequate sensitivity and specificity (Rajangam et al. 2018).
Metal nanoparticles containing elements such as gold (Au) and silver (Ag) are used in the detection of pesticides by affecting the dispersion and absolute state of solutions (Cao et al. 2020). Generally, gold nanoparticles (AuNPs) are red in a dispersed state, but change to steel-blue with thiocholine adsorption. Owing to the color change, different colorimetric methods using the hydrolytic reaction of AChE and AuNPs have been created. Numerous nanoparticles, notably AuNPs and platinum 4 nanoparticles (PINPs), exhibit peroxidase-like activity (Cao et al. 2020). Tetramethyl benzidine (TMB), which is highly sensitive to peroxidase activity, is often used in conjunction with nanoparticles in colorimetric tests. Hydrogen peroxide (H2O2) oxidizes tetramethyl benzidine from colourless to blue. However, the resulting sulfhydryl-containing thiocholine from the AChE reaction can reduce the catalytic efficiency of nanoparticles (Cao et al. 2020).
Rapid Test Card (Strip)
The rapid test card (strip) was devised by immobilizing protein, substrate, and chromogenic compounds on a paper system for visual confirmation of pesticides (Cao et al. 2020). This methodology has the benefits of the ability to transfer, rate, basic activity, and ease. Protein activity is viable for 2 months at 4 °C, but lower when stored at room temperature for a longer time (Sun et al. 2017). For visual detection of pesticide deposits, improving the shading goal implies improvement in the exactness (Guo et al. 2013). Examination on visual sharpness has shown that the visual cells of people are generally delicate to a frequency close to the blue-green. In this setting, Sun et al. (2011) planned a twofold film screening card, made out of glass fiber RB 65 and polyester fiber VL 78, which contains acetylcholinesterase and its substrate (indoxyl acetic acid derivation), respectively. The card was able to adsorb and deliver the full portion of protein or substrate (Cao et al. 2020).
Chemiluminescence and Fluorescence Methods
Fluorescence-Based Systems
Chemosensors can detect multiple compounds simultaneously. Cao et al. (2020) successfully predicted the concentrations of the oxyanions, oxalate, citrate, and malonate simultaneously by using the respective fluorescence changes. Contrasted and Ultraviolet-noticeable spectrophotometry, fluorescence spectrophotometry is more delicate (a few significant degrees), has a lower location limit, is less influenced by grid obstructions, and fluorescence synthetic compounds are steady at a level of durable fluorescence power (Cao et al. 2020). A few substances, (for example, indoxyl acetic acid derivation, a-naphthyl acetic acid derivation) have no fluorescence, but their hydrolysis products have, which can in a roundabout way be utilized to determine pesticide concentrations (Cao et al. 2020). Moreover, 7-acetoxy-l-methylquinolinium iodide (AMQI) was used as a fluorogenic substrate with a high fluorescence quantum yield. Quantum dot (QD) is a decent fluorescent test, whose regular fluorescence can be extinguished by the chemically created product thiocholine (Keshri et al. 2021).
Luminescent-Based Systems
The physical and substance strength of the luminescence is one of the necessities for identifying intensity. Thus, a low grapheme quantum dots (GQDs) was applied as a photoluminescence (PL) test for dichlorvos affirmation with the lowest detection limit of 0.172 mg L' using a bi-enzymatic catalysis instrument. Without organophosphorus compounds, the GODs/AChE/ChOx (choline oxidase) system showed “switch-off Photoluminescence (PL) radiation, due to the dousing of GQDs during HO creation”. With dichlorvos and methyl paraoxon, the system showed a strong PL signal as a result of the obstruction in H2O2. This strategy showed incredible recovery rates in the certifiable model assessment. Moreover, the presence of Hg2+ could impact the quenching of GQDs. Apart from PL-based procedures, chemiluminescence-based organophosphorus compounds detection methods have been applied. Overall, the chemiluminescence sensors worked on the light surge from the compound reactions with enhanced analytical displays in view of the shortfall of background sway. Thus, the advancement of LDH-@ ZIF-8 to the AChE/A TCh/Cho structure accomplished the time of OH free enthusiasts and expansion the chemiluminescence transmission through the creation of RhoB particles, while the relationship of diazinon to AChE perplexes the H2O2 creation in this manner decreases the flood of the chemical luminescence discharge.
Electrochemical Techniques
Electrochemical Biosensors
Colorimetric technique with high selectivity and affectability, which can be scaled down for on-site use (Cao et al. 2020). As prior examinations have stressed the exploration progress of compound hindrance-based biosensors for recognition of pesticides in different grids. Albeit extensive advances have been accomplished in this exploration heading, it has a specific separation from the method of useful applications (Van Dyk and Pletschke 2011). One reason is that the irreversible inhibitors structure covalent adducts with their protein targets. Nonetheless, it is feasible to reactivate the protein utilizing nucleophilic oximes, for example, Pyridine 2-aldoxime methochloride (2-PAM) (Cao et al. 2020). An electrochemical biosensor is dependent on the change in the impedance, voltage and current due to the presence of analyte on the electrode surface.
Conductometric-Based Enzyme Strategy
It can be used to study enzymatic reactions that produce changes in the concentration of charged species in a sample. Immobilized Arthrospira platensis cells on gold cathodes were used in an enzymatic conductometric biosensor to detect pesticides in water (Tekaya et al. 2013). Cholinesterase movement (AChE) was hindered by pesticides and varied neighboring conductivity was estimated after expansion of the substrate acetylthiocholine chloride (AChCl). The Michaelis–Menten steady (Km) was assessed to be 1.8 mM through an adjustment of acetylcholine chloride (AChCl) (Tekaya et al. 2013). Limitation of AChE was seen with paraoxon-methyl, parathion-methyl, triazine, and diuron with a respective recognizable detection limit of 10 − 18 M, 10 − 20 M, 10 − 20 M, and 10 − 12 M, and a respective half-maximal inhibitory concentration (IC 50) of 10 − 16 M, 10 − 20 M, 10 − 18 M, and 10 − 06 M (Tekaya et al. 2013). After 30 min of cell receptivity to pesticides, a crucial reduction in response time of 90% was seen for AChE reaction to AChCl (Tekaya et al. 2013).
Amperometry-Based Enzyme Strategy
It measures current resulting from the oxidation or reduction of an electroactive species in a biochemical reaction. For example, a sensitive amperometric technique for the identification of organophosphorus compounds was created (Ciucu et al. 2003). The procedure relies upon a ferophthalocyanine negatively changed carbon stick cathode with acetylcholinesterase and choline oxidase co-immobilized on the outside (Ciucu et al. 2003). The development of cholinesterase is immobilized with pesticides (Ciucu et al. 2003). The best inhibitory effect was found with a film containing low compound stacking and was used to improve amperometric biosensors for pesticides (Ciucu et al. 2003). The experiments were carried out using acetylcholine as a substrate; choline produced by hydrolysis in the enzymatic layer was oxidized by choline-oxidase, and the resulting hydrogen peroxide (H2O2) was detected electrochemically at + 0.35 V vs Ag/AgCl (Ciucu et al. 2003). The reducing of substrate predictable state current achieved by the development of pesticide was used for appraisal. With this technique, up to 10–10 M of paraoxon and carbofuran can be recognized (Ciucu et al. 2003).
