Abstract
A modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method combined with dispersive liquid-liquid microextraction (DLLME) pretreatment was developed for the determination of 5-hydroxymethyl-2-furaldehyde (HMF) in cooked japonica rice. Optimum conditions of the proposed method include MgSO4 (1 g), NaCl (0.5 g), C18 (60 mg), PSA (50 mg), the extraction solvent (CHCl3), dispersing solvent volume/extraction solvent volume (4:1), ultrapure water volume/the total volume of dispersing solvent volume and extraction solvent volume (1:1), and pH (7). HMF was concentrated by the clean-up method with the enrichment factor of 1.98. The separation of HMF was performed on an ACQUITY UPLC BEH-RP C18 column (2.1 mm × 100 mm, 1.7 μm) by the UPLC-ESI-MS/MS (ultraperformance liquid chromatography-electrospray ionization-tandem mass spectrometry). Acceptable values of HMF were achieved for the following parameters: recoveries between 82.87 and 110.48% with the relative standard deviation lower than 8.68%, accuracy in the range of 93.95–114.22%, precision (< 8.76%), limit of detection (LOD) (0.1 μg/L), and limit of quantification (LOQ) (0.3 μg/L). The concentrations of HMF were less than 1.13 μg/kg in rice samples, conspicuously increased with warming time. The color of rice samples, especially the b* value (in the range of 6.02–11.43), was well correlated with the content of HMF and the warming time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryzae sativa, L.), as a starch-rich commodity, is a staple food for the majority of the world’s populated countries (Villanova et al. 2017). There are varieties of rice grown throughout the world, yet japonica rice is mainly grown and studied in East Asia, Southeast Asia, and South Asia (Cai et al. 2007). There are different preparation techniques for rice production, including cooked rice (Buttery et al. 1983) and parboiled rice (Villanova et al. 2017). After rice cooking, the temperature of the electric cooker is maintained between 60–80 °C, which is referred to as the warming process. As higher temperature and water content persist, the Maillard reaction may occur and affect the color of products (Ferrer et al. 2005) and contribute to the formation of 5-hydroxymethyl-2-furaldehyde (HMF) (Lieve et al. 2008) during the cooking and warming processes. Certainly, the grain quality of rice is important to rice production, as well as after storage. According to Rez-Jiménez et al., the HMF content and value b* both increased during storage of infant rice cereal compared with the non-stored samples (Ramı́Rez-Jiménez et al. 2003). Therefore, the Maillard reaction and storage stage were both helpful to the formation of HMF in rice matrices.
Controlled browning is usually used to promote acceptable flavor and color properties in foods. Color properties are important attributes to the quality of cooked rice (Luangmalawat et al. 2008). Moreover, HMF is not only an advanced Maillard product (an undesired product) in foodstuffs exposed to heat treatment but is also a component of caramel color and a derivative of furan (Kroh 1994). On one hand, HMF is known as a heat-damage indicator and has been determined to evaluate the extent of the Maillard reaction in parboiled rice or other cereals during thermal processing (Lieve et al. 2008), and is also an indicator of the quality loss of sugar-containing food during storage (Madani-Tonekaboni et al. 2015). On the other hand, HMF has been investigated as a food contaminant with potential harmful properties in food (Kowalski et al. 2013). High amounts of HMF have been reported in an extensive variety of carbohydrate-rich foodstuffs such as cereal products (Degen et al. 2012), biscuit (Teixidó et al. 2006), and infant milk–based products (Morales and Jiménez-Pérez 2001). Especially, HMF levels in rice have been reported by Lamberts et al. (Lieve et al. 2008) and Villanova et al. (Villanova et al. 2017), while there have been no reports about the effective clean-up pretreatment and trace level determination and concentration of HMF in rice. Thus, it was highly essential to develop an accurate, rapid, and effective clean-up pretreatment to detect HMF in rice.
Generally, HMF analysis in foodstuffs has been reported in the previous literature, such as high performance liquid chromatography (HPLC) in follow-on milks and infant formulas (Er et al. 2015); ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) in fermented soy sauce (Zhang and Li 2019); gas chromatography coupled to massspectrometry (GC-MS) in several Spanish food samples (Teixidó et al. 2006); and capillary electrophoresis-ultraviolet (CE-UV) in vinegar and soy sauce (Wu et al. 2018). Especially, the LC-MS method for the determination of HMF has excellent repeatability and sensitivity in food samples. Therefore, ultra-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-ESI-MS/MS) method was further applied to analyze HMF in rice.
Sample preparation is an essential step to remove matrix interferences and improve extraction recovery (Kamalabadi et al. 2015). Traditional sample preparations including solid-phase extraction (SPE) (Teixidó et al. 2006) and liquid-liquid extraction (LLE) (Erika et al. 2008) were used for the determination of HMF in baby foods or other solid samples. However, LLE is time-consuming and non-economical and consumes high amounts of toxic solvents. Moreover, SPE cartridges are expensive and also time-consuming due to the evaporation step for concentration. In order to combat the above problem, the numerous benefits of trace analysis microextraction techniques were previously investigated and reported, including the ease of operation, excellent recovery, rapidity, low price, and high enrichment factor (Madani-Tonekaboni et al. 2015). Microextraction techniques for the analysis of HMF include headspace solid-phase microextraction (HS-SPME) (Giordano et al. 2003), headspace-liquid phase microextraction (HS-LPME) (Chaichi et al. 2013) and vortex-assisted liquid-liquid microextraction (VALLME) (Abu-Bakar et al. 2014). Especially, the dispersive liquid-liquid microextraction (DLLME), proposed in 2006 (Berijani et al. 2006), was widely applied because of the advantages of including a short extraction time and high enrichment factor (Yan and Wang 2013). Although the above techniques are simple, rapid, and inexpensive, they are not suitable for solid samples. Therefore, QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method, a new microextraction technique, has firstly been introduced by Anastassiades et al. (2003). Besides, the QuEChERS pretreatment has been modified by many authors for the determination of pesticide residue from different matrices like vegetables (Komasawa et al. 2010) and soil (Pang et al. 2016). The QuEChERS method was later modified for analysis of carbohydrate matrices in rice. In our previous report, DSPE-DLLME which was used only as a clean-up method had no effect on concentrating HMF in thermally treated abalone (Xu et al. 2018). To our best knowledge, there are no effective pretreatments for the determination and concentration of HMF in starch-rich products such as rice (solid sample).
