Abstract
A fluorometric immunochromatographic assay (FICA) is described where ZnCdSe/ZnS quantum dots (QDs) act as fluorescent label and gold nanoparticles (AuNPs) act as quencher. The assay works in the “turn-on” mode, i.e. the fluorescent signal (best measured at excitation/emission wavelengths of 302/525 nm) increases with the increase of analyte concentration. This assay can detect tetracycline antibiotics including tetracycline, oxytetracycline, chlortetracycline, and doxycycline. It is not interfered by other veterinary drugs. The visual limits of detection (LODs) for the tetracycline antibiotics are 2 μg·L−1 in buffer, 20 μg·L−1 in milk, and 40 μg·kg−1 in animal muscle tissue. The assay (including sample treatment) can be performed within 30 min. The FICA based on “turn on” mode is more sensitive than the colloidal gold-based immunochromatographic assay (CGICA) and quantum dot-based immunochromatographic assay (QDICA) based on “turn off” mode using either AuNPs or QDs as signal labels. One strip can simultaneously provide the fluorescent test results in the “turn on” mode on the basis of QD luminescence quenching under UV light. The colorimetric test is of the “turn off” mode based on the formation of a red coloration due to the use of AuNPs under natural light. The use of such a dual-functional test mode allows for rapid semi-quantitative determination of tetracycline antibiotics in milk and tissue samples.

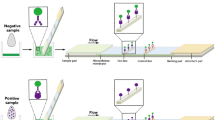
Schematoc of a fluorometric immunochromatographic assay (FICA) based on fluorescence quenching of quantum dot (QD) by gold nanoparticle (AuNP) combined with a dual-functional test mode under UV light (turn on mode) and natural light (turn off mode) to visually detect tetracycline antibiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetracycline antibiotics which inhibit protein synthesis of bacteria, are widely applied in the therapy of animal and human infections as broad-spectrum agents, for their low cost and favorable antimicrobial properties [1, 2]. Tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC), and doxycycline (DC) are the most common ones in veterinary medicine although the tetracycline antibiotics family have over 20 members [2]. Tetracycline antibiotics may be present in food, due to their frequently used in food-producing animals as both growth promoters and antibiotics, to treat, control, and prevent microbial diseases. That raises the concerns about tetracycline antibiotics residues, because of the drug resistance and risk to human health, such as liver damage, gastrointestinal disturbances, teeth yellowing, and allergic reaction [3, 4]. To ensure food safety, the European Union (EU) and Chinese Ministry of Agriculture have established the maximum residue limits (MRLs) of 100 μg·kg−1 in milk, animal muscle tissues, fish, and shrimp for tetracycline antibiotics, except the doxycycline is not for use in animals from which milk is produced for human consumption.
Many analytical methods have been used to detect tetracycline antibiotics in foods, mainly including high-performance liquid chromatographic [5, 6] coupled with mass spectrometry [7, 8], capillary electrophoresis [9], surface-enhanced Raman scattering [10], surface plasmon resonance sensor [11], thin-layer chromatography [12], fluorometric assay [13], and microbiological method [14]. Those instrumental analytical methods and some sensors have higher sensitivity and they can provide the quantitative data, but they need professional instrument and relatively complicated sample pretreatment. And although the microbiological method is general, it is insensitive and time-consuming.
Immunoassay is a kind of detecting technology based on the specific binding of antibody with antigen. And common immunoassays to detect tetracycline antibiotics contain enzyme-linked immunosorbent assay [15], fluorescence immunoassay [16], electrochemical immunosensor [17,18,19], and immunochromatographic assay [20,21,22,23,24,25]. Immunochromatographic assay has been widely used in on-site detection because it is low cost, simple operation, easy understanding test phenomena, and no need of apparatus. The gold nanoparticles have been mainly used as labels in the immunochromatographic assays reported to detect tetracycline antibiotics [20,21,22,23,24]. In addition, the dyed latex bead [22] and quantum dot [25] have been also reported as labels. However, these methods are based on a “turn off” mode, i.e. the concentration of tetracycline negatively correlates with the signal. More and more people interested in developing the immunochromatographic assay based on “turn on” mode. Anfossi et al. [26] had developed a lateral flow immunoassay in “turn on” mode to detect fumonisins based on quantum dots fluorescence quenching by metal nanoparticles. In this work, a combination of quantum dot as fluorescent signal source and gold nanoparticle as fluorescence quencher has been introduced into assay to develop an immunochromatographic assay based on a “turn on” mode, i.e. the concentration of tetracycline positively relates with the fluorescent signal. In particular, the remarkable characteristic of the assay is its dual-functional mode, i.e. “turn-on” mode under UV light and “turn-off” mode under natural light. The application of dual-functional test mode can achieve rapid semi-quantitative detection of tetracycline antibiotics residues in milk and animal muscle tissue samples.
