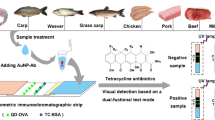
Abstract
Colloidal gold, quantum dots (QDs), and upconversion nanoparticles (UCNPs), which were respectively labeled with monoclonal antibody against norfloxacin (NOR), were employed as signal probes to establish three types of immunochromatographic strips, and the strips were evaluated according to their ability to detect NOR in milk samples. The results showed that the visual detection limit of NOR was 2.0 μg L−1 in the standard solution and 20.0 μg L−1 in milk samples when using colloidal gold-immunochromatographic strips (ICS). When QD-based fluorescence immunochromatographic strips (FICS) were used, the visual detection limit was 2.0 μg L−1 in the standard solution and 10.0 μg L−1 in milk samples. If UCNP-based FICS strips were employed, the visual limit of detection was 0.5 μg L−1 in the standard solution and 2.5 μg L−1 in milk samples. Besides, the results from the proposed methods showed high agreement with results yielded by using commercial ELISA kits, indicating the good accuracy of these strips. Compared with colloidal gold-ICS, QDs-FICS showed similar sensitivity in the standard solution and a two-fold higher sensitivity in milk samples. Meanwhile, UCNPs-FICS yielded the highest sensitivity both in standard solution and in milk samples, and they were also more stable in normal laboratory conditions (that is, they could be exposed to regular room light). Additionally, the two fluorescence nanoparticle-based test strips could be built with less antibody and coating antigens and can be cost-effective. In conclusion, the proposed QDs-FICS and UCNPs-FICS could be employed as cost-effective alternative methods for sensitive, rapid, and on-site detection of NOR in milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoroquinolones (FQs) are synthetic antibacterial agents with a broad-spectrum activity against gram-positive and gram-negative bacteria by destroying bacterial DNA gyrase and topoisomerase IV and are widely used for the treatment of bacterial diseases in humans and animals (Anaya-Gonzalez et al. 2019; Chawla et al. 2018; Cuprys et al. 2018). Long-term consumption of animal-derived foods that contain low-level fluoroquinolone antibacterial agents may reduce the efficacy of such compounds to treat infections, and it may also cause many side effects in humans, such as headache, dizziness, nausea, and vomiting (Kurrey et al. 2019; Tomas et al. 2019; Xu et al. 2019). The China Food and Drug Administration (CFDA) issued Announcement No. 79 in 2017 regarding fluoroquinolone drugs, and its clinical warnings include 17 active ingredients such as norfloxacin, ofloxacin, and ciprofloxacin. The U.S. Food and Drug Administration (FDA) banned the sale and use of enoxacin in poultry products in 2005 (Alhusban et al. 2019), and the European Union (EU) established maximum residue limits (MRLs) for certain veterinary medicines in foodstuffs of animal origin (Council Regulation EEC No. 2377/901990) (Sheng et al. 2011).
Various methods including microbiological assay (Tumini et al. 2017; Wagman et al. 2017), spectrofluorometry (Chen et al. 2016), capillary electrophoresis (Ibarra et al. 2012), electrochemical sensor (Abnous et al. 2017; Canales et al. 2019; Majdinasab et al. 2019), gas chromatography-mass spectrometry (GC-MS) (Caro et al. 2006), high-performance liquid chromatography (HPLC) with diode array (Zheng et al. 2014), fluorescence (Duan et al. 2017; Pochivalov et al. 2017), and mass spectrometry (MS) (Alhusban et al. 2019; Szerkus et al. 2017) had been utilized to detect FQ residues in animal-derived foods and biological samples. These methods have many advantages undoubtedly. Nevertheless, there was some inherent imperfectness in these technologies, such as being time consuming, its high cost, requiring professional operators, and complex manipulation. Compared with other analytic methods, rapid immunochromatographic strips (ICS) based on the specific recognition between antigen and antibody show significant advantages: simpler operation (one step), faster detection (within 10 min), and easy result judgment, which could be efficiently implemented in detecting bioactive components in natural products (Ding et al. 2019; Song et al. 2019; Wang et al. 2018; Wangman et al. 2016; Wu et al. 2017a).
Fluorescent nanoparticles, especially lanthanide-doped upconversion nanoparticles (UCNPs, which showed advantages of strong fluorescence in visible region under excitation by near-infrared light, typically 980 nm) (Rafique et al. 2019; Tian et al. 2019; Yan et al. 2018) and quantum dots (QDs, which were unique fluorescent semiconductor nanoparticles, highly versatile for biological applications) (Li et al. 2019; Liu et al. 2019; Tan et al. 2020), have attracted much attention in recent years. They have significant advantages as fluorescent bio-labels over traditional organic fluorophores owing to their attractive optical and chemical features, such as low autofluorescence, photochemical stability, low toxicity, large Stokes shifts, and high resistance to photobleaching. These properties render UCNPs and QDs robust signal labels for developing highly sensitive ICS.