The objective of the research was to establish a rapid, effective, and high enrichment factor pretreatment method to determine trace levels of HMF in rice matrices. Besides, this research also investigated the impact of cooking and warming steps in rice production on HMF concentrations and the color of cooked japonica rice. Moreover, correlation analysis was conducted between rice warming time, HMF levels, and color (b* values).
Materials and Methods
Chemicals
HMF (99%), dichloromethane (CH2Cl2), chloroform (CHCl3), tetrachloromethane (CCl4), chlorobenzene (C6H5Cl), magnesium sulfate (MgSO4), and sodium chloride (NaCl) were all purchased from Sigma-Aldrich Chemical Co. Ltd (Shanghai, China). Methanol (CH4O), formic acid (HCOOH), ammonium hydroxide (NH3·H2O), and acetonitrile (CH3CN) of HPLC grade were acquired from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Octadecylsilane (C18), primary secondary amine (Cleanert PSA), and graphitized carbon black (GCB) were obtained from Agela Technologies (Tianjin, China). Polished round-grained rice was bought from a local market in Wuxi, Jiangsu province, P.R. China. For all experiments, ultrapure water (UPW) was used.
HMF stock solutions (200 mg/L) were stored in the dark at 4 °C. A calibration curve was constructed by spiking different standard solutions (0.5, 1.0, 2.0, 5.0, 7.5, 10.0, 15.0, 20.0, 25.0, and 50.0 μg/L) into a practical sample matrix.
Sample Preparation and Modified QuEChERS Method Combined with DLLME
Sample Preparation
Japonica rice (JR) was purchased from a local market in Wuxi, Jiangsu province, P.R. China. The JR included fresh JR (abbreviation FJR) and fresh JR stored at the room temperature for 24 months (abbreviation FJRS). Six hundred grams of FJR or FJRS with 870 g of UPW were cooked by the electric cooker (FS4088, Midea, Guandong, China), and the cooked FJR or cooked FJRS were kept warm in an electric cooker for 0, 6, 12, 18, 24, and 30 h (temperature approximately about 60–80 °C). The samples were named cooked FJR-0h, FJR-6h, FJR-12h, FJR-24h, FJR-30h, FJRS-0h, FJRS-6h, FJRS-12h, FJRS-24h, and FJRS-30h, respectively. Cylindrical samples of 16.00 cm (diameter) × 1.5 cm (height) were chosen from the cooked FJR or cooked FJRS. Then, 10 g of cooked FJR or cooked FJRS were extracted with 10 mL of acetonitrile, and homogenized for 60 s × 3 at 8000 rpm by a digital ULTRA-TURRAX (T 25, IKA, Germany). The sample was filtered through a 0.22 μm filter membrane and then obtained for UPLC-ESI-MS/MS analysis. The cooked FJR-0h spiked with 500.0 μg/L HMF without clean-up was prepared as the above procedure.
Modified QuEChERS Method Combined with DLLME
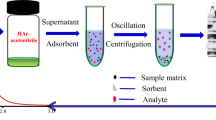
The single factor design experiment was performed to optimize the sample preparation procedure. The detailed information of optimization was listed as follows: the amounts of MgSO4 (1, 2, 3, and 4 g), the amounts of NaCl (0, 0.5, 1, and 1.5 g), the amounts of C18 (20, 40, 60, and 80 mg), the amounts of PSA (20, 50, 80, and 110 mg), the amounts of GCB (0, 10, 15, and 20 mg), the optimization of extraction solvent (CH2Cl2, CHCl3, CCl4, and C6H5Cl), the volume ratio 1 (dispersing solvent volume/extraction solvent volume, 0.25:1, 1:1, 4:1, and 8:1), the volume ratio 2 (UPW volume/the total volume of dispersing solvent volume and extraction solvent volume, 0.5:1, 1:1, 2:1, and 4:1) and the optimization of pH (3, 7, 8, and 10). The sample mixture, with the addition of 1 g of MgSO4 and 0.5 g of NaCl, was immediately shaken for 1 min and then centrifuged at 8000 rpm for 5 min by a multifunctional centrifuge (5810R, eppendorf, Hamburg, Germany). The clear acetonitrile layer was added 60 mg of C18 and 50 mg of PSA (for modified QuEChERS method), then the mixture was rapidly shaken using a vortex mixer (MX-S, IKA, Germany) for 1 min and centrifuged at 8000 rpm for 5 min.