Experimental
Materials and instruments
Tetracycline hydrochloride (97.7%), chlortetracycline hydrochloride (99.5%), oxytetracycline hydrochloride (96.5%), doxycycline hyclate (98.7%) standards, ovalbumin (OVA), and goat anti-mouse IgG were purchased from Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com). Carboxyl-functional ZnCdSe/ZnS quantum dots (QDs) (emission maximun at 525 nm) were obtained from Jiayuan Quantum Dots Co., Ltd. (Wuhan, China, www.qds.net.cn). Anti-TC monoclonal antibody and tetracycline coating antigen (TC-BSA) were prepared in our laboratory. The commercial ELISA kit was obtained from REAGEN LLC (Moorestown, New Jersey, USA, www.reagen.us). Poly(vinyl chloride) (PVC) sheet, absorbent pad, conjugate pad, sample pad, nitrocellulose (NC) membrane, automatic guillotine cutter (ZQ2000) and XYZ dispenser (HM3035) were purchased from Kinbio Tech. Co., Ltd. (Shanghai, China, www.goldbio.cn). A portable UV lamp was purchased from JiaPeng Technology Co., Ltd. (Shanghai, China, shjiapeng.testmart.cn).
Preparation of OVA labeled with QDs (QD-OVA)
The conjugation of QD and OVA is based on the covalent binding between the carboxyl on the surface of QD and the amino on the OVA by the active ester method, and the chemical reaction is provided in Supplementary Material (Fig. S1). The preparation procedure of QD-OVA is detailed as follow. First, 10 mg of OVA and 10 mg of EDC were dissolved in 1 mL of boric acid-borax buffer (8.58 g Na2B4O7∙10H2O and 6.80 g H3BO3 in 1 L deionized water; pH 8.4). Then, 50.0 μL of QD (8 μmol·L−1), 23.0 μL of EDC solution, and 17.2 μL of OVA solution mentioned above were added into 309.8 μL of borate buffer successively. The mixture was incubated on a shake table for 2 h (200 rpm, 25 °C) and concentrated to 200 μL using an ultrafilter. Finally, the QD-OVA conjugates were obtained.
Preparation of antibody against TC labeled with AuNPs (AuNP-Ab)
The synthesis protocol of AuNPs (surface plasmon maximum at 520 nm) is provided in Supplementary Material. The preparation process of AuNP-Ab is detailed as follow. First, 1 μL of K2CO3 (0.2 mol·L−1) was added into 1 mL of the AuNPs solution for adjusting the pH to the optimal value (6–6.5). Subsequently, 3 μL of antibody (7.2 mg·mL−1) was mixed with the above solution and the mixture was placed for 1 h at 4 °C. Second, 10 μL of PEG-20000 aqueous solution (20%, w/v) and 20 μL of BSA aqueous solution (20%, w/v) were added in the aforesaid mixture followed by incubation at 25 °C for 30 min. Then the solution was centrifuged (425 rcf, 4 °C) for 15 min in order to remove agglomerates from the AuNPs. And the supernatant was collected followed by centrifugation (6797 rcf, 4 °C) for 30 min. Finally, the precipitate was re-suspended using working buffer and the AuNP-Ab conjugate was obtained.
Preparation of the FICA strips
Figure 1a displays the assembly form of the FICA strip. NC membrane was glued on the PVC sheet firstly. Then the absorbent pad was glued on the top of the PVC sheet, which had a 2 mm of overlap with NC membrane. The conjugate pad was glued below the NC membrane and had a 2.5 mm of overlap with the NC membrane. Analogously, the sample pad was glued on the bottom of the PVC sheet and had a 2.5 mm of overlap with the conjugate pad.
The QD-OVA was sprayed on the NC membrane by the XYZ dispenser as the C line (control line) with a 0.8 μL·cm−1 dosage. And the mixture of coating antigen (TC-BSA) and QD-OVA was sprayed on the NC membrane by the XYZ dispenser as the T line (test line) with a 0.8 μL·cm−1 dosage. After dried six hours at 37 °C, the assembled plate was cut to 3.7 mm width of strips by automatic guillotine cutter.