Various ICS detection systems based on UCNPs and QDs have been established (Di Nardo et al. 2016; Wu et al. 2017b; Yang et al. 2011; Zhang et al. 2019). However, few studies have focused on the performance of different labels for the same analyte. A systematic and comprehensive study of the performance of different labels, including UCNPs, QDs, and colloidal gold, is presented in this paper for visual detection of norfloxacin (NOR) in milk samples, boosting the application of fluorescent nanomaterials in immunochromatography.
Materials and Methods
Reagents and Chemicals
Rare earth nitrates used in this work, including C6H9Er.xH2O and C6H9O6Yb.4H2O, were of 99.9% purity. Fluoroquinolone standards, N-hydroxysuccinimide (NHS), 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDC), ovalbumin (OVA), bovine serum albumin (BSA), and poly(acrylic acid) (PAA) were of analytical grade. All abovementioned reagents and goat anti-mouse IgG were purchased from Sigma-Aldrich (St. Louis, MO, USA). C6H17O10Yb was purchased from Aladdin Industrial Inc. (Shanghai, China). The carboxyl-modified QDs were from Wuhan Jiayuan Quantum Dots Co., Ltd. (Wuhan, China). The commercial ELISA kits were purchased from Reagent Technology Co., Ltd. (Shenzhen, China). Anti-NOR monoclonal antibody (McAb) was purchased from Shandong Lvdu Bioscience and Technology Co., Ltd. (Shandong, China). All other reagents used in this experiment were purchased from Tianjin No.1 Chemical Reagent Factory (Tianjin, China). The solutions were prepared with deionized water from a Milli-Q system (Millipore, Billerica, MA, USA).
Nitrocellulose (NC) membranes (HF90, HF135, and HF180) were purchased from Millipore (Bedford, MA, USA). Glass fiber membrane (SB 06), absorbent pad, polyvinyl chloride sheets, and filter paper were purchased from Kinbio Tech Co. (Shanghai, China).
Apparatus
The size and morphology of the nanoparticles were determined using a JEM-2010 FEF transmission electron microscope (TEM) (JEOL Ltd., Japan) operated at 200 kV and a scanning electron microscope (SEM) (1530VP, LEO, Germany). The upconversion fluorescence spectra were measured using an F-4500 fluorescence spectrophotometer (Hitachi Co., Japan) modified with an external 1-W adjustable continuous wave 980-nm laser (Beijing Hi-Tech Optoelectronics Co., China). The strips were prepared using a guillotine cutter (ZQ2000) and dispenser (HM3035) (Shanghai Kinbio Tech. Co., Ltd., China), and UCNP-based ICS were determined using a 6-W adjustable continuous wave 980-nm laser. The QD-based ICS were determined by using a portable analyzer with a UV lamp at 254 nm (Shanghai Jiapeng Technology Co., China).
Preparation of NOR-Protein Conjugates
The NOR-protein conjugates were prepared by the active ester method as described in a previous literature (Cui et al. 2011). At first, 11.7 mg of NOR powder, 70.5 mg of EDC·HCl, and 21.2 mg of NHS were dissolved in anhydrous dimethylformamide by continuous mixing at room temperature in a blender for 12 h. After centrifugation for 10 min, the supernate of the mixture was then added dropwise to a mixture of 5 mL phosphate buffer solution (PBS) (0.01 M, pH 7.4) and 33.0 mg OVA. At the next step, the resultant solution was subjected to dialysis against 1 L of PBS for 3 days. In the final step, the coating antigen (NOR-OVA) was obtained, stored at 4 °C, and ready to be used.
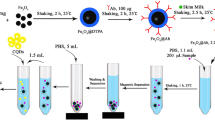
Preparation of Signal Probes Based on Different Nanoparticles
The steps of preparation of different probes are summarized in Table 1. To prepare colloidal gold-based signal probes, colloidal gold particles of 20-nm-diameter uniform spheres and high monodispersion were first synthesized by trisodium citrate reduction method (Sheng et al. 2011). Then 0.2 mol L−1 K2CO3 was added to the prepared solution (1 mL) to adjust the pH value to 8.5, and then 18 μL antibody against NOR (1 mg mL−1) was added dropwise under stirring. The mixture was gently mixed and incubated for 1 h at room temperature, and then 20 μL of 20% BSA solution and 10 μL of 20% PEG-20,000 were added and incubated at room temperature for another 30 min to block the residual surfaces of the colloidal gold. The solution was then placed in a centrifugal separator running at 10,000 rpm, 4 °C for 30 min. The supernatant was discarded while the precipitate was re-suspended in an appropriate amount (50 μL) of dilution buffer (0.1 mol L−1 Tris-HCl buffer (pH 8.5) containing 5% sucrose, 0.5% BSA, and 0.05% NaN3) and stored at 4 °C before use.