Microfluidic Device
Biological laboratory work can be miniaturized onto a few square centimetre cards using microfluidic devices (Cao et al. 2020). Hence, it is named “chip research office” or “lab-on-a-chip.” In the early and mid-1990s, the microfluidic chip was made with thin electrophoresis. Owing to the natural advantages of conveyance ability, low model/reagent use and wastage, low working cost, and high throughput, microfabricated devices are important in various fields (Oedit et al. 2015). Currently, significant advances in food processing have been accomplished. For instance, Duford et al. arranged two diffusive microfluidic devices (one for vegetables and one for soil) to detect pesticides (Cao et al. 2020).
Nanoparticle-Based Biosensors
Carbon nanomaterials, metal oxides, conducting polymers, and clays-based materials have been proved to be effective as electrode materials for the detection of toxic pesticides and herbicides (Ramachandran et al. 2015; Abd Elkodous et al. 2021). Vohra et al. (2020), Srivastava et al. (2018), and Bucur et al. (2018) illustrated that the high sensitivity, stability, and efficiency of nanoparticle-based biosensors make them very suitable for pesticide detection on-site. As pesticide analysis is essential to enhance and improve crop yield and their impacts on human health, biosensor research led to the utilization of nanoparticles due to their selective and sensitive detection of pesticides. Silver, titanium, and gold on graphene nanocomposites and titanium dioxide (TiO2) on graphene have been used to detect small molecules including pesticides. A sensitive electrochemical sensor was developed by Govindasamy et al. (2017) using silver particles supported graphene nanoribbons for detection of Organophosphorus pesticide methyl parathion (MP) on fruits and vegetables.
Organophosphate pesticides like malathion and rhodamine, herbicides like simazine, and insecticides like monocrotophos, paraoxon, methyl parathion, phoxim, and carbofuran have been detected in water, food, and soil (Bucur et al. 2018). Interestingly, gold nanoparticles (AuNP) absorb and also scatter light at their surface plasmon resonance (SPR) wavelength region. This characteristic as well as the affordability makes AuNP a most useful optical probe (Vohra et al. 2020). The SPR characteristic of nanoparticles facilitates the identification of pesticides at very low concentrations and maintains sensor activity to a large extent with a good shelf-life. Table 3 represents an overview of nanoparticle-based biosensors reported in literature.
Nanomaterials in Biosensors Design
Maghsoudi et al. 2021a, 2021b) and Rajaji et al. (2021) illustrate that a wide range of metals like gold, silver, and copper nanoparticles, non-metallic carbon-based compounds such as graphene, carbon nanotubes, and graphite can be used in the design of biosensors (Fig. 6A, and 6B). Kucherenko et al. (2019) illustrated that the most common inorganic nanomaterials are nano particles of metals and metal oxides (TiO2, Al2O3, Fe2O3, ZrO2, MoO3, and CeO2), quantum dots and zeolites. They can be produced either physically (by fragmentation of the initial material to nano-scaled particles) or chemically (by synthesis from precursors). Inorganic nanomaterials show promise in the development of electrochemical enzyme-based biosensors, since they have unique physical and chemical properties, are easily produced, catalysed by chemical reactions, can have various surface modifications, accelerated electron transfer, improve enzyme immobilization, and biocompatibility (Kucherenko et al. 2019).
Kucherenko et al. (2019) explained that the organic nanomaterials represented by carbon nanotubes, graphene, carbon nanofibers, calixarenes, fullerenes, organic quantum dots, and inter alia are used in developing electrochemical biosensors. Organic nanomaterials have a high level of biocompatibility, but are often lower than inorganic nanomaterials. Aromatic molecules can be non-covalently bound to the surface of graphene and carbon nanotubes via strong π–π interactions. Organic nanomaterials’ hydrophobic surfaces may interact with hydrophobic compounds. Fouling, which is a serious challenge for biological applications of graphene- or carbon nanotube-based biosensors, could be the effect of this (Zhai et al. 2021).
Kucherenko et al. (2019) mentioned that nanomaterials in biosensors enhance important features such as the limit of detection, sensitivity, linear detection range, reproducibility, selectivity, response time and stability. Some valuable features of nanomaterials such as the high surface to volume ratio, ensures a remarkable rise in the surface sensitivity of the transducer and efficiency in enzyme immobilization. In addition, nanomaterials are known for their high electrical conductivity, catalytic activity and magnetic properties, which are essential for biosensors (Kucherenko et al. 2019; Zhai et al. 2021).
Embedding of Nanoparticles in Enzyme Biosensors
Kucherenko et al. (2019) explained that nanomaterials could be directly generated on the transducer surface through the application of a constant or a variable voltage, and the enzyme is then adsorbed or immobilized on the nanomaterials. Nanomaterials are integrated by immobilizing the enzyme on the material and then by attaching the enzyme/nanomaterial composite to the electrode (Fig. 7A, B, C, D). Nanomaterials in biosensors enhance electron transport, which are produced or used in the enzymatic reaction among the enzyme and transducer surface; increases the sensor surface sensitivity, enabling the adsorption of a greater number of enzyme molecules; enhances the stability of the enzyme and efficiency; and catalyzes several additional chemical reactions (Kucherenko et al. 2019).
Illustration of the embedding of nanoparticles in enzyme biosensors. (A) Enzyme immobilization on the nanomaterial-modified electrode. (B) Schematic of a biosensor based on phosphor triesterase (PTE) immobilized via glutaraldehyde on a graphene surface with platinum nanoparticles. (C) Enzyme/nanomaterial co-immobilization on the electrode. (D) Schematic of a biosensor based on glucose oxidase encapsulated in a chitosan-kappa-carrageenan bio nano-composite (Modified from Kucherenko et al. 2019 with permission)
Therefore, type of the biosensor is dependent on the utilized signal transduction technique. Because of their cheap cost, high sensitivity, tiny size, and simple design, electrochemical transducers have been frequently used in biosensors for pesticide detection (Audrey et al. 2012). In case of enzyme inhibition biosensors, type of detection technique is dependent on type of utilized substrate. For instance, potentiometric detection is favoured when acetylcholine and butyrylcholine salts are utilised as substrates for cholinesterase because of the production of H+ ions following the enzymatic reaction (Rajangam et al. 2018). However, when thiocholine and acetate salts are utilised as substrates, amperometric and piezoelectric transducers are the recommended detection techniques. The production of electroactive species and changes in mass due to the precipitation of 3-indoxyl, 4-aminophenyl, nitrophenyl, and indophenyl acetate after hydrolysis are the basis of their sensing method (Rajangam et al. 2018). Utilizing optical biosensors is limited because it is only effective in detecting organophosphorus pesticides. Fluorescence-based systems are also limited because some substances have no fluorescence (Cao et al. 2020). Luminescent biosensor relies on evaluation the intensity of light emitted of a chemiluminescent substrate. This strategy has proved its efficiency in enzyme-based biosensors, but the need for entrapping luminescent cosubstrates in case of non-luminescent substrates is a difficulty (Blum and Coulet 2000). The present demand for food contamination detection necessitates a food sample analysis that is fast, on-site, and preferably naked eye pesticide detection (Chiou et al. 2015). Hence, rapid test strip is a good choice for on-site pesticide detection, despite its low detection limit (LOD). Consequently, it is recommended to combine rapid testing screening with conventional testing methods such as chromatography for obtaining best results (Chiou et al. 2015). Whitesides (2006) was the first to suggest the concept of a “microfluidic paper analysis device” in 2006. Amazingly, application of microfluidic devices in food safety sensing is the most effective technique due to its less sample utilization, quick detection, multi-functional integration, easy handling, and mobility are all advantages of this strategy. In the future, the industrialization of microfluidic analysis system combined with aptamer, nanomaterials, and new biomolecules will become a development trend. Microfluidic chips will undergo more in-depth basic research in the coming years in many fields, especially in food safety analysis.