Subsequently, the mixture of CHCl3 (as extraction solvent) and UPW (2.5 mL) were added into 2 mL of the acetonitrile (as the dispersive solvent for the DLLME). After centrifugation at 8000 rpm for 5 min, the organic phase (lower layer) appeared at the bottom of centrifuge tube and was collected and volatilized in a mild stream of nitrogen. Lastly, the UPW-redissolved sample was kept in the dark for UPLC-ESI-MS/MS analysis. The cooked FJR-0h spiked with 500.0 μg/L HMF with clean-up (modified QuEChERS method-DLLME) was completely finished. All samples were prepared at 4 °C in triplicate. The enrichment factor (EF) of modified QuEChERS method combined with DLLME was measured by the following equation: EF=CA/CB, and CA was the peak area of cooked FJR-0h spiked with 500.0 μg/L HMF with clean-up (modified QuEChERS method-DLLME), CB was the peak area of cooked FJR-0h spiked with 500.0 μg/L HMF without clean-up.
UPLC-ESI-MS/MS Conditions
The rapid determination of HMF was conducted by ultra-performance liquid chromatography (UPLC, Waters ACQUITY binary solvent manager, America) equipped with an electrospray ionization (ESI) and coupled with a triple quadrupole mass spectrometer (Waters ACQUITY TQ Detector, America). An ACQUITY UPLC BEH-RP C18 column (2.1 mm × 100 mm, 1.7 μm) with gradient elution was used for HMF analysis. Mobile phase A and B were water (100%) and methanol (100%), respectively. The mobile phase was set as follows: 5% methanol for 0–1 min, 5 to 50% methanol for 1–5 min, and then from 50 to 100% methanol for 5–10 min, then reduced to 5% methanol for 10–10.5 min, and finally, 5% methanol (the initial condition) was kept constant for 3 min. The flow rate was 0.3 mL/min. The column temperature was set at 40 °C, and the injected volume was 10 μL.
The mass spectrometer was operated in the positive electrospray ionization (ESI+) mode, and the data was acquired from the multiple reaction monitoring (MRM) mode. The acquisition electrospray ionization (ESI) source parameters were as follows: entrance potential (EP) = 10 V, collision energy (CE) = 15 V, spray voltage = 5500 V, ion source temperature = 600 °C, source gas and auxiliary gas were set at 15 and 18 L/min, respectively. High purity nitrogen (99%) was used as the gas source. The MS/MS product ion parameters for analysis of HMF were listed as follows: the precursor ion (m/z) was 127.1, the produce ion (m/z) including 109.2 (m/z, quantitation ion), 53.1 (m/z, confirmation ion), and 81.1 (m/z, confirmation ion).
Color Measurement
Color measurements were conducted in rice samples by a colorimeter (UltraScan PRO, HunterLab, USA) using the Hunter scale. Particularly, b* values measure blueness to yellowness (b*, negative = blue, positive = yellow) (Chen et al. 2016). All analyses were performed in triplicate, and 6 measurements were performed on each sample.
Statistical Analysis
Mean and standard deviation (SD) of the data was calculated for each experiment and analyzed by SPSS software package (SPSS 22.0 for Windows). Analysis of one-way variance (ANOVA) was used to determine significant differences among applied treatments (Bewick et al. 2004). Moreover, a Pearson correlation coefficient test was applied to examine the correlations between all the analyzed parameters. The correlation coefficient (r) was also calculated by the Pearson correlation analysis (Nitin and Hui 2005). The level of significant differences for the comparison test as well as for the correlation analysis was set at ***(P < 0.001); **(P < 0.01); *(P < 0.05), which were all considered statistically significant.
Results and Discussion
Optimization of modified QuEChERS method combined with DLLME condition
As shown in Fig. 1 A, the modified QuEChERS method conditions including MgSO4, NaCl, C18, PSA, and GCB were optimized. MgSO4 and NaCl were added into the acetonitrile to promote the separation of water and organic phases without dilution with non-polar solvents and were used to obtain high recoveries (DãEz et al. 2006). Besides, the addition of MgSO4 was used to remove residual water (Paz et al. 2015); MgSO4 and sodium chloride (NaCl) were also used to provide a well-defined phase separation to obtain a high recovery (DãEz et al. 2006). As shown in Fig. 1, MgSO4 varied from 1 to 4 g, NaCl varied from 0 to 1.5 g, and the highest EF of HMF was achieved when 1 g of MgSO4 and 0.5 g of NaCl were added.
Optimization of modified QuEChERS method combined with dispersive liquid-liquid microextraction (DLLME) for the determination of cooked FJR-0h sample spiked with 5-hydroxymethyl-2-furaldehyde (HMF) standard (500.0 μg/L). Volume ratio 1, dispersing solvent volume/extraction solvent volume; volume ratio 2, UPW volume/the total volume of dispersing solvent volume and extraction solvent volume. The enrichment factor (EF) was calculated by the followed equation: EF = CA/CB, in which CA and CB were the peak area of cooked FJR-0h spiked with 500.0 μg/L HMF with and without clean-up (modified QuEChERS method-DLLME), respectively
The frequently used sorbents include C18, PSA, and GCB in the modified QuEChERS procedure. C18 had shown the ability to remove lipids and non-polar interferences (Paz et al. 2015). As C18 increased from 20 to 60 mg, the EF of HMF was also increased. However, the increasing amount of C18 apparently decreased the EF of HMF. Thus, 60 mg of C18 was selected as the appropriate amount of sorbent.