For the preparation of NaYF4:Yb, Er UCNP-based signal probes, UCNPs with good performance were first synthesized. In our study, one type of UCNPs was prepared by using thermal decomposition method, and the second type was produced by solvothermal method. The performance of the two types of particles was evaluated. Method 1 (thermal decomposition method) (Hu et al. 2016, 2015): 1-mM LnAc3 (Y:Yb:Er = 79 mol%:19 mol%:2 mol%) was mixed with 6 mL of oleic acid (OA) and 17 mL of 1-octadecene under agitation. Degassing at 100 °C for 10 min, the solution was heated to and kept at 160 °C for 30 min in argon gas and then cooled back to room temperature. Subsequently, 4.0 mM NH4F and 2.5 mM NaOH were dissolved in 10 mL of methanol solution and stirred for 30 min. The solution was first heated to 80 °C then to 300 °C for 1 h under argon protection. The solution was then cooled down to room temperature, washed afterward with ethanol, and collected through centrifugal separation. In the end, OA-capped UCNPs (OA-UCNPs) were obtained, which were “Type 1” UCNPs. Method 2 (solvothermal method) (Liu et al. 2018; Yao et al. 2019): NaOH (0.7 g), oleic acid (8.0 mL), and ethanol (8.0 mL) were mixed by agitation to form a homogeneous solution. Five milliliters of H2O was dropped into the solution, and then 3.0 mL H2O containing 0.303 g NaF was added with vigorous stirring until a translucent solution was produced. Subsequently, 1.1 mL of 0.80 mol L−1 Y(NO3)3, 0.35 mL of 0.63 mol L−1 Yb(NO3)3, and 0.05 mL of 0.40 mol L−1 Er(NO3)3 were mixed together. After aging in room temperature for 10 min, the mixture was transferred to a 50-mL Teflon-lined autoclave; the samples were annealed at 230 °C for 12 h. The system was naturally cooled to room temperature, and the products were deposited at the bottom of the vessel. The resulting UCNPs were washed with ethanol; after filtering and drying, the obtained UCNPs were preserved in the desiccator, which were “Type 2” UCNPs. As shown in Fig. 1, the Type 1 nanoparticles were characterized by well-dispersed and spherical nanoparticles with very smooth surfaces and average uniform diameters of about 30 nm, and the Type 2 nanoparticles were characterized by hexagonal nanorods with uniform diameters of about 110 nm and uniform lengths of about 2 μm, which reduce the particle immigration significantly. By comparing the fluorescence intensity, the Type 1 spherical nanoparticles, whose upconversion efficiency was far higher than that of the Type 2 nanoparticles, were found to be a more efficient upconversion material because they would increase the sensitivity of the established method. Additionally, they were of smaller size. Therefore, the particles prepared by method 1 were chosen for the experiment. Then, the obtained OA-UCNPs by method 1 were converted into biocompatible PAA-capped UCNPs (PAA-UCNPs) through a ligand exchange process described in our previous study (Hu et al. 2015).
The UCNP-based signal probes were built by the active ester method. Five milligrams of PAA-UCNPs, 4 mM EDC, and 10 mM NHS was dispersed in 2 mL of 2-(N-morpholino)ethanesulfonic acid buffer (MES, 10 mM, pH 5.5) and were incubated at 30 °C for 2 h with continuous agitation. After being washed three times with water, the precipitate was re-dispersed into 2 mL of N-(2-hydroxyethyl)piperazine-N-ethanesulfonic acid buffer (HEPES, 10 mM, pH 7.2) containing 0.2 mg of McAb against NOR. The linkage reaction was processed at 4 °C overnight. Subsequently, 15 mg of BSA was added to block nonspecific sites. In the final step, the resultant compounds were subjected to centrifugation, were washed and dispersed in 1 mL of HEPES buffer that contained 0.1% BSA, and were ready to be used.
For the preparation of QD-based signal probes, 100 μL carboxyl-modified QDs (8 μmol L−1) was first diluted into a final concentration of 1 μM using 10 mM sodium borate (pH 8.6). Subsequently, 100 μL of anti-NOR antibody (2.8 mg mL−1) and 23 μL of EDC (10 mg mL−1) were added. The mixture was then placed on a shaking table at 200 rpm for 4 h at room temperature. Then the conjugates were concentrated to 600 μL in an ultrafiltration centrifuge tube and stored at 4 °C without being exposed to sunlight until use.