Test principle and procedure
The absorption spectrum of the AuNP (surface plasmon maximum at 520 nm) has a large degree of overlap with the emission spectrum of the QD (emission maximum at 525 nm) (Fig. 1d). When the QD and AuNP are close to each other, the emission light of the QD can be absorbed by the AuNP, which can lead to QD fluorescence quenching. When TC is absence in sample (negative sample), the AuNP-Ab will be captured by the TC-BSA on the T line. This results in fluorescence quenching on the T line, and clear fluorescence on the C line is observed under UV light, and that clear red signal on the T line is observed under natural light (Fig. 1b). When TC is present in sample (positive sample), the TC competes with the TC-BSA on the T line to combine with the AuNP-Ab. This results in that green fluorescence on the T line recovers gradually, and clear green fluorescence on the C line still is observed under UV light. And this also results in that red signal on the T line disappears gradually under natural light. When there is enough TC in sample, both green fluorescence bands on the T and C line are observed under UV light, and no red T line is observed under natural light (Fig. 1c). The visual LOD of the FICA is the minimum concentration of TC which causes the green fluorescence on the T line to recover under UV light based on “turn on” mode or the red band on the T line to disappear completely under natural light based on “turn off” mode.
The FICA test was carried out according to the following procedure. One hundred microliters of TC standard or sample solution and 5 μL of AuNP-Ab were mixed and then added onto the sample pad. After 10 min, the fluorescent test results based on “turn on” mode can be observed by unaided eye under UV light by using a portable UV lamp, and the colorimetric test results based on “turn off” mode also can be observed by unaided eye under natural light.
Sample treatment
For the muscle tissue samples (shrimp, grass carp, weever, carp, chicken, pork, beef), 1 g of tissue sample and 2.0 mL 3% trichloroacetic acid aqueous solution were added into a 15 mL centrifuge tube followed by vortexing for 5 min. The mixture was centrifuged for 10 min at 10,621 rcf. Then, NaOH (1 mol·L−1) was added to adjust the pH of the collected supernatant to neutral. Last, the resulting supernatant was diluted 10-fold with 0.01 mol·L−1 PBS (phosphate-buffered saline, 8.0 g NaCl, 0.2 g KCl, 1.42 g Na2HPO4, and 0.24 g KH2PO4 in 1 L deionized water, pH 7.4) and detected using the FICA strips.
For the milk samples, 1.0 mL of whole milk and 1.0 mL 3% trichloroacetic acid aqueous solution were transferred into a 15 mL centrifuge tube followed by vortexing for 5 min. The mixture was centrifuged for 10 min at 10,621 rcf. Then, NaOH (1 mol·L−1) was added to adjust the pH of the collected supernatant to neutral. Last, the resulting supernatant was diluted 5-fold with PBS and detected using the FICA strips.
Results and discussion
Choice of nanomaterials and assay mode
The spherical gold nanoparticle [20,21,22,23,24] is the most common used as signal label in lateral flow immunochromatographic assays (LFICAs). In addition, gold nanocage [27], flower-like gold nanoparticle [28], carbon nanoparticle [29], dyed latex bead [22, 30], quantum dot [25, 30], dye-doped nanoparticle [31] have been reported as signal label to develop the LFICAs. These LFICAs have all used single kind of nanoparticle and their test principle are all based on signal “turn-off” mode, i.e. the target concentration negatively correlates with the signal. In the visual immunochromatographic assays based on “turn-off” mode, the corresponding minimum concentration of target is served as the visual LOD when the signal on the T line disappears. Here, a relatively high target concentration is required to cause the signals to disappear completely, reflecting the sensitivity is relatively low. For the visual immunochromatographic assays based on “turn-on” mode (the target concentration positively correlates with the signal), a relatively low target concentration can cause a visible weak signal to appear, reflecting the sensitivity is relatively high. Due to unique optical feature of quantum dot (QD) and good fluorescence quenching ability of gold nanoparticle (AuNP), QD-AuNP has been widely used as a fluorescence quenching group to develop the analytical methods. In this work, the carboxyl-functional QD (ZnCdSe/ZnS, emission maximum at 525 nm) as fluorescence signal label and the spherical AuNP (diameters of about 18 nm) as fluorescence quencher have been selected to develop a FICA based on “turn-on” mode to more sensitively detect TC. It is worth mentioning, the assay has a remarkable characteristic of dual-functional test mode, that is to say, one strip can give simultaneously the fluorescent test results in “turn on” mode on the base of QD luminescence quenching