Parameters Influencing the Performance of Biosensors
Impact of pH
pH is a significant determinant in the characterization of the free and immobilized components of biosensors. The ionization of amino acids depends on pH, and consequently the immobilization of proteins and compounds to grids or terminal surfaces (biosensor) (Rajangam et al. 2018). The impact of pH-based framework has not received as much attention as amperometric biosensors, especially those comprising screen-printed cathodes (Rajangam et al. 2018). The research concentrated on immobilization frameworks dependent on film techniques, because the process causes adjustments in the reaction of the sensors. A potentiometric butyrylcholine sensor for organophosphate pesticides was analyzed by Imato and Ishibashi (1995) where they determined the influence of pH ranging from 2.0 to 10.5 on biosensors. The best results were recorded in the pH range 4.0 to 8.0 (Rajangam et al. 2018).
Zhang et al. (2001) developed an amperometric enzymatic biosensor for the detection of paraoxon and determined the effect of pH (ranging from 4.0 to 10.0) on the process. The authors recorded an optimal pH range between 6.5 and 7.5 with reduced detection under pH 6.0 and hydrolysis above pH 8.0. Andreescu et al. (2002) identified organophosphorus compounds in insect sprays with compound sensors (AChE and tyrosinase) (Pogačnik and Franko 2003). Different pH ranges were tested in an amperometric sensor with a phosphate buffer with the best results recorded for pH ranging from 5 to 9 (Rajangam et al. 2018). The ideal pH for the tyrosinase range was 6 to 6.5, while for AChE it was at 8.0. The detection of carbamate pesticides and organophosphates on vegetables was determined by Pogačnik and Franko (2003) with a photothermal biosensor (Rajangam et al. 2018). Two buffers were examined, namely Tris (pH 7.4) and phosphate buffer (pH 8.0) for optimal conditions for cholinesterase. Phosphate buffer was superior to Tris (Rajangam et al. 2018). A nanostructured polyacrylonitrile layer for immobilization of AChE was created by Marinov et al. (2009) and the effect of pH range 6.0 to 9.0 was tested. The ideal pH is 8.0, though diverse for immobilized AChE.
Impact of Substrate Concentration
Biosensor processes are highly dependent on substrate concentration. A biosensor’s conductometric process is used to determine organophosphorus compounds. The effect of an acetylcholine center during immobilization and substrate combinations of 8 mM was chosen for pesticide detection with reaction periods of 10–30 min. The effect of substrate concentration was minimal for carbon nanotube biosensors. A stream-dependent biosensor system for the detection of carbofuran and carbaryl was devised and the influence of the substrate centre was determined with varying acetylcholine from 0.5 to 7.0 mM; the optimum concentration was 2.5 mM (Rajangam et al. 2018).
Impact of Enzyme Concentration
Optimum biosensor procedures are determined by varying enzyme concentrations keeping in mind that smaller concentrations will irreversibly result in higher inhibitions (Rajangam et al. 2018). A potentiometric polyaniline biosensor was developed comprising of different enzymes, in Bovine Serum Albumin (BSA) and inter-linked with glutaraldehyde, with 1 uL of enzyme solution giving acceptable results (Rajangam et al. 2018). In other studies, Siriwuan et al. (in Rajangam et al. 2018) used enzyme concentrations of 200 and 150 units in potentiometric and conductometric biosensor experiments; a BSA stabilized biosensor was used and enzyme concentration of 1 U/μL. The influence of immobilization procedures such as precipitation, sol–gel, gelatine membranes, and graphite micro-particles on enzyme concentration has been studied. Precipitation of organophosphorus compounds exhibited good inhibition responses (Pohanka et al. 2011). Song et al. (2011) developed an amperometric biosensor where Prussian blue-chitosan was electropolymerized. on a carbon electrode to detect carbaryl. The authors found that an increase in enzyme concentration, increased the biosensor response until 25 U/ml after which it decreased.
Impact of Organic Solvents
Organic solvents are often used in pesticide extraction and inhibition studies, each with distinct solubilities. Thus, the influence of organic solvents on free and immobilized enzymes during biosensor reactions must be considered. Organic solvents are also the substrate, compound, and immobilized network. Subsequently, the impact of nonpolar and polar solvents on components of biosensor reactions is examined (Campanella et al. 1999).
A bienzymatic framework was created comprising choline oxidase and butyryl cholinesterase for the recognition of aldicarb and paraoxon in a 50% water-immersed chloroform-hexane combination. The biosensor reacted well in the solvent combination with a 4.5 ug/L detection limit for aldicarb and paraoxon (Rajangam et al. 2018). Andreescu et al. (2002) as cited in Rajangam et al. 2018) investigated the effect of hexane on bienzyme framework with a phenyl acetate substrate. Hexane did not inhibit the biosensor process and could thus be used for pesticide extraction. Schulze et al. (2002) created a dispensable sensor to detect the presence of paraoxon in orange juice with iso-octane as solvent. In this study, iso-octane reduced immobilized enzyme activity by 3% within 30 min of incubation. An amperometric biosensor for methyl paraoxon, carbaryl, dichlorvos, and carbofuran incubated in 20%, 10%, 5%, and 1% acetonitrile with a phosphate buffer was studied. The biosensor’s activity was low for all acetonitrile concentrations, but better in the 5% and 1% concentrations (Wilkins et al. 2000). Thus, 5% acetonitrile was used in further experiments with no change in activity. Another amperometric biosensor comprising AChE immobilized on graphite cathodes was examined and electron properties were improved by adding Prussian blue (Rajangam et al. 2018). The biosensor was tested with cyclohexanone, ethanol, propanol and benzene, of which ethanol was the best. The activity of the immobilized enzyme in 10% water–ethanol exhibited better activity than in an organic solvent framework. The dielectric constant of the enzyme activity decreased and variation in the water-polar framework could enhance electron movement in the framework (Rajangam et al. 2018).