Construction of Colloidal Gold-ICS, UCNPs-FICS, and QDs-FICS
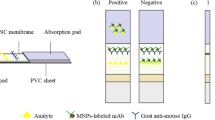
The test strip from top to bottom was made up of an absorbent pad, NC membrane, and a sample pad. The PVC sheet (20 cm × 2.5 cm) was used as the backing plate for the test strip.
NOR-OVA conjugates with a suitable dilution were used on the test line (T line) as capture reagent; goat anti-mouse immunoglobulin was used on the control line (C line) as capture reagent. They were separately immobilized on the NC membrane at 0.8 μL cm−1. The treated membrane was dried overnight at 37 °C. Then the sample pad, NC membrane, and absorption pad were assembled and cut into 0.37-cm wide individual strips. All the strips were stored in a plastic bag containing a desiccant gel at room temperature before use.
The theory of the assay was based on indirect competitive ELISA (ic-ELISA), as shown in Fig. 2. First, signal probes and standard or sample solutions (100 μL) were mixed in the microplate and were dropped onto the test strips. The solution would migrate, and when they reach the T line, signal probes would be trapped by the coating antigen. A clear visible red test line or a bright fluorescence test line would appear under proper excitation. The excess signal probes would migrate further and would be trapped by the goat anti-mouse IgG to form the C line. The visible changes of color intensity or the fluorescence intensity on the T line could be observed after 10 min. If NOR was present in the sample, it would compete with the immobilized NOR-OVA on the T line, causing the color or the fluorescence intensity to appear weaker. The more the NOR was in the sample, the weaker the color or fluorescence intensity of T line would be. As a result, the samples would be considered “negative” for NOR (−) if the T line and C line appeared to have the same color or fluorescence intensity. If the color or fluorescence intensity of the T line was weaker than that of the negative control, the test result would be considered as weakly positive (±). When there was no band at the T line, the sample would be defined to be positive (+).
Sample Preparation
Milk samples were purchased from a local super market. The samples (which were certified to be without NOR) were spiked with a known amount of NOR (0, 5, 10, and 50 μg L−1), and they were diluted directly with phosphate buffered saline supplemented with Tween 20 (PBST) at a ratio of 1:10, 1:5, and 1:5 before being analyzed using colloidal gold-ICS, QDs-FICS, and UCNPs-FICS, respectively. When commercial ELISA kits were employed for analysis, fat was first removed from the milk samples by centrifugation for 5 min, and then the samples were mixed with 35% methanol/sample extraction buffer at a ratio of 1:4 according to the instruction of the kit.
Results and Discussion
Optimization of the Immunochromatographic Assay
The chromatographic conditions were studied and optimized. Factors that might affect the performance and sensitivity of the test were investigated, such as NC membranes, blocking solutions, and dilution ratio of the coating antigens, gold-labeled antibody, and goat anti-mouse IgG. For colloidal gold-based ICS, as shown in Table 2, HF135 was found to be the best among the three types of NC membranes (HF180, HF135, and HF90) that were tested for all the established ICS. Similarly, blocking agents such as BSA or sucrose solution were also tested to avoid nonspecific interactions. In this study, satisfactory results could also be obtained without blocking for all the ICS. While for colloidal gold-based ICS, the dilution ratio of gold-labeled anti-NOR antibody, NOR-OVA conjugates (T line), and goat anti-mouse IgG (C line) was 1:20 with PBS, 1:11 with PBS, and 1:8 with PBS, respectively. For UCNPs-FICS, the dilution ratio of UCNP-labeled anti-NOR antibody, NOR-OVA conjugates (T line), and goat anti-mouse IgG (C line) was 1:50 with PBST containing 10% dilution buffer (0.1 mol L−1 Tris-HCl buffer (pH 8.5) containing 5% sucrose, 0.5% BSA, and 0.05% NaN3), 1:400 with PBS, and 1:60 with PBS, respectively. For QDs-FICS, the dilution ratio of QD-labeled anti-NOR antibody, NOR-OVA conjugates (T line), and goat anti-mouse IgG (C line) was 1:30 with PBST, 1:40 with PBS, and 1:30 with PBS. In these conditions, the quantity of NOR in these strips can be kept low enough to achieve good sensitivity but also high enough to provide an acceptable signal.