under UV light and the colorimetric test results in “turn off” mode on the base of red colour of surface plasmon of AuNP under natural light. The fluorescence quenching mechanisms include energy transfer, inner filter effect, photoinduced electron transfer, and so on [32]. The emission spectrum of the fluorescent material (donor) overlaps with the absorption spectrum of the quencher (acceptor), the fluorescence lifetime of the fluorescent material will decrease and the distance between fluorescence donor and acceptor is in the range of 1–10 nm can demonstrate that the quenching mechanism is the fluorescence resonance energy transfer (FRET). The inner-filter effect (IFE) occurs when the excitation or emission spectra of fluorescent material overlap with the absorption spectrum of the quencher. Some published researches consider that the main cause of fluorescence quenching in strip assay is attributed to the FRET between fluorescence donor and acceptor [33, 34]. However, some published researches have provided different opinions. Jiang et al. [35] suggest that the IFE is the main cause of fluorescence quenching, they consider that the optimal distance between the fluorescence donor and acceptor is unlikely achieved in the strip assay. Anfossi et al. [26] have investigated whether the FRET or the IFE quenching mechanism occurred and which one predominated in LFICA based on the fluorescence quenching of QD by gold and silver nanoparticles, and they think that the larger contribution to QD quenching is due to IFE. In our work, the mixture of QD-OVA and TC-BSA is sprayed onto the strip as T line, the binding of the antibody labeled with AuNP and TC-BSA is difficult to insure the optimal distance between QD and AuNP to generate FRET, but the minor contribution from FRET to QD quenching may exist. Here, the IFE is considered to be the primary cause of fluorescence quenching. Whether due to the FRET or the IFE quenching mechanism, the “turn-on” test mode based on fluorescence quenching is expected to be good candidates as test strategy for improving sensitivity of LFICA.
Visual evaluation of assay sensitivity
The working conditions of the assay were optimized to develop the FICA. Under optimized conditions (Table S1), a series of concentrations of TC standard solutions was detected by the FICA. The result is shown in Fig. 2. As shown in Fig. 2a, the intensity of green florescence on the T line gradually became strong with the TC concentration increasing. The fluorescence on the T line was quenched by AuNPs, and no fluorescence was observed when the concentration of TC was 0 μg·L−1. And a green florescence band was observed when the concentration of TC was 2 μg·L−1. Therefore, the visual LOD of this assay based on “turn on” mode was 2 μg·L−1 under UV light. The same strips were observed under natural light and the results were exhibited in Fig. 2b. The red T line of strip gradually became weak with the TC concentration increasing. The red T line completely disappeared when the concentration of TC was 10 μg·L−1. Therefore, the visual LOD of this assay based on “turn off” mode was 10 μg·L−1 under natural light.
Detection of TC based on the dual-functional mode. a fluorescent test results of strips based on “turn on” mode under UV light, b colorimetric test results of strips based on “turn off” mode under natural light. A series of dilutions (0, 0.5, 1, 2, 3, 5, and 10 μg·L−1) of TC is prepared in 0.01 mol·L−1 PBS (pH 7.4)
The same TC coating antigen and anti-TC antibody were used to develop the QD-based immunochromatographic assay (QDICA) based on “turn off” mode and colloidal gold-based immunochromatographic assay (CGICA) based on “turn off” mode. The optimal working conditions and the visual test results of two assays are shown in Supplementary Material (Table S2 and Fig. S2). The visual LODs of the QDICA and CGICA were 5 μg·L−1 (Fig. S2a) and 10 μg·L−1 (Fig. S2b), respectively. The FICA based on “turn on” mode was more sensitive than the QDICA and CGICA based on “turn off” mode for the detection of TC when the same anti-TC antibody and TC coating antigen were used.
Specificity of the FICA
To investigate the specificity of the FICA, four tetracycline antibiotics and nine kinds of common veterinary drug were tested by the FICA strips. As shown in Fig. 3, the green fluorescence bands on the T lines were observed when the concentrations of TC, oxytetracycline, chlortetracycline, and doxycycline were all at 2 μg·L−1, indicating the FICA can equivalently recognize TC, oxytetracycline, chlortetracycline, and doxycycline. No fluorescence bands on the T lines were observed to detect nine kinds of common veterinary drug at 1000 μg·L−1, which proved the high specificity of the FICA for tetracycline antibiotics. Therefore, this FICA can be used to detect the tetracycline antibiotics and will not be affected by other common veterinary drugs.