Enzymes Previously Used in Biosensor Pesticide Detection
Research on enzyme inhibition methods for pesticide detection started in the 1980s. Kindervater et al. (1990) detected carbofuran in European drinking water using acetylcholinesterase and a flow-injection device. Many sensors were designed for organophosphorus and carbamate insecticides detection using cholinesterase with various detection methods such as semi-conductors (Vlasov et al. 1991), fiber-optics (Rogers et al. 1991), amperometric (Razumas et al. 1981), and potentiometric (Durand and Thomas 1984). Marty et al. (1993) designed biosensors with a one enzyme system, namely acetylcholinesterase for detection of organophosphorus and carbamate insecticides, aldehyde dehydrogenase for dithiocarbamate fungicides and acetolactate synthase for sulfonylurea and imidazolinone herbicides. Through the years, many enzymes other than cholinesterase were investigated in pesticide detection methods (Table 4). Pesticide detection was carried out with classic methods until 2014 when a Chinese research team developed a novel tablet based on colorimetric detection of pesticides in plants. The enzyme tablet was composed of acetylcholinesterase that hydrolyzed indoxyl acetate into indole, which was oxidized quickly in the air to form a blue-green color. In the case of pesticide persistence, the colour could not be formed (Zhu et al. 2014).
Conclusion
Biosensors measure enzyme activity to detect pesticides in food and environmental samples. Although much research had been done on developing an efficient pesticide detection method, the envisaged outcome has not been achieved yet. Biosensor methods for pesticide detection could become a useful tool for monitoring and screening contaminants and harmful substances in the agricultural sector. Pesticide detection using enzyme inhibition method is mostly dependent on cholinesterase that is mainly inhibited by organophosphate and carbamate pesticides. Although electrochemical, luminescent-based, and rapid testing strategies have proved their efficiency in food safety analysis, the microfluidic chip can condense numerous phases of sample detection on a microchip that has very high throughput compared to the traditional methods. Mass production of microfluidic analytic systems combining aptamer, nanomaterials, and novel biomolecules will become a growing trend in the coming years.
Way Forward
The review suggests that the use of enzyme inhibition for pesticide detection is useful in sustainable farming practices. Rather than relying on traditional methods of pest detection, a broader, more systemic approach is needed. Overall, eco-friendly production of crops in any nation will need conservation agricultural practices with proper pest control. Therefore, farmers should be actively engaged in environmental, ecological, and economic management, which are vital to sustainable agriculture. Researchers need to contribute to conservation agriculture to maintain optimum production levels. With regard to pesticide detection, an effective approach would be rapid test cards or microfluidic chips using enzyme inhibition. Researchers should collaborate in developing pesticide detection processes, which benefit farmers and ensure healthy crop cultivation. Although pesticide detection using enzyme inhibition technique has high conductive efficiency and detection sensitivity, biological enzymes still have limitations such as poor stability and tolerance. The sensitivity and detection limit of the enzyme inhibition-based biosensor could be enhanced in the future as follows: 1) by combining effective immobilisation technologies such as self-assembled monolayers and thin polymer films, 2) by integrating carbon nanotubes or metal nanoparticles such as Au nanomaterials, 3) by selecting the suitable transducer, 4) by modifying the natural enzymes using genetic engineering technologies to generate high tolerant and stable enzymes or 5) by employing highly effective artificial mimic enzymes to eliminate false positives results in the detection process.
References
Abd Elkodous M, El-Husseiny HM, El-Sayyad GS, Hashem AH, Doghish AS, Elfadil D, ... Matsuda A (2021) Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth. Nanotechnology Reviews 10(1): 1662-1739. https://doi.org/10.1515/ntrev-2021-0099
Abrera AT, Sützl L, Haltrich D (2020) Pyranose oxidase: a versatile sugar oxidoreductase for bioelectrochemical applications. Bioelectrochemistry 132:107409. https://doi.org/10.1016/j.bioelechem.2019.107409
Alsanosi SMM, Skiffington C, Padmanabhan S. Pharmacokinetic Pharmacogenomics (2014). Handbook of Pharmacogenomics and Stratified Medicine. Academic Press. 365–83. https://doi.org/10.22271/ed.book.795
Ambrus A, Füzesi I, Susan M, Dobi D, Lantos J, Zakar F, Katavics L (2005) A cost-effective screening method for pesticide residue analysis in fruits, vegetables, and cereal grains. J of Envir Sci and Heal 40(2):297–339. https://doi.org/10.1081/PFC-200045554
Amine A, Mohammadi H, Bourais I, Palleschi G (2006) Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens Bioelectron 21(8):1405–1423. https://doi.org/10.1016/j.bios.2005.07.012
Andreescu S, Barthelmebs L, Marty J (2002) Immobilization of acetylcholinesterase on screen-printed electrodes: comparative study between three immobilization methods and applications to the detection of organophosphorus insecticides. Anal Chim Acta 464(2):171–180. https://doi.org/10.1016/S0003-2670(02)00518-4
Arduini F, Ricci F, Tuta C, Moscone D, Amine A, Palleschi G (2006) Detection of carbamic and organophosphorous pesticides in water samples using a cholinesterase biosensor based on Prussian Blue-modified screen-printed electrode. Anal Chim Acta 580(2):155–162. https://doi.org/10.1016/j.aca.2006.07.052
Audrey S, Beatriz PS, Jean-Louis M (2012) Biosensors for pesticide detection: new trends. Am J Anal Chem 3:210–232. https://doi.org/10.4236/ajac.2012.33030
Avramescu A, Rouillon R, Carpentier R (1999) Potential for use of a cyanobacterium Synechocystis sp. immobilized in poly (vinylalcohol): Application to the detection of pollutants. Biotechnology Techniques. 13(8):559–562. https://doi.org/10.1023/A:1008991531206
Arduini F, Amine A, Moscone D, Palleschi G (2010) Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B 1 detection. Microchim Acta 170(3–4):193–214. https://doi.org/10.1007/s00604-010-0317-1
Bedair H (2020) Composition and pattern of wild trees and shrubs in the Egyptian flora. M. Sc. Thesis. Botany Department, Faculty of Science, Tanta University