Visual Assessment of Strip Sensitivity
The visual limit of detection (LOD) of the ICS was defined as the minimum concentration resulting in the disappearance of the band on the T line. As shown in Fig. 3, the sensitivity of the strips was studied using different concentrations of NOR. For colloidal gold-ICS, a faint band was observed on the T line at 1.0 μg L−1 of NOR, and no band was observed at 2.0 μg L−1. Therefore, two bands would indicate that the concentration of NOR was below 2.0 μg L−1, the result of which was defined as negative results (−). Oppositely, if only one band appeared on the C line, the concentration was at or above 2.0 μg L−1, the result of which was defined as positive result (+). Accordingly, 2.0 μg L−1 of NOR in PBST was the visual LOD for colloidal gold-ICS. For QD-based FICS, no fluorescence band was observed at the same concentration of 2.0 μg L−1 of NOR. Therefore, 2.0 μg L−1 of NOR in PBST was the visual LOD for QDs-FICS. As for UCNPs-FICS, the visual LOD was 0.5 μg L−1, which was four times more sensitive than the other two methods.
Cross-Reactivity
The cross-reactivity was performed to determine the specificity of the antibody against NOR. Because we were using the same antibody, the cross-reactivity was only studied using UCNPs-FICS. Twelve analogues of FQs were studied as follows: ciprofloxacin (CIP), nalidixic acid (NAL), enrofloxacin (ENR), ofloxacin (OFL), oxolinic acid (OXI), flumequine (FLU), fleroxacin (FLE), lomefloxacin (LOM), enoxacin (ENO), marbofloxacin (MAR), cinoxacin (CIN), and sarafloxacin (SAR). Standard solutions of each compound at concentrations of 5.0, 20.0, and 400.0 μg L−1 were applied to the test strips, as shown in Fig. 4. The fluorescence of the T line disappeared when the concentration of CIP, NAL, ENR, OFL, OXI, FLU, FLE, and LOM was 5.0 μg L−1. For ENO and MAR, the fluorescence of the T line disappeared at concentrations of 20.0 μg L−1. While for CIN and SAR, at concentrations of 400.0 μg L−1, the fluorescence of the T line would disappear. The results indicated that the test strips could be employed to detect not only NOR but also 12 other FQs at certain concentrations.
Specificity analysis using the UCNPs-FICS. Compounds from left to right: PBST, NOR (0.5 μg L−1), ciprofloxacin (CIP, 5.0 μg L−1), nalidixic acid (NAL, 5.0 μg L−1), enrofloxacin (ENR, 5.0 μg L−1), ofloxacin (OFL, 5.0 μg L−1), oxolinic acid (OXI, 5.0 μg L−1), flumequine (FLU, 5.0 μg L−1), fleroxacin (FLE, 5.0 μg L−1), lomefloxacin (LOM, 5.0 μg L−1), enoxacin (ENO, 20.0 μg L−1), marbofloxacin (MAR, 20.0 μg L−1), cinoxacin (CIN, 400.0 μg L−1), and sarafloxacin (SAR, 400.0 μg L−1)
Sample Analysis
The proposed strips were validated using commercial ELISA kits. Table 3 showed the results obtained using the ELISA kit and the proposed strips for the spiked milk samples. The samples were spiked with NOR at concentrations of 5.0, 10.0, and 50.0 μg L−1, respectively. In the visual assessment of the immunochromatographic strips, samples spiked with 5.0 μg L−1 of NOR showed positive results by using UCNPs-FICS. When the concentration reached 10.0 μg L−1, positive results were obtained by using UCNPs-FICS and QDs-FICS. Finally, when the concentration reached 50.0 μg L−1, positive results were obtained by all the ICS. Also, all the results tested by the commercial ELISA kit agreed closely with those of the spiked levels. The results indicated a strong correlation between the results of the visual assessment performed using the test strips and those obtained using the ELISA kit. Besides, the UCNPs-FICS (the detection limit was 2.5 μg L−1 in milk samples) showed higher sensitivity compared to QDs-FICS (the detection limit was 10.0 μg L−1 in milk samples) or conventional colloidal gold-ICG (the detection limit was 20.0 μg L−1 in milk samples).
Conclusions
In this study, three types of labeling materials, colloidal gold, QDs, and UCNPs, were studied and applied in immunochromatographic assays to determine NOR, respectively. Compared to the traditional colloid gold-ICS, the QDs-FICS showed similar sensitivity in the standard solution but showed higher sensitivity in milk samples since the use of QDs enhanced the interference-free capacity of that method. While UCNPs-FICS showed the highest sensitivity both in the standard solution and in milk samples, it was also more stable in regular laboratory conditions, that is, no need to be kept from visible light. This might be because of the strong and unique luminescence properties of UCNPs: any autofluorescence originating from biomolecules possibly contained in a solution could be avoided efficiently. In addition, the results obtained by the developed test strips were in good agreement with those obtained using an ELISA kit (as a reference method). We also found that the QDs and UCNP-based test strips cost less and could be easily produced in a large quantity because they have the capacity of saving antibody (both monoclonal antibody against norfloxacin and goat anti-mouse IgG) and coating antigen. Our results indicated that this novel approach using QDs and UCNPs as fluorescence signal probes was suitable for rapid, sensitive, visual, and on-site detection of low concentrations of small molecules present in complex samples.