Specificity analysis of the FICA. 1 PBS, 2 TC, 3 oxytetracycline, 4 chlortetracycline, 5 doxycycline, 6 3-amino-2-oxazolidinone, 7 sulfadimidine, 8 sulfamethoxazole, 9 norfloxacin, 10 enrofloxacin, 11 gentamycin, 12 salbutamol, 13 metronidazole, 14 chloramphenicol. The concentrations of TC, oxytetracycline, chlortetracycline, and doxycycline are 2 μg·L−1, other common veterinary drugs are at 1000 μg·L−1
Sample analysis by the FICA
To remove the matrix effects, the sample extraction solution and the dilution ratio of extracted sample were optimized. One milliliter of milk sample was extracted using 1 mL of 3% trichloroacetic acid aqueous solution and 1 mL of citric acid buffer, respectively. The extracted sample solutions were diluted 5-fold and 10-fold using PBS, respectively. The undiluted and diluted extracted sample solutions were analyzed by the FICA strips. The fluorescence on the T line was quenched to detect extracted sample solution with 5-fold dilution using 3% trichloroacetic acid aqueous solution as extraction solution (Fig. S3a). And the extracted sample solution using citric acid buffer as extraction solution need to be diluted 10-fold to remove the matrix effects (Fig. S3b). Therefore, 3% trichloroacetic acid aqueous solution was used as extraction solution and a 5-fold dilution of extracted sample solution was applied in further experiment. For muscle tissue sample (shrimp as an example), 3% trichloroacetic acid aqueous solution was used as extraction solution and a 10-fold dilution of extracted sample solution can remove the matrix effects (Fig. S3c, d).
The spiked milk and muscle tissue samples were detected by the FICA strips. The visual test results are shown in Fig. 4. The fluorescence on the T line was quenched and only one green fluorescence band on the C line can be observed, which was judged as negative result. The result was judged as positive, when two green fluorescence bands on the T and C lines were observed simultaneously. For muscle tissue sample (Fig. 4a-g), when the spiked concentration of TC was equal to, or higher than 40 μg·kg−1, we can observe the positive test results. The visual LODs of the FICA based on “turn on” mode for the muscle tissue samples were 40 μg·kg−1. For milk samples (Fig. 4h), when the spiked concentration of TC was equal to, or higher than 20 μg·L−1, we can observe the positive test results. The visual LOD of the FICA based on “turn on” mode for the milk sample was 20 μg·L−1. In addition, the results of the spiked samples by the FICA well agreed with that results by HPLC, indicating the good accuracy of the FICA for detection of TC in milk and animal muscle tissue samples (Table S3).
Detection of TC in the spiked samples based on the “turn on” mode with the FICA strips. a shrimp, b grass carp, c weever, d carp, e chicken, f pork, g beef, h milk. The spiked concentrations of TC from left to right are 0, 20, 40, and 100 μg·kg−1 (a-g, tissue sample) or 0, 10, 20, 50 μg·L−1 (h, milk sample)
Analytical performance of the FICA
The parameters of this work and some reported immunoassays to detect tetracycline antibiotics are summarized in Table 1. Among these methods, ELISA, fluorescence immunoassay, and electrochemical immunosensor display higher sensitivity and they can provide the quantitative data. But these methods require relatively time-consuming incubation and washing operations, and special instruments. The visual LODs of some reported immunochromatographic assays to detect tetracycline antibiotics using gold nanoparticle, latex bead, or quantum dot as labels are higher than that of the FICA in this work. Table 2 indicates the analytical performance of the commercial ELISA test kit with three immunochromatographic assays (FICA, QDICA, and CGICA) for the rapid determination of TC in milk and animal muscle tissue samples. The ELISA test kit is more sensitive and can provide a quantitative result. However, the ELISA test kit required 130 min to finish the entire test process including sample treatment and test, it is far more than that required by three immunochromatographic assays (30 min). Compared with the ELISA test kit, the FICA, QDICA, and CGICA are more time-saving and convenient. In addition, the visual LODs of the QDICA and CGICA based on “turn off” mode using same TC coating antigen and anti-TC antibody are 50 μg·L−1 and 100 μg·L−1 for milk, respectively; 100 μg·kg−1 and 200 μg·kg−1 for animal muscle tissue, respectively. The sensitivities of the FICA based on “turn on” mode to detect TC in milk and animal muscle tissue samples are improved two point five times for the QDICA based on “turn off” mode and five times for the CGICA based on “turn off” mode. Particularly, the FICA also can provide a dual-functional test mode. To utilize the difference between the visual LODs of the assay based on “turn on” mode and “turn off” mode can achieve semi-quantitative detection of TC and one-step judge of limits of TC are violated or not.