Bedair H, Shaltout K, Ahmed D, Sharaf El-Din A, El-Fahhar R (2020) Characterization of the wild trees and shrubs in the Egyptian Flora. Egypt J of Botany 60(1):147–168. https://doi.org/10.21608/ejbo.2019.6982.1276
Besombes J, Cosnier S, Labbé P, Reverdy G (1995) A biosensor as warning device for the detection of cyanide, chlorophenols, atrazine and carbamate pesticides. Anal Chim Acta 311(3):255–263. https://doi.org/10.1016/0003-2670(94)00686-G
Bhalla V, Zhao X, Zazubovich V (2011) Detection of explosive compounds using Photosystem II-based biosensor. J Electroanal Chem 657(1–2):84–90. https://doi.org/10.1016/j.jelechem.2011.03.026
Bhavadharani M, Janaki P, Roseleen R, Sheeba Joyce Arulmozhiselvan K, Ejilane J. (2019).Evaluation of rapid enzyme inhibition test for pesticides detection and validation by spectrophotometer and GC/MS. Pharm. Innov, 8:1069–1074. http://www.thepharmajournal.com/
Blum LJ, Coulet PR (2000). Luminescent biosensors. In Biosensors and Their Applications (pp. 213–223). Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-4181-3_12
Braham Y, Barhoumi H, Maaref A (2013) Urease capacitive biosensors using functionalized magnetic nanoparticles for atrazine pesticide detection in environmental samples. Anal Methods 5(18):4898–4904. https://doi.org/10.1039/C3AY40579F
Bucur B, Munteanu D, Marty L, Vasilescu A (2018) Advances in enzyme-based biosensors for pesticide detection. Biosensors 8(2):27. https://doi.org/10.3390/bios8020027
Cao J, Wang M, Yu H, She Y, Cao Z, Ye J, Lao S (2020) An overview on the mechanisms and applications of enzyme inhibition-based methods for determination of organophosphate and carbamate pesticides. J Agric Food Chem 68(28):7298–7315. https://doi.org/10.1021/acs.jafc.0c01962
Campanella L, De Luca S, Sammartino P, Tomassetti M (1999) A new organic phase enzyme electrode for the analysis of organophosphorus pesticides and carbamates. Anal Chim Acta 385(1–3):59–71. https://doi.org/10.1016/S0003-2670(98)00806-X
Chiou J, Leung HH, Lee HW, Wong WT (2015) Rapid testing methods for food contaminants and toxicants. J Integr Agric 14(11):2243–2264. https://doi.org/10.1016/S2095-3119(15)61119-4
Chowdhury Z, Zain M, Khan A, Rafique F, Khalid K (2012) Batch and fixed bed adsorption studies of lead (II) cations from aqueous solutions onto granular activated carbon derived from Mangostana garcinia shell. BioResources 7(3):2895–2915. https://doi.org/10.15376/biores.7.3.2895-2915
Chowdhury Z, Fakhruddin M, Islam N, Moniruzzaman M, Gan H, Alam K (2013) Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography–mass spectrometry. Food Control 34(2):457–465. https://doi.org/10.1016/j.foodcont.2013.05.006
Ciucu A, Negulescu C, Baldwin P (2003) Detection of pesticides using an amperometric biosensor based on ferophthalocyanine chemically modified carbon paste electrode and immobilized bienzymatic system. Biosens Bioelectron 18(2–3):303–310. https://doi.org/10.1016/S0956-5663(02)00173-2
Clarisse BR, Susanne BM, Schulze H, Bachmann TT et al (2010) Analysis of phosphorothionate pesticides using a choroperoxidase pretreatment and acetylcholinesterase biosensor detection. J Agric Food Chem 58:8748–8756
Del Carlo M, Pepe A, Mascini M, De Gregorio M, Visconti A, Compagnone D (2005) Determining pirimiphos-methyl in durum wheat samples using an acetylcholinesterase inhibition assay. Anal Bioanal Chem 381(7):1367–1372. https://doi.org/10.1007/s00216-004-3013-3
Diehl-Faxon J, Ghindilis L, Atanasov P, Wilkins E (1996) Direct electron transfer based tri-enzyme electrode for monitoring of organophosphorus pesticides. Sens Actuators, B Chem 36(1–3):448–457. https://doi.org/10.1016/S0925-4005(97)80112-8
Duford A, Xi Y, Salin D (2013) Enzyme inhibition-based determination of pesticide residues in vegetable and soil in centrifugal microfluidic devices. Anal Chem 85(16):7834–7841. https://doi.org/10.1021/ac401416w
Durand P, Thomas D (1984) Use of immobilized enzyme coupled with an electrochemical sensor for the detection of organophosphates and carbamates pesticides. Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer 5(4–5):51–57 (PMID:6520739)
Ellman G, Courtney D, Andres V, Featherstone M (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Fatunsin OT, Oyeyiola AO, Moshood MO, Akanbi LM, Fadahunsi DE (2020) Dietary risk assessment of organophosphate and carbamate pesticide residues in commonly eaten food crops. Scientific African 8:e00442. https://doi.org/10.1016/j.sciaf.2020.e00442
Fukuto R (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environmental health perspectives 87:245–254. https://doi.org/10.1289/ehp.9087245
Ghosh S, Falyouna O, Malloum A, Othmani A, Bornman C, Bedair H, ... & Ahmadi S (2021) A general review on the use of advance oxidation and adsorption processes for the removal of furfural from industrial effluents. Microporous and Mesoporous Materials, 111638. https://doi.org/10.1016/j.micromeso.2021.111638
Gai K, Qi H, Zhu X, Wang M. (2019). Preparation of Ag-Fe3O4 nanoparticles sensor and application in detection of methomyl. In E3S Web of Conferences (Vol. 118, p. 01002). EDP Sciences. https://doi.org/10.20964/2019.02.43
Giardi T, Guzzella L, Euzet P, Rouillon R, Esposito D (2005) Detection of herbicide subclasses by an optical multibiosensor based on an array of photosystem II mutants. Environ Sci Technol 39(14):5378–5384. https://doi.org/10.1021/es040511b
Giardi T, Scognamiglio V, Rea G, Rodio G, Antonacci A, Lambreva M, Johanningmeier U (2009) Optical biosensors for environmental monitoring based on computational and biotechnological tools for engineering the photosynthetic D1 protein of Chlamydomonas reinhardtii. Biosens Bioelectron 25(2):294–300. https://doi.org/10.1016/j.bios.2009.07.003
Govindasamy M, Mani V, Chen SM, Chen TW, Sundramoorthy AK (2017) Methyl parathion detection in vegetables and fruits using silver@ graphene nanoribbons nanocomposite modified screen printed electrode. Sci Rep 7(1):1–11
Grimalt S, Dehouck P (2016) Review of analytical methods for the determination of pesticide residues in grapes. J Chromatogr A 1433:1–23. https://doi.org/10.1016/j.chroma.2015.12.076
Guo X, Zhang X, Cai Q, Shen T, Zhu S (2013) Developing a novel sensitive visual screening card for rapid detection of pesticide residues in food. Food Control 30(1):15–23. https://doi.org/10.1016/j.foodcont.2012.07.015