PSA, an ion exchange sorbent, was usually applied to remove sugars, organic acids, and matrix coextractants (Paz et al. 2015). The addition of various amounts of PSA including 20 mg, 50 mg, 80 mg, and 110 mg were performed. Application of 50 mg of PSA resulted in the maximum EF for HMF. Thus, 50 mg of PSA was chosen to be the optimal amount of sorbent.
GCB can effectively remove most of the hydrophobic compounds such as pigments (Anastassiades et al. 2003). However, the EF of HMF decreased with the increase of the amount of GCB. In terms of sensitivity, GCB was not added in the modified QuEChERS procedure after further optimization. The results suggested that the combination of C18, PSA, and GCB could give an excellent clean-up effect.
In this study, four procedures including extraction solvent, volume ratio 1 (dispersing solvent volume/extraction solvent volume), volume ratio 2 (UPW volume/the total volume of dispersing solvent volume and extraction solvent volume), and pH were tested based on the DLLME. The effects of different DLLME conditions were investigated as shown in Fig. 1 B.
High density organic solvents (such as CH2Cl2, CHCl3, CCl4, and C6H5Cl) were selected as extraction solvents due to acceptable operation parameters and ideal physical properties, including density, boiling point, and solubility. The EF of CHCl3 had the highest value among samples, demonstrated applicable extraction properties, and was subsequently selected as the extraction solvent.
The increase of the volume ratio 1 from 0.25:1 to 4:1 remarkably increased the EF of HMF. Therefore, 4:1 was selected to be the optimal volume ratio 1. When the volume ratio 2 was carried out from 0.5:1 to 1:1, the EF of HMF increased with the increase of the volume ratio 2. Nevertheless, the EF of HMF was relatively lower under the volume ratio 2 of 4:1. The results were consistent to our previous study (Xu et al. 2018); the volume ratio 1 and the volume ratio 2 were 4:1, 1:1, respectively. There was no concentration of HMF in the previous study; however, the EF value of about 1.98 could be obtained in cooked FJR-0h spiked with 500.0 μg/L HMF using modified QuEChERS method-DLLME.
The effect of pH on the EF of HMF was evaluated under different pH values (including pH 3, 7, 8, and 10). Findings revealed that the maximum EF of HMF was obtained after increasing the pH to 7. However, after further increasing the pH to 8 and 10, the EF of HMF was not obviously increased. Thus, when pH was applied at 7, the maximum EF for HMF was obtained.
The Selectivity and Enrichment of Modified QuEChERS Method Combined with DLLME
The chromatograms of HMF obtained under optimal experimental conditions with or without clean-up were shown in Fig. 2. Firstly, the influence of the matrix effect on the peak of HMF standards spiking into the cooked FJR-0h could be ignored with the clean-up (Fig. 2b, d). Nevertheless, there were dissymmetric peak and tailing peak in the chromatograms of HMF standards spiked into the cooked FJR-0h without clean-up (Fig. 2a, c). Secondly, the larger peak area was obtained in HMF standards after clean-up procedure such as Fig. 2 c and d.
Chromatograms of 5-hydroxymethyl-2-furaldehyde (HMF) in cooked FJR-0h with or without clean-up. a, cooked FJR-0h spiked with 50 μg/L HMF without clean-up; b, cooked FJR-0h spiked with 50 μg/L HMF with modified QuEChERS method-DLLME; c, cooked FJR-0h spiked with 500 μg/L HMF without clean-up; d, cooked FJR-0h spiked with 500 μg/L HMF with modified QuEChERS method-DLLME
Moreover, the cooked FJR-30h sample was also performed by different clean-up processes including modified QuEChERS, DLLME, and modified QuEChERS-DLLME. As shown in Fig. 3, the small peak of HMF was eluted with the obvious background noise when the cooked FJR-30h sample was only treated by the modified QuEChERS.
Finally, the results demonstrated that the modified QuEChERS method combined with DLLME could give an effective clean-up and concentration effect compared to the sample without clean-up, with only modified QuEChERS or DLLME in the determination of HMF.
Evaluation of the Method Performance
Under optimal experimental conditions, the performance of modified QuEChERS method-DLLME combined with UPLC-ESI-MS/MS method was investigated in accordance with the following parameters, including linearity, coefficient of determination (R2), limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, repeatability, and recovery.
Calibration curves were generated from cooked FJR-0h samples spiked with calibration standards in the range between 0.5 and 50.0 μg/L (Table 1). Excellent linearity with R2 of 0.9995 (better than 0.998) was attained using a modified QuEChERS method-DLLME procedure for HMF in the concentration range of 0.5–50.0 μg/L, which confirmed method reliability. The signal-to-noise ratio (S/N) was usually applied to calculate the analytical limits of the method. Besides, the cooked FJR-0h samples spiked with decreasing standard solution concentrations were subjected to a modified QuEChERS method-DLLME combined with UPLC-ESI-MS/MS analysis. The LOD and LOQ were determined at a S/N = 3 and 10, respectively (Hu et al. 2016). The LOD and LOQ of HMF were 0.1 and 0.3 μg/L (as reported in Table 1, equal to 0.01, 0.03 μg/kg, respectively) under complex matrix conditions, which are lower or close to those previous reports. For example, the LOD obtained in the present study was apparently lower than those presented by Masoumeh Madani-Tonekaboni (Madani-Tonekaboni et al. 2015) and Malene W. Poulsen (Spano et al. 2009) for baby formula using DLLME combined with HPLC and for honey without clean-up for RP-HPLC, with 1.9 μg/kg and 3 μg/kg, respectively. This LOQ was slightly lower than that covered by Jinyuan Wang (Wang and Schnute 2012) using UHPLC-MS/MS to detect HMF (LOQ = 1 μg/L) in beverages with no clean-up method. The LOQ value was close to our previous report (Xu et al. 2018) (LOQ = 0.3 μg/L) using DSPE-DLLME by HPLC-MS/MS in thermally treated abalone, but our previous report was carried out in HMF standard solutions and not in complex sample matrices.