References
Abnous K, Danesh NM, Alibolandi M, Ramezani M, Taghdisi SM, Emrani AS (2017) A novel electrochemical aptasensor for ultrasensitive detection of fluoroquinolones based on single-stranded DNA-binding protein. Sensors Actuators B Chem 240:100–106. https://doi.org/10.1016/j.snb.2016.08.100
Alhusban AA, Tarawneh OA, Dawabsheh SO, Alhusban AA, Abumhareb FW (2019) Liquid chromatography–tandem mass spectrometry for rapid and selective simultaneous determination of fluoroquinolones level in human aqueous humor. J Pharmacol Toxicol 97:36–43. https://doi.org/10.1016/j.vascn.2019.03.001
Anaya-Gonzalez C, Soldevila S, Garcia-Lainez G, Bosca F, Andreu I (2019) Chemical tuning for potential antitumor fluoroquinolones. Free Radic Biol Med 141:150–158. https://doi.org/10.1016/j.freeradbiomed.2019.06.010
Canales C, Ramos D, Fierro A, Antilén M (2019) Electrochemical, theoretical and analytical studies of the electro-oxidation of sulfamerazine and norfloxacin on a glassy carbon electrode. Electrochim Acta 318:847–856. https://doi.org/10.1016/j.electacta.2019.06.035
Caro E, Marcé RM, Cormack PAG, Sherrington DC, Borrull F (2006) Direct determination of ciprofloxacin by mass spectrometry after a two-step solid-phase extraction using a molecularly imprinted polymer. J Sep Sci 29:1230–1236. https://doi.org/10.1002/jssc.200500439
Chawla K, Kumar A, Shenoy VP, Chakrabarty S, Satyamoorthy K (2018) Genotypic detection of fluoroquinolone resistance in drug-resistant Mycobacterium tuberculosis at a tertiary care centre in south Coastal Karnataka, India. J Glob Antimicrob Resist 13:250–253. https://doi.org/10.1016/j.jgar.2018.01.023
Chen J, Shanin IA, Lv S, Wang Q, Mao C, Xu Z, Sun Y, Wu Q, Eremin SA, Lei H (2016) Heterologous strategy enhancing the sensitivity of the fluorescence polarization immunoassay of clinafloxacin in goat milk. J Sci Food Agric 96:1341–1346. https://doi.org/10.1002/jsfa.7228
Cui J, Zhang K, Huang Q, Yu Y, Peng X (2011) An indirect competitive enzyme-linked immunosorbent assay for determination of norfloxacin in waters using a specific polyclonal antibody. Anal Chim Acta 688:84–89. https://doi.org/10.1016/j.aca.2010.12.030
Cuprys A, Pulicharla R, Brar SK, Drogui P, Verma M, Surampalli RY (2018) Fluoroquinolones metal complexation and its environmental impacts. Coord Chem Rev 376:46–61. https://doi.org/10.1016/j.ccr.2018.05.019
Di Nardo F, Anfossi L, Giovannoli C, Passini C, Goftman VV, Goryacheva IY, Baggiani C (2016) A fluorescent immunochromatographic strip test using quantum dots for fumonisins detection. Talanta 150:463–468. https://doi.org/10.1016/j.talanta.2015.12.072
Ding Y, Hua X, Chen H, Gonzalez-Sapienza G, Barnych B, Liu F, Wang M, Hammock BD (2019) A dual signal immunochromatographic strip for the detection of imidaclothiz using a recombinant fluorescent-peptide tracer and gold nanoparticles. Sensors Actuators B Chem 297:126714. https://doi.org/10.1016/j.snb.2019.126714
Duan R, Jiang J, Liu S, Yang J, Qiao M, Shi Y, Hu X (2017) Determination of norfloxacin in food by an enhanced spectrofluorimetric method. J Sci Food Agric 97:2569–2574. https://doi.org/10.1002/jsfa.8077
Hu G, Sheng W, Zhang Y, Wu X, Wang S (2015) A novel and sensitive fluorescence immunoassay for the detection of fluoroquinolones in animal-derived foods using upconversion nanoparticles as labels. Anal Bioanal Chem 407:8487–8496. https://doi.org/10.1007/s00216-015-8996-4
Hu G, Sheng W, Zhang Y, Wang J, Wu X, Wang S (2016) Upconversion nanoparticles and monodispersed magnetic polystyrene microsphere based fluorescence immunoassay for the detection of sulfaquinoxaline in animal-derived foods. J Agric Food Chem 64:3908–3915. https://doi.org/10.1021/acs.jafc.6b01497