Guodong L, Yuehe L (2006) Biosensor based on self-assembling acetylcholinesterase on carbon nanotubes for flow injection amperometric detection of organophosphate pesticides and nerve agents. Anal Chem 78:835–843
Hagstrom D, Hirokawa H, Zhang L, Radic Z, Taylor P, Collins S (2017) Planarian cholinesterase: in vitro characterization of an evolutionarily ancient enzyme to study organophosphorus pesticide toxicity and reactivation. Arch Toxicol 91(8):2837–2847. https://doi.org/10.1007/s00204-016-1908-3
Huertas-Pérez F, García-Campaña M (2008) Determination of N-methylcarbamate pesticides in water and vegetable samples by HPLC with post-column chemiluminescence detection using the luminol reaction. Anal Chim Acta 630(2):194–204. https://doi.org/10.1016/j.aca.2008.09.047
Imato T, Ishibashi N (1995) Potentiometric butyrylcholine sensor for organophosphate pesticides. Biosens Bioelectron 10(5):435–441. https://doi.org/10.1016/0956-5663(95)96890-B
Ivanov N, Evtugyn A, Gyurcsányi RE, Toth K, Budnikov HC (2000) Comparative investigation of electrochemical cholinesterase biosensors for pesticide determination. Anal Chim Acta 404(1):55–65. https://doi.org/10.1016/S0003-2670(99)00683-2
Ivanov A, Evtugyn G, Budnikov H, Ricci F, Moscone D, Palleschi G (2003) Cholinesterase sensors based on screen-printed electrodes for detection of organophosphorus and carbamic pesticides. Anal Bioanal Chem 377(4):624–631. https://doi.org/10.1007/s00216-003-2174-9
Kaoutit E, Bouchta D, Zejli H, Izaoumen N, Temsamani R (2004) A Simple Conducting Polymer-Based Biosensor for the Detection of Atrazine. Analytical Letters 37(8):1671–1681. https://doi.org/10.1081/AL-120037595
Kartal F, KilinÇ A, Timur S (2007) Lipase biosensor for tributyrin and pesticide detection. Int J Environ Anal Chem 87(10–11):715–722. https://doi.org/10.1080/03067310701327741
Kaur J, Singh K (2020) Enzyme-based optical biosensors for organophosphate class of pesticide detection. Phys Chem Chem Phys 22(27):15105–15119. https://doi.org/10.1039/D0CP01647K
Keshri K, Mandal K, Kumar Y, Yadav D, Mukhopadhyay P (2021) Naphthalenediimides with High Fluorescence Quantum Yield: Bright-Red, Stable, and Responsive Fluorescent Dyes. Chemistry (weinheim an Der Bergstrasse, Germany) 27(23):6954–6962. https://doi.org/10.1002/chem.202100020
Kesik M, Kanik E, Turan J, Kolb M, Timur S, Bahadir M, Toppare L (2014) An acetylcholinesterase biosensor based on a conducting polymer using multiwalled carbon nanotubes for amperometric detection of organophosphorous pesticides. Sensors and Actuators B: Chemical 205:39–49. https://doi.org/10.1016/j.snb.2014.08.058
Khaled E, Kamel S, Hassan A, Abdel-Gawad H, Aboul-Enein Y (2014) Performance of a Portable Biosensor for the Analysis of Ethion Residues. Talanta 119:467–472. https://doi.org/10.1016/j.talanta.2013.11.001
Kindervater R, Künnecke W, Schmid D (1990) Exchangeable immobilized enzyme reactor for enzyme inhibition tests in flow-injection analysis using a magnetic device. Determination of pesticides in drinking water. Anal Chim Acta 234:113–117. https://doi.org/10.1016/S0003-2670(00)83545-X
Koblížek M, Malý J, Masojídek J, Komenda J, Kučera T, Giardi T, Pilloton R (2002) A biosensor for the detection of triazine and phenylurea herbicides designed using Photosystem II coupled to a screen-printed electrode. Biotechnol Bioeng 78(1):110–116. https://doi.org/10.1002/bit.10190
Koblizek M, Masojidek J, Komenda J, Kucera T, Pilloton R, Mattoo K, Giardi T (1998) A sensitive photosystem II-based biosensor for detection of a class of herbicides. Biotechnol Bioeng 60(6):664–669. https://doi.org/10.1002/(SICI)1097-0290(19981220)60:6%3c664::AID-BIT3%3e3.0.CO;2-B
Kolakowski M, Miller L, Murray A, Leclair A, Bietlot H, van de Riet M (2020) Analysis of glyphosate residues in foods from the Canadian retail markets between 2015 and 2017. J Agric Food Chem 68(18):5201–5211. https://doi.org/10.1021/acs.jafc.9b07819
Kucherenko S, Soldatkin O, Kucherenko Y, Soldatkina V, Dzyadevych V (2019) Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Advances 1(12):4560–4577. https://doi.org/10.1039/C9NA00491B
Kuusk E, Rinken T (2004) Transient phase calibration of tyrosinase-based carbaryl biosensor. Enzyme Microb Technol 34(7):657–661. https://doi.org/10.1016/j.enzmictec.2004.03.004
LeDoux M (2011) Analytical methods applied to the determination of pesticide residues in foods of animal origin. A review of the Past Two Decades. J Chromatogr A 1218(8):1021–1036. https://doi.org/10.1016/j.chroma.2010.12.097
Maly J, Masojidek J, Masci A, Ilie M, Cianci E, Foglietti V, Pilloton R (2005) Direct mediatorless electron transport between the monolayer of photosystem II and poly (mercapto-p-benzoquinone) modified gold electrode-new design of biosensor for herbicide detection. Biosens Bioelectron 21(6):923–932. https://doi.org/10.1016/j.bios.2005.02.013
Marinov I, Gabrovska K, Velichkova J, Godjevargova T (2009) Immobilization of acetylcholinesterase on nanostructure polyacrylonitrile membranes. Int J Biol Macromol 44(4):338–345. https://doi.org/10.1016/j.ijbiomac.2009.01.008
Maghsoudi S, Hassani S, Mirnia K, Abdollahi M (2021) Recent advances in nanotechnology-based biosensors development for detection of arsenic, lead, mercury, and cadmium. International Journal of Nanomedicine 16:803. https://doi.org/10.2147/IJN.S294417
Marty L, Mionetto N, Noguer T, Ortega F, Roux C (1993) Enzyme sensors for the detection of pesticides. Biosens Bioelectron 8(6):273–280. https://doi.org/10.1016/0956-5663(93)85007-B
Masojídek J, Souček P, Máchová J, Frolík J, Klem K, Malý J (2011) Detection of photosynthetic herbicides: Algal growth inhibition test vs. electrochemical photosystem II biosensor. Ecotoxicology and Environmental safety 74(1):117–122. https://doi.org/10.1016/j.ecoenv.2010.08.028
Mazzei L, Ciurli S, Zambelli B (2016) Isothermal titration calorimetry to characterize enzymatic reactions. Methods Enzymol 567:215–236. https://doi.org/10.1016/bs.mie.2015.07.022
Moro L, Pezzotti G, Turemis M, Sanchís J, Farré M, Denaro R, Giardi T (2018) Fast pesticide pre-screening in marine environment using a green microalgae-based optical bioassay. Mar Pollut Bull 129(1):212–221. https://doi.org/10.1016/j.marpolbul.2018.02.036
Mushtaq W, Bedair H, Shakeel A (2020) Halophytes: A Phytoremediation Tool for Salt-Affected Soils with Special Reference to Indian Subcontinent. Handbook of Halophytes: from Molecules to Ecosystems towards Biosaline Agriculture 1:16. https://doi.org/10.1007/978-3-030-17854-3_95-1