Precision was established by repeatability (intraday precision) and intermediate precision (interday precision) (Cunha et al. 2016). As shown in Table 2, repeatability for the cooked FJR-0h samples spiked with five preparations of standard solutions (2.0, 5.0, 10.0, 20.0, and 50.0 μg/L) was addressed by six repeated measurements and expressed as relative standard deviation (RSD) (Rizelio et al. 2012) whose values ranged from 0.88 to 5.24%. Furthermore, intermediate precision was obtained from cooked FJR-0h samples spiked with three preparations of standard solutions (5.0, 10.0, and 50.0 μg/L) at different 5 days, with six consecutive replicates (Cunha et al. 2016). The results were in the range of 1.05% and 8.76%. The precision obtained in our measurements for rice samples (average of 3.52% and 5.96% for repeatability and intermediate precision, respectively) is comparable with the precision of measurements performed in the HMF beverages analyses (average of 5.10%) by Jinyuan Wang (Wang and Schnute 2012): 5.10% for HMF standard (not samples spiked with HMF standards) by the UHPLC-MS/MS with no clean-up method. Besides, the result is also closely comparable with the precision data measured in our previous study: average of 2.54% for HMF standard solutions (not samples spiked with HMF standards) by the HPLC-MS/MS in abalone samples with clean-up procedure. Furthermore, accuracy was expressed by % accuracy (calculated by observed amount/specified amount × 100%) (Wang and Schnute 2012). These values are also shown in Table 2 and their values ranged from 93.95 to 114.22%.
Generally, the matrix matched calibration is used to take matrix effect into account (Kittlaus et al. 2013). Recovery was performed by spiking actual samples at different standard solutions and was used to evaluate the method’s performance in different matrices (Madani-Tonekaboni et al. 2015). As shown in Fig. 4 B, HMF was eluted at 3.3 min with a symmetrical and no-tail peak. To confirm matrix effects in rice samples, the spiked HMF standards were added into the cooked FJR-30h sample (Fig. 4 B-b) and cooked FJRS-24h samples (Fig. 4 B-d) after the modified QuEChERS method-DLLME procedure. Besides, the spiking levels of HMF standards of the cooked rice samples did not induce shoulder peak formation. Moreover, the MS/MS spectrum of HMF in cooked FJRS-24h is shown in Fig. 4 A. The precursor ion (m/z, 127.1) and the produce ion (quantitation ion, confirmation ion: m/z, 109.2, 53.1, and 81.1, respectively) were exactly detected in actual rice samples.
MS/MS spectrum of HMF in cooked FJRS-24h was showed in Fig. 4 A. Chromatograms of 5-hydroxymethyl-2-furaldehyde (HMF) in japonica rice matrices (as shown in Fig. 4 B); a, the sample of HMF standard (10.0 μg/L); b, the sample of cooked FJR-30h; c, the sample of cooked FJR-30h spiked with HMF standards (2.0 μg/L); d, the sample of cooked FJRS-24h; e, the sample of cooked FJRS-24h spiked with HMF standards (20.0 μg/L)
Rice samples spiked with different concentration levels of HMF (2.0–20.0 μg/L) were used and three replicate analyses were performed on each concentration level. Table 3 shows the detailed values of recovery data for HMF in rice matrices. The obtained recoveries using the proposed method of HMF ranged from 82.87 to 104.86% for cooked FJR-30h samples and from 95.02 to 110.48% for cooked FJRS-24h samples. RSDs were in the range of 6.90% and 8.04% for cooked FJR-30h samples, 5.14–8.68% for cooked FJRS-24h samples, and below 9% for all analytes. The results are better than the previous reported data or in agreement with reported literature data. For example, the recoveries in this work were in agreement with the previously reported recoveries in apple cider samples using SPE by high performance liquid chromatography-diode array detector (HPLC-DAD) (95.1–102.0%) (Hu et al. 2013), in oil using LLE by HPLC-DAD (94.33–99.60%) (Durmaz and Gökmen 2010), also in fruit juices using VALLME by HPLC (96.0–105%) (Abu-Bakar et al. 2014), and in fruit juice and dried fruit samples using in-tube SPME by LC-MS (92.5–94.4%) (Kataoka et al. 2009). Besides, the recoveries obtained in this work were relatively better than previously reported recoveries in wine samples using headspace-SPME combined with GC-MS (56.2–61.1%) (Olivero and Trujillo 2010) and in wine samples using SPE by HPLC-DAD (63.9–97.2%) (Hu et al. 2013).
Analysis of 5-Hydroxymethyl-2-Furaldehyde and Color in Cooked Japonica Rice
Determination of 5-Hydroxymethyl-2-Furaldehyde in Cooked Japonica Rice
Starch is the major compound in rice (more than 70%) and also important in human nutrition. Besides, starch contains two types of polysaccharides, amylose, and amylopectin (Wang et al. 2010). Moreover, the protein content was in the range of 2.6–3.2% in rice. The above compounds could slightly interact each other accompanied by the Maillard reaction during the storage of rice in room temperature for 24 months. Moreover, the levels of HMF are detected, and the chromatograms of the FJR and FJRS samples before the cooking process are shown in Fig. S1 (in Supplementary Material). Thus, the above results illustrated that the HMF content in JRS samples was 0.04 μg/kg, while the FJR sample (non-stored sample) has no HMF prior to cooking (lower than LOQ).