Ibarra IS, Rodriguez JA, Páez-Hernández ME, Santos EM, Miranda JM (2012) Determination of quinolones in milk samples using a combination of magnetic solid-phase extraction and capillary electrophoresis. Electrophoresis 33:2041–2048. https://doi.org/10.1002/elps.201100559
Kurrey R, Mahilang M, Deb MK, Nirmalkar J, Shrivas K, Pervez S, Rai MK, Rai J (2019) A direct DRS-FTIR probe for rapid detection and quantification of fluoroquinolone antibiotics in poultry egg-yolk. Food Chem 270:459–466. https://doi.org/10.1016/j.foodchem.2018.07.129
Li C, Li Y, Zhou T, Xie R (2019) Ultrasonic synthesis of Mn-doped CsPbCl3 quantum dots (QDs) with enhanced photoluminescence. Opt Mater 94:41–46. https://doi.org/10.1016/j.optmat.2019.04.053
Liu Y, Zhang C, Liu H, Li Y, Xu Z, Li L, Whittaker A (2018) Controllable synthesis of up-conversion nanoparticles UCNPs@MIL-PEG for pH-responsive drug delivery and potential up-conversion luminescence/magnetic resonance dual-mode imaging. J Alloys Compd 749:939–947. https://doi.org/10.1016/j.jallcom.2018.03.355
Liu Y, Zhang F, He X, Ma P, Huang Y, Tao S, Sun Y, Wang X, Sun D (2019) A novel and simple fluorescent sensor based on AgInZnS QDs for the detection of protamine and trypsin and imaging of cells. Sensors Actuators B Chem 294:263–269. https://doi.org/10.1016/j.snb.2019.05.057
Majdinasab M, Mitsubayashi K, Marty JL (2019) Optical and electrochemical sensors and biosensors for the detection of quinolones. Trends Biotechnol 37:898–915. https://doi.org/10.1016/j.tibtech.2019.01.004
Pochivalov A, Timofeeva I, Vakh C, Bulatov A (2017) Switchable hydrophilicity solvent membrane-based microextraction: HPLC-FLD determination of fluoroquinolones in shrimps. Anal Chim Acta 976:35–44. https://doi.org/10.1016/j.aca.2017.04.054
Rafique R, Kailasa SK, Park TJ (2019) Recent advances of upconversion nanoparticles in theranostics and bioimaging applications. TrAC Trends Anal Chem:115646. https://doi.org/10.1016/j.trac.2019.115646
Sheng W, Li Y, Xu X, Yuan M, Wang S (2011) Enzyme-linked immunosorbent assay and colloidal gold-based immunochromatographic assay for several (fluoro)quinolones in milk. Microchim Acta 173:307–316. https://doi.org/10.1007/s00604-011-0560-0
Song X, Shukla S, Kim M (2019) An immunoliposome-based immunochromatographic strip assay for the rapid detection of Cronobacter species. J Microbiol Methods 159:91–98. https://doi.org/10.1016/j.mimet.2019.02.006
Szerkus O, Jacyna J, Gibas A, Sieczkowski M, Siluk D, Matuszewskib M, Kaliszan R, Markuszewski MJ (2017) Robust HPLC–MS/MS method for levofloxacin and ciprofloxacin determination in human prostate tissue. J Pharmaceut Biomed 132:173–183. https://doi.org/10.1016/j.jpba.2016.10.008
Tan X, Li Q, Yang J (2020) CdTe QDs based fluorescent sensor for the determination of gallic acid in tea. Spectrochim Acta A 224:117356. https://doi.org/10.1016/j.saa.2019.117356
Tian R, Zhao S, Liu G, Chen H, Ma L, You H, Liu C, Wang Z (2019) Construction of lanthanide-doped upconversion nanoparticle-Uelx Europaeus Agglutinin-I bioconjugates with brightness red emission for ultrasensitive in vivo imaging of colorectal tumor. Biomaterials 212:64–72. https://doi.org/10.1016/j.biomaterials.2019.05.010
Tomas A, Stilinović N, Sabo A, Tomić Z (2019) Use of microdialysis for the assessment of fluoroquinolone pharmacokinetics in the clinical practice. Eur J Pharm Sci 131:230–242. https://doi.org/10.1016/j.ejps.2019.02.032
Tumini M, Nagel O, Molina MP, Althaus R (2017) Microbiological assay with Bacillus licheniformis for the easy detection of quinolones in milk. Int Dairy J 64:9–13. https://doi.org/10.1016/j.idairyj.2016.08.008