Maghsoudi S, Hassani Mirnia K, Abdollahi M (2021) Recent advances in nanotechnology-based biosensors development for detection of arsenic, lead, mercury, and cadmium. International Journal of Nanomedicine 16:803. https://doi.org/10.2147/IJN.S294417
Nguyen HH, Lee SH, Lee UJ, Fermin CD, Kim M (2019) Immobilized enzymes in biosensor applications. Materials 12(1):121. https://doi.org/10.3390/ma12010121
Nishant N, Upadhyay R (2016) Presence of pesticide residue in vegetable crops: A review. Agricultural Reviews 37(3):173–185
Oedit A, Vulto P, Ramautar R, Lindenburg W, Hankemeier T (2015) Lab-on-a-Chip hyphenation with mass spectrometry: strategies for bioanalytical applications. Curr Opin Biotechnol 31:79–85. https://doi.org/10.1016/j.copbio.2014.08.009
Oliveira C, Moccelini K, Castilho M, Terezo J, Possavatz J, Magalhães R, Dores F (2012) Biosensor Based on Atemoya Peroxidase Immobilised on Modified Nanoclay for Glyphosate Biomonitoring. Talanta 98:130–136. https://doi.org/10.1016/j.talanta.2012.06.059
Pandey R, Joshi K, Joshi R, Shrestha J, Dhakal M (2017). Package of Practices for Climate Resilient Value Chain Development of Major Vegetables in Udayapur, Nepal. t www.icimod.org/himaldoc
Pogačnik L, Franko M (2003) Detection of organophosphate and carbamate pesticides in vegetable samples by a photothermal biosensor. Biosens Bioelectron 18(1):1–9. https://doi.org/10.1016/S0956-5663(02)00056-8
Pohanka M, Drobik O, Krenkova Z, Zdarova-Karasova J, Pikula J, Cabal J, Kuca K (2011) Voltammetric biosensor based on acetylcholinesterase and different immobilization protocols: A simple Tool for Toxic Organophosphate Assay. Anal Lett 44(7):1254–1264. https://doi.org/10.1080/00032719.2010.511745
Rajangam B, Daniel K, Krastanov I (2018a) Progress in Enzyme Inhibition Based Detection of Pesticides. Eng Life Sci 18(1):4–19. https://doi.org/10.1002/elsc.201700028
Ramachandran R, Mani V, Chen S, kumar G, Govindasamy M, (2015) Recent developments in electrode materials and methods for pesticide analysis-an overview. Int J Electrochem Sci 10:859–869
Rasmussen M, Minteer D (2013) Self-powered herbicide biosensor utilizing thylakoid membranes. Anal Methods 5(5):1140–1144. https://doi.org/10.1039/C3AY26488B
Rasmussen M, Wingersky A, Minteer D (2014) Comparative study of thylakoids from higher plants for solar energy conversion and herbicide detection. Electrochim Acta 140:304–308. https://doi.org/10.1016/j.electacta.2014.02.121
Ramadan MF, Abdel-Hamid M, Altorgoman MM, AlGaramah HA, Alawi MA, Shati AA, ... & Awwad NS (2020). Evaluation of pesticide residues in vegetables from the Asir Region, Saudi Arabia.Molecules 25(1):205. https://doi.org/10.3390/molecules25010205
Razumas V, Kulys J, Malinauskas A (1981) High-sensitivity bioamperometric determination of organophosphate insecticides. Environ Sci Technol 15(3):360–361. https://doi.org/10.1021/es00085a015
Reddy G, Madhavi G, Swamy K (2014) Mobilized lipase enzymatic biosensor for the determination of Chlorfenvinphos and Malathion in contaminated water samples: A voltammetric study. J Mol Liq 198:181–186. https://doi.org/10.1016/j.molliq.2014.06.019
Rajaji U, Chinnapaiyan S, Chen SM, Govindasamy M, Oliveira Filho JID, Khushaim W, Mani V (2021) Design and Fabrication of Yttrium Ferrite Garnet-Embedded Graphitic Carbon Nitride: A Sensitive Electrocatalyst for Smartphone-Enabled Point-of-Care Pesticide (Mesotrione) Analysis in Food Samples. ACS Appl Mater Interfaces 13:24865–24876. https://doi.org/10.1021/acsami.1c04597
Rogers R, Cao J, Valdes J, Eldefrawi T, Eldefrawi ME (1991) Acetylcholinesterase fiber-optic biosensor for detection of anticholinesterases. Toxicol Sci 16(4):810–820. https://doi.org/10.1093/toxsci/16.4.810
Sacks V, Eshkenazi I, Neufeld T, Dosoretz C, Rishpon J (2000) Immobilized parathion hydrolase: An amperometric sensor for parathion. Anal Chem 72(9):2055–2058. https://doi.org/10.1021/ac9911488
Sahin A, Dooley K, Cropek M, West C, Banta S (2011) A dual enzyme electrochemical assay for the detection of organophosphorus compounds using organophosphorus hydrolase and horseradish peroxidase. Sens Actuators, B Chem 158(1):353–360. https://doi.org/10.1016/j.snb.2011.06.034
Salam F, Puat A, Abd Rahman G, Ramli M, Mohamed A, Kadir A, Wan C (2016) Comparative study of pesticides analysis using enzyme inhibition sensor and gas chromatography methods. Procedia Chemistry 20:33–39. https://doi.org/10.1016/j.proche.2016.07.005
Schulze H, Schmid D, Bachmann T (2002) Rapid detection of neurotoxic insecticides in food using disposable acetylcholinesterase-biosensors and simple solvent extraction. Anal Bioanal Chem 372(2):268–272. https://doi.org/10.1007/s00216-001-1137-2
Scognamiglio V, Pezzotti I, Pezzotti G, Cano J, Manfredonia I, Buonasera K, Giardi T (2013) A new embedded biosensor platform based on micro-electrodes array (MEA) technology. Sens Actuators, B Chem 176:275–283. https://doi.org/10.1016/j.snb.2012.09.101
Scognamiglio V, Raffi D, Lambreva M, Rea G, Tibuzzi A, Pezzotti G, Giardi T (2009) Chlamydomonas reinhardtii genetic variants as probes for fluorescence sensing system in detection of pollutants. Anal Bioanal Chem 394(4):1081–1087. https://doi.org/10.1007/s00216-009-2668-1
Shan D, Wang Y, Zhu M, Xue H, Cosnier S, Wang C (2009) Development of a high analytical performance-xanthine biosensor based on layered double hydroxides modified-electrode and investigation of the inhibitory effect by allopurinol. Biosens Bioelectron 24(5):1171–1176. https://doi.org/10.1016/j.bios.2008.07.023
Singh S, Kumar V, Chauhan A, Datta S, Wani AB, Singh N, Singh J (2018) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16(1):211–237. https://doi.org/10.1007/s10311-017-0665-8
Song Y, Zhang M, Wang L, Wan L, Xiao X, Ye S, Wang J (2011) A novel Biosensor Based on Acetylecholinesterase/prussian Blue–chitosan Modified Electrode for Detection of Carbaryl Pesticides. Electrochim Acta 56(21):7267–7271. https://doi.org/10.1016/j.electacta.2011.06.054
Songa A, Waryo T, Jahed N, Baker G, Kgarebe V, Iwuoha I (2009) Electrochemical nanobiosensor for glyphosate herbicide and its metabolite. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis 21(3–5):671–674. https://doi.org/10.1002/elan.200804452
Srivastava K, Dev A, Karmakar S (2018) Nanosensors and nanobiosensors in food and agriculture. Environ Chem Lett 16(1):161–182. https://doi.org/10.1007/s10311-017-0674-7
Sun J, Guo L, Bao Y, Xie J (2011) A simple, label-free AuNPs-based colorimetric ultrasensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosens Bioelectron 28(1):152–157. https://doi.org/10.1016/j.bios.2011.07.012