The cooking and warming stage of the electric cooker includes temperature increase to 100 °C and is then maintained at 60–80 °C for 0–30 h. Therefore, Maillard reaction may occur in the electric cooker during the cooking and warming procedure. The concentrations of HMF in cooked FJR and cooked FJRS samples are shown in Table 4. Prolonging the warming time from 0 to 30 h, the amounts HMF remarkably increased both in the cooked FJR samples or cooked FJRS samples. The HMF was not detectable during 0 to 6 h; however, HMF concentration was in the range of 0.11–0.22 μg/kg in the cooked FJR samples. Besides, HMF levels ranged from 0.07 to 1.13 μg/kg in the cooked FJRS samples, which were relatively higher than that in cooked FJR samples.
HMF, an advanced Maillard reaction product, could be facilitated and accumulated by heat treatment and storage (Rada-Mendoza et al. 2004). On one hand, temperature and water content (about 60%) were necessary to form HMF during the Maillard reaction (Arribas-Lorenzo and Morales 2010), and the warming stage (60–80 °C) promoted the development of variable amounts of HMF. On the other hand, reaction time was also a significant determinant of the formation of Maillard reaction products and favored HMF formation (Alberto et al. 2012). In other words, the HMF levels of the cooked FJR and cooked FJRS samples strongly correlated with the Maillard reaction temperature and time.
Color in Cooked Japonica Rice
Mean b* values in the cooked FJR and cooked FJRS samples in the warming stage for 0–30 h are reported in Table 4. Significant differences (P < 0.05) were detected in the cooked FJR samples among the six warming times suggesting that with longer warming times, the greater b* values will increase (mean b* values for 0–30 h in the cooked FJR samples were 6.02, 6.83, 7.89, 8.16, 8.78, and 9.01, respectively). Furthermore, the values indicating the degree of yellowness of the samples were significantly higher in the cooked FJRS samples than those in cooked FJR samples. Comparing the cooked FJR-24h and cooked FJRS-24h samples, there were conspicuous differences in color data, the b* values ranged from 8.78 to 9.88. Especially, the b* values both increased observably with a prolonged warming time of cooked FJR or cooked FJRS samples. The results demonstrated that the b* (yellowness) value was facilitated by the Maillard reaction time. In other words, the b* values of rice samples were observably changed by the keeping warm time and increased with the extension of time.
Interestingly, significant differences in HMF contents were also observed during the warming period. The HMF levels in the cooked FJR and cooked FJRS samples were less than 0.22 μg/kg and ranged from 0.07 to 1.13 μg/kg for 0–30 h, respectively. The HMF levels in Table 4 were in the same rising tendency to the ones reported in the color (the b* value). Capuano et al. (Capuano and Fogliano 2011) and Toker et al. (Toker et al. 2013) also illustrated that HMF, as the precursors of polymeric products, might be involved in reaction leading to the development of brown color. Moreover, color changes occur at parboiled rice mainly due to the Maillard browning and pigment diffusion during soaking and steaming. The result revealed that the color of cooked FJR or cooked FJRS samples seem to correlate with the content of HMF and the time.
Correlation Analysis in Cooked Japonica Rice
As shown in Table 5, the correlation analysis displayed positive correlations between the warming time and HMF levels (r = 0.970; **P < 0.01), between the warming time and b* values (r = 0.969; **P < 0.01), also between HMF levels and b* values (r = 0.969; **P < 0.01) in cooked FJR samples. Furthermore, the correlation analysis revealed that the warming time was positively correlated with HMF levels (r = 0.989; **P < 0.01) and b* values (r = 0.963; **P < 0.01) in cooked FJRS samples, respectively. Additionally, HMF levels showed positive effects on b* values with r = 0.970 (**P < 0.01) in cooked FJRS samples. Therefore, the results illustrated that the warming time is essential for HMF levels and b* values with strong positive correlations. Moreover, it was apparent that HMF levels, in cooked FJR samples or cooked FJRS samples, do have effects on b* values.
Conclusions
A rapid and effective modified QuEChERS method combined with DLLME using UPLC-ESI-MS/MS was proposed for HMF analysis in cooked japonica rice. Moreover, the pretreatment method was helpful to give an acceptable clean-up and concentration effect compared to the sample without clean-up, with only modified QuEChERS or with only DLLME in the determination of HMF. Besides, the established pretreatment method was rapid and accurate for the trace HMF determination in rice with excellent repeatability, linearity, accuracy, and satisfactory recovery rate. The HMF levels were increased with longer warming times with a positive correlation (r = 0.970), and HMF levels in cooked FJRS samples were higher than that in cooked FJR samples. The b* values of cooked japonica rice samples strongly correlated with the content of HMF (r = 0.970) and the warming time (r = 0.969). HMF provided guidance for monitoring color changes in rice.