Wagman AS, Cirz R, McEnroe G, Aggen J, Linsell MS, Goldblum AA, Lopez S, Gomez M, Miller G, Simons LJ, Belliotti TR, Harris CR, Poel T, Melnick MJ, Gaston RD, Moser HE (2017) Synthesis and microbiological evaluation of novel tetracyclic fluoroquinolones. ChemMedChem 12:1687–1692. https://doi.org/10.1002/cmdc.201700426
Wang Z, Guo L, Liu L, Kuang H, Xu C (2018) Colloidal gold-based immunochromatographic strip assay for the rapid detection of three natural estrogens in milk. Food Chem 259:122–129. https://doi.org/10.1016/j.foodchem.2018.03.087
Wangman P, Longyant S, Utari HB, Senapin S, Pengsuk C, Sithigorngul P, Chaivisuthangkura P (2016) Sensitivity improvement of immunochromatographic strip test for infectious myonecrosis virus detection. Aquaculture 453:163–168. https://doi.org/10.1016/j.aquaculture.2015.11.041
Wu W, Li M, Chen M, Li L, Wang R, Chen H, Chen F, Mi Q, Liang W, Chen H (2017a) Development of a colloidal gold immunochromatographic strip for rapid detection of Streptococcus agalactiae in tilapia. Biosens Bioelectron 91:66–69. https://doi.org/10.1016/j.bios.2016.11.038
Wu Y, Peng W, Zhao Q, Piao J, Zhang B, Wu X, Wang H, Shi Z, Gong X, Chang J (2017b) Immune fluorescence test strips based on quantum dots for rapid and quantitative detection of carcino-embryonic antigen. Chin Chem Lett 28:1881–1884. https://doi.org/10.1016/j.cclet.2017.07.026
Xu X, Feng L, Li J, Yuan P, Feng J, Wei L, Cheng X (2019) Rapid screening detection of fluoroquinolone residues in milk based on turn-on fluorescence of terbium coordination polymer nanosheets. Chin Chem Lett 30:549–552. https://doi.org/10.1016/j.cclet.2018.11.026
Yan Q, Chen Z, Xue S, Han X, Lin Z, Zhang S, Shi G, Zhang M (2018) Lanthanide-doped nanoparticles encountering porphyrin hydrate: boosting a dual-mode optical nanokit for Cu2+ sensing. Sensors Actuators B Chem 268:108–114. https://doi.org/10.1016/j.snb.2018.04.080
Yang Q, Gong X, Song T, Yang J, Zhu S, Li Y, Cui Y, Li Y, Zhang B, Chang J (2011) Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosens Bioelectron 30:145–150. https://doi.org/10.1016/j.bios.2011.09.002
Yao H, Shen H, Tang Q (2019) Highly luminescent up/down conversion thin films prepared by a room temperature process. Thin Solid Films 683:1–7. https://doi.org/10.1016/j.tsf.2019.05.010
Zhang S, Yao T, Wang S, Feng R, Chen L, Zhu V, Hu G, Zhang H, Yang G (2019) Upconversion luminescence nanoparticles-based immunochromatographic assay for quantitative detection of triamcinolone acetonide in cosmetics. Spectrochim Acta A 214:302–308. https://doi.org/10.1016/j.saa.2019.02.053
Zheng H, Mo J, Zhang Y, Gao Q, Ding J, Yu Q, Feng Y (2014) Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples. J Chromatogr A 1329:17–23. https://doi.org/10.1016/j.chroma.2013.12.083
Funding
This study was funded by the Natural Science Foundation of Hebei Province (grant number C2019208188), the Doctoral Research Foundation Project of Hebei University of Science and Technology (grant numbers 81/1181288 and 81/1181289), and by the PT Foundation of Hebei University of Science and Technology (grant number 82/1182218).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gaoshuang Hu declares that she has no conflict of interest. Shan Gao declares that he has no conflict of interest. Xue Han declares that she has no conflict of interest. Lixin Yang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
A statement regarding informed consent is not applicable for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, G., Gao, S., Han, X. et al. Comparison of Immunochromatographic Strips Using Colloidal Gold, Quantum Dots, and Upconversion Nanoparticles for Visual Detection of Norfloxacin in Milk Samples. Food Anal. Methods 13, 1069–1077 (2020). https://doi.org/10.1007/s12161-020-01725-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01725-3