Semi-quantitative detection of TC in sample based on the dual-functional mode
In our work, the sensitivities of the FICA based on single “turn on” mode for milk and animal muscle tissue samples are enough to detect TC, but it only provides a qualitative signal, i.e. any concentration of TC above 20 μg·L−1 in milk sample or 40 μg·kg−1 in muscle tissue sample will give a positive result, reflecting the TC is present in sample. Here, a dual-function test mode (“turn on” and “turn off”) of the assay has been applied to rapidly semi-quantitatively detect TC concentration in the milk and animal muscle tissue samples.
For the milk sample, under UV light, the test results of the FICA strips based on “turn-on” mode are observed (Fig. 5a) with a visual LOD at 20 μg·L−1. The same strips are observed under natural light, the test results based on “turn-off” mode are obtained (Fig. 5b) with a visual LOD at 100 μg·L−1. In summary, one strip is observed under UV light and natural light, respectively, if the strip both provides a negative result, it means that the concentration of TC in the milk sample is lower than 20 μg·L−1, and if the strip both provides a positive result, it means that the concentration of TC in the milk sample is equal to, or higher than 100 μg·L−1, reflecting the limit is violated (the MRL of TC in milk at 100 μg·L−1). If the strip provides a positive result under UV light and that provides a negative result under natural light, indicating the concentration of TC in the milk sample is 20 μg·L−1 or ranges from 20 μg·L−1 to 100 μg·L−1.
Detection of TC in the spiked samples based on the dual-functional mode. a fluorescent test results of strips for milk sample based on “turn on” mode under UV light, b colorimetric test results of strips for milk sample based on “turn off” mode under natural light, c fluorescent test results of strips for shrimp sample based on “turn on” mode under UV light, d colorimetric test results of strips for shrimp sample based on “turn off” mode under natural light
For the muscle tissue sample (shrimp as an example), the test results based on “turn-on” mode and “turn-off” mode are shown in Fig. 5c, d, with the visual LODs at 40 μg·kg−1 and 200 μg·kg−1, respectively. In the same way, we can judge that the concentration of TC in muscle tissue sample is lower than 40 μg·kg−1 when the strip both provides a negative result, and that is equal to, or higher than 200 μg·kg−1 (the MRL of 100 μg·kg−1 is violated) when the strip both provides a positive result under UV light and natural light. If the strip provides a positive result under UV light and that provides a negative result under natural light, indicating the concentration of TC in the animal muscle tissue sample is 40 μg·kg−1 or ranges from 40 μg·kg−1 to 200 μg·kg−1. In order to know if limits are violated or not, the sample requires a 2.5-fold dilution to analyze by the FICA strip under UV light. If the strip provides a negative result, it means that the concentration of TC in the animal muscle tissue sample is lower than 100 μg·kg−1, and if the strip provides a positive result, it means that the concentration of TC in the animal muscle tissue sample is equal to, or higher than 100 μg·kg−1, reflecting the limit is violated (the MRL of TC in the animal muscle tissue at 100 μg·kg−1).
Therefore, the fluorometric immunochromatographic assay combined with a dual-functional mode can be applied as a useful screening tool for rapid, qualitative and semi-quantitative to visually detect TC residues in milk and animal muscle tissue samples.
Conclusion
A fluorometric immunochromatographic assay is described that is based on “turn on” mode using QD as fluorescence signal label and AuNP as fluorescence quencher. This FICA can be used to detect the tetracycline antibiotics with the visual LODs of 2 μg·L−1 when working in assay buffer, 20 μg·L−1 for milk, and 40 μg·kg−1 for animal muscle tissues. The FICA based on “turn on” mode is more sensitive than the CGICA and QDICA based on “turn off” mode using sole AuNP or QD as signal label for the detection of tetracycline antibiotics. One strip can provide simultaneously the fluorescent test signals based on “turn on” mode under UV light and the color test signals based on “turn off” mode under natural light. According to the test results in dual-functional test mode (“turn on” and “turn off”), we can judge directly if limits of tetracycline in milk and animal muscle tissue samples are violated or not. However, the limitation of this FICA is that it only provides the visual qualitative and semi-quantitative results, i.e. whether tetracycline antibiotics are present or not in the sample, and the MRLs are violated or not. In order to know the accurate concentration of analyte, the strip reader device is needed to achieve quantitative analysis of analyte contents in the sample. Therefore, the FICA combined with a dual-functional mode can serve as a useful screening tool to monitor tetracycline antibiotics residues in milk and animal muscle tissue samples.