References
Abu-Bakar N-B, Makahleh A, Saad B (2014) Vortex-assisted liquid-liquid microextraction coupled with high performance liquid chromatography for the determination of furfurals and patulin in fruit juices. Talanta 120:47–54
Alberto F et al (2012) Controlling the Maillard reaction by reactant encapsulation: sodium chloride in cookies. J Agric Food Chem 60:10808–10814
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Arribas-Lorenzo G, Morales FJ (2010) Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J Agric Food Chem 58:2966–2972
Berijani S, Assadi Y, Anbia M, Hosseini MRM, Aghaee E (2006) Dispersive liquid-liquid microextraction combined with gas chromatography-flame photometric detection: very simple, rapid and sensitive method for the determination of organophosphorus pesticides in water. J Chromatogr A 1123:1–9
Bewick V, Cheek L, Ball J (2004) Statistics review 9: one-way analysis of variance. Crit Care 8(2):130–136
Buttery RG, Ling LC, Juliano BO, Turnbaugh JG (1983) Cooked rice aroma and 2-acetyl-1-pyrroline. J Agric Food Chem 31:823–826
Cai X, Liu J, Qiu Y, Zhao W, Song Z, Baorong LU (2007) Differentiation of Indica-Japonica rice revealed by insertion/deletion (InDel) fragments obtained from the comparative genomic study of DNA sequences between 93-11 (Indica) and Nipponbare (Japonica). Front Biol China 2:291–296
Capuano E, Fogliano V (2011) Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci Technol 44:793–810
Chaichi M, Mohammadi A, Hashemi M (2013) Optimization and application of headspace liquid-phase microextraction coupled with gas chromatography-mass spectrometry for determination of furanic compounds in coffee using response surface methodology. Microchem J 108:46–52
Chen YT, Shiau SY, Fu JT (2016) Physicochemical properties of dough and steamed bread made from regular and whole wheat flour international. J Food Eng 12:411–419
Cunha SC, Senra L, Cruz R, Casal S, Fernandes JO (2016) 4-Methylimidazole in soluble coffee and coffee substitutes. Food Control 63:15–20
DãEz C, Traag WA, Zommer P, Marinero P, Atienza J (2006) Comparison of an acetonitrile extraction/partitioning and “dispersive solid-phase extraction” method with classical multi-residue methods for the extraction of herbicide residues in barley samples. J Chromatogr A 1131:11–23
Degen J, Hellwig M, Henle T (2012) 1,2-dicarbonyl compounds in commonly consumed foods. J Agric Food Chem 60:7071–7079
Durmaz G, Gökmen V (2010) Determination of 5-hydroxymethyl-2-furfural and 2-furfural in oils as indicators of heat pre-treatment. Food Chem 123:912–916
Er DB, Demirhan B, Sönmez C, Torul H, Tamer U, Yentür G (2015) Short communication: determination of potential 5-hydroxymethyl-2-furaldehyde and 2-furaldehyde compounds in follow-on milks and infant formulas using the high-performance liquid chromatography method. J Dairy Sci 98:818–822
Erika T, Encarnación M, F Javier S, M Teresa G (2008) Liquid chromatography multi-stage mass spectrometry for the analysis of 5-hydroxymethylfurfural in foods. J Chromatogr A 1185:102–108
Ferrer E, Alegría A, Farré R, Clemente G, Calvo C (2005) Fluorescence, browning index, and color in infant formulas during storage. J Agric Food Chem 53:4911–4917
Giordano L, Calabrese R, Davoli E, Rotilio D (2003) Quantitative analysis of 2-furfural and 5-methylfurfural in different Italian vinegars by headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry using isotope dilution. J Chromatogr A 1017:141–149
Hu G, Hernandez M, Zhu H, Shao S (2013) An efficient method for the determination of furan derivatives in apple cider and wine by solid phase extraction and high performance liquid chromatography-Diode array detector. J Chromatogr A 1284:100–106
Hu G, Zhu Y, Hernandez M, Koutchma T, Shao S (2016) An efficient method for the simultaneous determination of furan, 2-methylfuran and 2-pentylfuran in fruit juices by headspace solid phase microextraction and gas chromatography-flame ionisation detector. Food Chem 192:9–14
Kamalabadi M, Ghaemi E, Mohammadi A, Alizadeh N (2015) Determination of furfural and hydroxymethylfurfural from baby formula using headspace solid phase microextraction based on nanostructured polypyrrole fiber coupled with ion mobility spectrometry. Food Chem 181:72–77
Kataoka H, Itano M, Ishizaki A, Saito K (2009) Determination of patulin in fruit juice and dried fruit samples by in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J Chromatogr A 1216:3746–3750
Kittlaus S, Schimanke J, Kempe G, Speer K (2013) Development and validation of an efficient automated method for the analysis of 300 pesticides in foods using two-dimensional liquid chromatography-tandem mass spectrometry. J Chromatogr A 1283:98–109
Komasawa N, Ueki R, Kaminoh Y, Nishi SI (2010) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A 1217:2548–2560
Kowalski S, Lukasiewicz M, Duda-Chodak A, Zięć G (2013) 5-Hydroxymethyl-2-furfural (HMF)-heat-induced formation, occurrence in food and biotransformation-a review. Pol J Food Nutr Sci 63:207–225
Kroh LW (1994) Caramelisation in food and beverages. Food Chem 51:373–379
Lieve L, Ine R, Kristof B, Kurt G, Jana D (2008) Impact of parboiling conditions on Maillard precursors and indicators in long-grain rice cultivars. Food Chem 110:916–922