Sun Z, Tian L, Guo M, Xu X, Li Q, Weng H (2017) A double-Film Screening Card for Rapid Detection of Organophosphate and Carbamate Pesticide Residues by one Step in Vegetables and Fruits. Food Control 81:23–29. https://doi.org/10.1016/j.foodcont.2017.05.012
Suwansa-ard S, Kanatharana P, Asawatreratanakul P, Limsakul C, Wongkittisuksa B, Thavarungkul P (2005) Semi disposable reactor biosensors for detecting carbamate pesticides in water. Biosens Bioelectron 21(3):445–454. https://doi.org/10.1016/j.bios.2004.11.005
Tekaya N, Saiapina O, Ouada B, Lagarde F, Ouada B, Jaffrezic-Renault N (2013) Ultra-sensitive conductometric detection of pesticides based on inhibition of esterase activity in Arthrospira platensis. Environ Pollut 178:182–188. https://doi.org/10.1016/j.envpol.2013.03.013
Tibuzzi A, Rea G, Pezzotti G, Esposito D, Johanningmeier U, Giardi T (2007) A new miniaturized multiarray biosensor system for fluorescence detection. J Phys: Condens Matter 19(39):395006. https://doi.org/10.1088/0953-8984/19/39/395006
Touloupakis E, Boutopoulos C, Buonasera K, Zergioti I, Giardi T (2012) A photosynthetic biosensor with enhanced electron transfer generation realized by laser printing technology. Anal Bioanal Chem 402(10):3237–3244. https://doi.org/10.1007/s00216-012-5771-7
Touloupakis E, Giannoudi L, Piletsky A, Guzzella L, Pozzoni F, Giardi T (2005) A multi-biosensor based on immobilized Photosystem II on screen-printed electrodes for the detection of herbicides in river water. Biosens Bioelectron 20(10):1984–1992. https://doi.org/10.1016/j.bios.2004.08.035
Van Dyk S, Pletschke B (2011) Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 82(3):291–307. https://doi.org/10.1016/j.chemosphere.2010.10.033
Ventrella A, Catucci L, Placido T, Longobardi F, Agostiano A (2011) Biomaterials based on photosynthetic membranes as potential sensors for herbicides. Biosens Bioelectron 26(12):4747–4752. https://doi.org/10.1016/j.bios.2011.05.043
Vlasov Y, Bratov A, Levichev S, Tarantov Y (1991) Enzyme semiconductor sensor based on butyrylcholinesterase. Sens Actuators, B Chem 4(3–4):283–286. https://doi.org/10.1016/0925-4005(91)80123-2
Vohra T, Grover H , Saxena S, Verma K, Rameshwari R (2020). A Review on Nanoparticles Based Biosensors for Pesticide Detection in Water 1–10. http://meddocsonline.org/
Wang L, Xia Q, Zhang P, Hu Y, Lin M (2012) Determination of organophosphorus pesticide residues in vegetables by an enzyme inhibition method using α-naphthyl acetate esterase extracted from wheat flour. J Zhejiang Univ Sci B 13(4):267–273. https://doi.org/10.1631/jzus.B11a0180
Whitesides MG (2006) The origins and the future of microfluidics. Nature 442:368–373
Wilkins E, Carter M, Voss J, Ivnitski D (2000) A quantitative determination of organophosphate pesticides in organic solvents. Electrochemistry Communications 2(11):786–790. https://doi.org/10.1016/S1388-2481(00)00122-3
Xuejiang W, Ling C, Siqing X, Zhiliang Z et al (2006) Tyrosinase biosensor based on interdigited electrodes for herbicides determination. Int J Electrochem Sci 1:55–61
Yang Q, Qu Y, Bo Y, Wen Y, Huang S (2010) Biosensor for atrazin based on aligned carbon nanotubes modified with glucose oxidase. Microchim Acta 168(3–4):197–203. https://doi.org/10.1007/s00604-009-0272-x
Yazgan I, Aydin T, Odaci D, Timur, (2008) Use of pyranose oxidase enzyme in inhibitor biosensing. Anal Lett 41(11):2088–2096. https://doi.org/10.1080/00032710802209276
Yu Z, Zhao G, Liu M, Lei Y, Li M (2010) Fabrication of a novel atrazine biosensor and its subpart-per-trillion levels sensitive performance. Environ Sci Technol 44(20):7878–7883. https://doi.org/10.1021/es101573s
Zamaleeva I, Sharipova R, Shamagsumova V, Ivanov N, Evtugyn A, Ishmuchametova G, Fakhrullin F (2011) A whole-cell amperometric herbicide biosensor based on magnetically functionalised microalgae and screen-printed electrodes. Anal Methods 3(3):509–513. https://doi.org/10.1039/C0AY00627Kn
Zhai R, Chen G, Liu G, Huang X, Xu X, Li L, ... & Abd El-Aty A M (2021). Enzyme inhibition methods based on Au nanomaterials for rapid detection of organophosphorus pesticides in agricultural and environmental samples: A review. Journal of Advanced Research. https://doi.org/10.1016/j.jare.2021.08.008
Zhang S, Zhao H, John R (2001) Development of a quantitative relationship between inhibition percentage and both incubation time and inhibitor concentration for inhibition biosensors-theoretical and practical considerations. Biosens Bioelectron 16(9–12):1119–1126. https://doi.org/10.1016/S0956-5663(01)00240-8
Zhang J, Luo A, Liu P, Wei S, Wang G, Wei S (2009) Detection of organophosphorus pesticides using potentiometric enzymatic membrane biosensor based on methylcellulose immobilization. Anal Sci 25(4):511–515. https://doi.org/10.2116/analsci.25.511
Zhu S, Zhou C, He J, Zhang X, Guo X (2014) Rapid colormetric detection of pesticide residues based on enzyme inhibition method. Transactions of the Chinese Society of Agricultural Engineering 30(6):242–248. https://doi.org/10.3969/j.issn.1002-6819.2014.06.029
Acknowledgements
The authors would like to thank Tanta University, Tanta, Egypt, for its support in the current work. The authors also acknowledge the support of Alexandria University, Alexandria, Egypt, and Paklihawa Campus, Institute of Agriculture and Animal Science, Tribhuvan University, Bhairahawa, Lumbini, Nepal. Finally, the authors would like to acknowledge Universidad Autónoma de Nuevo León, Mexico, and the University of the Free State, Bloemfontein, South Africa, for their valuable support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Informed Consent
Informed consent not applicable.
Conflict of Interest
Heba Bedair declares that she has no conflict of interest. Hadeer Abdulrahman Rady declares that she has no conflict of interest. Aya Misbah Hussien declares that she has no conflict of interest. Meena Pandey declares that she has no conflict of interest. Wilgince Apollon declares that he has no conflict of interest. Samar Sami AlKafaas declares that she has no conflict of interest. Soumya Ghosh declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bedair, H., Rady, H.A., Hussien, A.M. et al. Pesticide Detection in Vegetable Crops Using Enzyme Inhibition Methods: a Comprehensive Review. Food Anal. Methods 15, 1979–2000 (2022). https://doi.org/10.1007/s12161-022-02254-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-022-02254-x