References
Chopra I, Roberts M (2001) Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260
Granados-Chinchilla F, Rodríguez C (2017) Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem 2017:1315497
Gokulan K, Cerniglia CE, Thomas C, Pineiro SA, Khare S (2017) Effects of residual levels of tetracycline on the barrier functions of human intestinal epithelial cells. Food Chem Toxicol 109:253–263
Pérez-Rodríguez M, Pellerano RG, Pezza L, Pezza HR (2018) An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 182:1–21
Liu Y, Yang HL, Yang S, Hu QW, Cheng HB, Liu HY, Qiu YS (2013) High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp. J Chromatogr B Anal Technol Biomed Life Sci 917:11–17
Chico J, Meca S, Companyó R, Prat MD, Granados M (2008) Restricted access materials for sample clean-up in the analysis of trace levels of tetracyclines by liquid chromatography application to food and environmental analysis. J Chromatogr A 1181:1–8
Nebot C, Guarddon M, Seco F, Iglesias A, Miranda JM, Franco CM, Cepeda A (2014) Monitoring the presence of residues of tetracyclines in baby food samples by HPLC-MS/MS. Food Control 46:495–501
Liu HC, Chen LG, Ding J (2017) A core-shell magnetic metal organic framework of type Fe3O4@ZIF-8 for the extraction of tetracycline antibiotics from water samples followed by ultra-HPLC-MS analysis. Microchim Acta 184:4091–4098
Islas G, Rodriguez JA, Perez-Silva I, Miranda JM, Ibarra IS (2018) Solid-phase extraction and large-volume sample stacking-capillary electrophoresis for determination of tetracycline residues in milk. J Anal Methods Chem 2018:5394527
Li R, Zhang H, Chen QW, Yan N, Wang H (2011) Improved surface-enhanced Raman scattering on micro-scale Au hollow spheres: synthesis and application in detecting tetracycline. Analyst 136:2527–2532
Verma R, Gupta BD (2013) Optical fiber sensor for the detection of tetracycline using surface plasmon resonance and molecular imprinting. Analyst 138:7254–7263
Meisen I, Wisholzer S, Soltwisch J, Dreisewerd K, Mormann M, Müthing J, Karch H, Friedrich AW (2010) Normal silica gel and reversed phase thin-layer chromatography coupled with UV spectroscopy and IR-MALDI-o-TOF-MS for the detection of tetracycline antibiotics. Anal Bioanal Chem 398:2821–2831
Mao YN, Wu YF, Nie YY, Wang J, Liu YY, Xu SH, Luo XL (2018) A two-wavelength fluorescence recovery method for the simultaneous determination of aureomycin and oxytetracycline by using gold nanocrystals modified with serine and 11-mercaptoundecanoic acid. Microchim Acta 185:222
Kurittu J, Lönnberg S, Virta M, Karp M (2000) A group-specific microbiological test for the detection of tetracycline residues in raw milk. J Agric Food Chem 48:3372–3377
Pastor-Navarro N, Morais S, Maquieira Á, Puchades R (2007) Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues: application to honey samples. Anal Chim Acta 594:211–218
García-Fernández J, Trapiella-Alfonso L, Costa-Fernández JM, Pereiro R, Sanz-Medel A (2014) A quantum dot-based immunoassay for screening of tetracyclines in bovine muscle. J Agric Food Chem 62:1733–1740
Liu X, Zheng S, Hu YX, Li ZJ, Luo F, He Z (2016) Electrochemical immunosensor based on the chitosan-magnetic nanoparticles for detection of tetracycline. Food Anal Methods 9:2972–2978
Que XH, Chen X, Fu LB, Lai WQ, Zhuang JY, Chen GN, Tang DP (2013) Platinum-catalyzed hydrogen evolution reaction for sensitive electrochemical immunoassay of tetracycline residues. J Electroanal Chem 704:111–117
Liu BQ, Zhang B, Chen GN, Tang DP (2014) Biotin-avidin-conjugated metal sulfide nanoclusters for simultaneous electrochemical immunoassay of tetracycline and chloramphenicol. Microchim Acta 181:257–262