Luangmalawat P, Prachayawarakorn S, Nathakaranakule A, Soponronnarit S (2008) Effect of temperature on drying characteristics and quality of cooked rice. LWT-Food Sci Technol 41:716–723
Madani-Tonekaboni M, Kamankesh M, Mohammadi A (2015) Determination of furfural and hydroxymethyl furfural from baby formula using dispersive liquid–liquid microextraction coupled with high performance liquid chromatography and method optimization by response surface methodology. J Food Compos Anal 40:1–7
Morales FJ, Jiménez-Pérez S (2001) Hydroxymethylfurfural determination in infant milk-based formulas by micellar electrokinetic capillary chromatography. Food Chem 72:525–531
Nitin B, Hui L (2005) Correlation between gene expression profiles and protein-protein interactions within and across genomes. Bioinformatics 21:2730–2738
Olivero SJP, Trujillo JPP (2010) A new method for the determination of carbonyl compounds in wines by headspace solid-phase microextraction coupled to gas chromatography-ion trap mass spectrometry. J Agric Food Chem 58:12976–12985
Pang N, Wang T, Hu J (2016) Method validation and dissipation kinetics of four herbicides in maize and soil using QuEChERS sample preparation and liquid chromatography tandem mass spectrometry. Food Chem 190:793–800
Paz M, Correia-Sá L, Becker H, Longhinotti E, Domingues VF, Delerue-Matos C (2015) Validation of QuEChERS method for organochlorine pesticides analysis in tamarind (Tamarindus indica) products: Peel, fruit and commercial pulp. Food Control 54:374–382
Rada-Mendoza M, Sanz ML, An O, Villamiel M (2004) Formation of hydroxymethylfurfural and furosine during the storage of jams and fruit-based infant foods. Food Chem 85:605–609
Ramı́Rez-Jiménez A, Guerra-Hernández E, Garcı́A-Villanova B (2003) Evolution of non-enzymatic browning during storage of infant rice cereal. Food Chem 83:219–225
Rizelio VM, Gonzaga LV, Borges GDSC, Micke GA, Fett R, Costa ACO (2012) Development of a fast MECK method for determination of 5-HMF in honey samples. Food Chem 133:1640–1645
Spano N et al (2009) A direct RP-HPLC method for the determination of furanic aldehydes and acids in honey. Talanta 78:310–314
Teixidó E, Santos FJ, Puignou L, Galceran MT (2006) Analysis of 5-hydroxymethylfurfural in foods by gas chromatography-mass spectrometry. J Chromatogr A 1135:85–90
Toker OS, Dogan M, Ersöz NB, Yilmaz MT (2013) Optimization of the content of 5-hydroxymethylfurfural (HMF) formed in some molasses types: HPLC-DAD analysis to determine effect of different storage time and temperature levels. Ind Crop Prod 50:137–144
Villanova FA, Vanier NL, De AMN, Pesek J, Matyska-Pesek M, Elias MC, De OM (2017) Improvement of the quality of parboiled rice by using anti-browning agents during parboiling process. Food Chem 235:51–57
Wang J, Schnute WC (2012) Simultaneous Quantitation of 2-Acetyl-4-tetrahydroxybutylimidazole, 2-and 4-Methylimidazoles, and 5-Hydroxymethylfurfural in Beverages by ultrahigh-performance liquid chromatography–tandem mass spectrometry. J Agric Food Chem 60:917–921
Wang L, Xie B, Shi J, Xue S, Deng Q, Wei Y, Tian B (2010) Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll 24:208–216
Wu J-H, Feng TT, Liang X, Yu CP, Dong L, du M, Tan MQ, Xu XB (2018) Determination of HMF in vinegar and soy sauce using two-step ultrasonic assisted liquid–liquid micro-extraction coupled with capillary electrophoresis-ultraviolet detection. Food Anal Methods 11:479–485
Xu X, Feng T, Zhang J, Ma Y, Song L (2018) A rapid clean-up method for the quantitation of 5-hydroxymethyl-2-furaldehyde in thermally treated abalone (Haliotis discus) muscle by HPLC-MS/MS. Anal Methods 10:5091–5096
Yan H, Wang H (2013) Recent development and applications of dispersive liquid-liquid microextraction. J Chromatogr A 1295:1–15
Zhang L, Li C (2019) Simultaneous analysis of 2-methylimidazole, 4-methylimidazole, and 5-hydroxymethylfurfural potentially formed in fermented soy sauce by “quick, easy, cheap, effective, rugged, and safe” purification and UHPLC with tandem mass spectrometry. J Sep Sci 42:501–508
Acknowledgments
All authors acknowledge the National key R & D program (2016YFD0400801), the National First-class Discipline of Food Science and Technology (JUFSTR20180204), and the program of “Collaborative innovation center of food safety and quality control in Jiangsu Province”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Tingting Feng declares that she has no conflict of interest. Qingxia Zhang declares that she has no conflict of interest. Xuejiao Wang declares that she has no conflict of interest. Shuqin Xia declares that she has no conflict of interest. Zhen Fang declares that he has no conflict of interest. Jing Li declares that she has no conflict of interest. Xiaoming Zhang declares that he has no conflict of interest. Jingyang Yu declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
A statement regarding informed consent is not applicable for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 369 kb)
Rights and permissions
About this article
Cite this article
Feng, T., Zhang, Q., Wang, X. et al. Determination of 5-Hydroxymethyl-2-Furaldehyde in Cooked Japonica Rice Using a Modified QuEChERS Method Combined with Dispersive Liquid-Liquid Microextraction Followed by UPLC-ESI-MS/MS. Food Anal. Methods 12, 1838–1848 (2019). https://doi.org/10.1007/s12161-019-01533-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01533-4