Zhou JY, Nie W, Chen YQ, Yang CJ, Gong L, Zhang C, Chen Q, He LD, Feng XY (2018) Quadruplex gold immunochromatogaraphic assay for four families of antibiotic residues in milk. Food Chem 256:304–310
Chen YN, Kong DZ, Liu LQ, Song SS, Kuang H, Xu CL (2016) Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Anal Methods 9:905–914
Wang C, Li XM, Peng T, Wang ZH, Wen K, Jiang HY (2017) Latex bead and colloidal gold applied in a multiplex immunochromatographic assay for high-throughput detection of three classes of antibiotic residues in milk. Food Control 77:1–7
Le T, Yu H, Wang XL, Ngom B, Guo YC, Bi DR (2011) Development and validation of an immunochromatographic test strip for rapid detection of doxycycline residues in swine muscle and liver. Food Agric Immunol 22:235–246
Nail L, Sharma R, Mann B, Lata K, Rajput YS, Nath BS (2017) Rapid screening test for detection of oxytetracycline residues in milk using lateral flow assay. Food Chem 219:85–92
Xie Y, Zhang L, Yang X, Le T (2017) Development of a quantum dot-based immunochromatography test strip for rapid screening of oxytetracycline and 4-epi-oxytetracycline in edible animal tissues. Food Addit Contam Part A 34:371–378
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Spano G, Speranskaya ES, Goryacheva IY, Baggiani C (2018) A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim Acta 185:94
Yang YH, Ozsoz M, Liu GD (2017) Gold nanocage-based lateral flow immunoassay for immunoglobulin G. Microchim Acta 184:2023–2029
Fu XQ, Chu YX, Zhao K, Li JG, Deng AP (2017) Ultrasensitive detection of the β-adrenergic agonist brombuterol by a SERS-based lateral flow immunochromatographic assay using flower-like gold-silver core-shell nanoparticles. Microchim Acta 184:1711–1719
Wiriyachaiporn N, Sirikett H, Maneeprakorn W, Dharakul T (2017) Carbon nanotag based visual detection of influenza a virus by a lateral flow immunoassay. Microchim Acta 184:1827–1835
Sheng W, Li SJ, Liu Y, Wang JP, Zhang Y, Wang S (2017) Visual and rapid lateral flow immunochromatographic assay for enrofloxacin using dyed polymer microspheres and quantum dots. Microchim Acta 184:4313–4321
Bamrungsap S, Apiwat C, Chantima W, Dharakul T, Wiriyachaiporn N (2014) Rapid and sensitive lateral flow immunoassay for influenzaantigen using fluorescently-doped silica nanoparticles. Microchim Acta 181:223–230
Zu FL, Yan FY, Bai ZJ, Xu JX, Wang YY, Huang YC, Zhou XG (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914
Fu QQ, Liang JJ, Lan CF, Zhou KN, Shi CY, Tang Y (2014) Development of a novel dual-functional lateral-flow sensor for on-site detection of small molecule analytes. Sensors Actuators B Chem 203:683–689
Shi CY, Deng N, Liang JJ, Zhou KN, Fu QQ, Tang Y (2015) A fluorescent polymer dots positive readout fluorescent quenching lateral flow sensor for ractopamine rapid detection. Anal Chim Acta 854:202–208
Jiang H, Li XM, Xiong Y, Pei K, Nie LJ, Xiong YH (2017) Silver nanoparticle-based fluorescence-quenching lateral flow immunoassay for sensitive detection of ochratoxin A in grape juice and wine. Toxins 9:83
Acknowledgements
This work was supported by the Tianjin Municipal Science and Technology Commission (Project No. 16PTSYJC00130), the National Key R and D Program of China (Project No. 2016YFD0401204), and the International Science and Technology Cooperation Program of China (Project No. 2014DFR30350).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2575 kb)
Rights and permissions
About this article
Cite this article
Sheng, W., Chang, Q., Shi, Y. et al. Visual and fluorometric lateral flow immunoassay combined with a dual-functional test mode for rapid determination of tetracycline antibiotics. Microchim Acta 185, 404 (2018). https://doi.org/10.1007/s00604-018-2945-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2945-9