Abstract
Biofortification of crops with selenium (Se) is an effective strategy to increase the consumption of this micronutrient. Inductively coupled plasma mass spectrometry (ICP-MS) is one of the most used techniques in quantifying Se. However, due to some disadvantages (high cost, long analysis time, and being destructive), there is a need for new alternatives to Se quantification. This study aimed at establishing instrumental parameters for Se quantification, using two energy-dispersive X-ray fluorescence spectrometry (EDXRF) techniques (benchtop and handheld) in Se-biofortified broccoli seedlings and comparing it with ICP-MS. The results showed that the selection of proper filters for both EDXRF systems was crucial for determining Se and, when compared with ICP-MS, presented similar results for selenium-biofortified broccoli seedling treatments (BS50Se). In addition, the EDXRF techniques presented a Se limit of detection (LOD) at 0.6–0.9 mg kg−1. This study demonstrates that EDXRF systems were successfully applied to concentration measurement of Se in biofortified samples and linked to low cost and shorter analysis time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium—at low concentrations—is an essential metalloid in the human diet (Nunes et al. 2012). Scientific studies have already demonstrated its essential role in various functions, such as antioxidant defense (Rayman 2000), immune function (Khoso et al. 2015), thyroid hormone formation (Wichman et al. 2016), and fertility and reproduction (Mehdi et al. 2013). In addition, in the last 20 years, studies demonstrate an inverse relationship between Se levels and cancer incidence (Babaknejad et al. 2014; Bachiega et al. 2016; Jayaprakash and Marshall 2011; Roman et al. 2014). Although the recommended daily intake of selenium is only 55 μg day−1, there are reports stating that Se deficiency affects 800 million people worldwide (Malagoli et al. 2015; Otten et al. 2006); for example, in many European countries, this recommended daily intake is not achieved (Ivory and Nicoletti 2017). In this scenario, biofortification becomes an effective, economical, and sustainable method to improve Se in the food chain (Alfthan et al. 2015; Bouis and Saltzman 2017).
The quantification of selenium is an important step in the biofortification process. Different analytical techniques have been used to quantify this micronutrient in biofortified matrices, such as hydride generation atomic fluorescence spectrophotometry (HG-AFS) in brown rice (Liu et al. 2011); hydride generation atomic absorption spectroscopy (HG-AAS) in lamb’s lettuce (Hawrylak-Nowak et al. 2018); and inductively coupled plasma optical emission spectrometry (ICP-OES) in lentil seeds (Rahman et al. 2013) and in lettuce (Smoleń et al. 2014). The literature also highlights the high sensitivity of inductively coupled plasma mass spectrometry (ICP-MS), which is commonly used for quantification of Se in foods such as broccoli and carrots (Bañuelos et al. 2015), rice (Reis et al. 2018), and flour and bread (Hart et al. 2011). However, this technique requires the complete destruction of the matrix, which increases the time and cost of analysis. Moreover, the sample’s digestion with strong acid reagents requires great caution during sample preparation (Noda et al. 2006).
In this study, we proposed X-ray fluorescence (XRF) technique, especially benchtop and handheld energy-dispersive X-ray fluorescence spectrometry (EDXRF) for the determination of Se in Se-enriched broccoli seedlings. These techniques are promising for nutrient plant analysis, offering a simple, fast, and non-destructive method of nutrient analysis in a variety of sample matrices (Goodlaxson et al. 2018; Jolly et al. 2017; Perring and Monard 2010), especially for Se (Hanley et al. 2017; Paltridge et al. 2012). In addition, the use of the portable X-ray fluorescence (pXRF) system in measuring the concentration of many elements in different matrices has increased dramatically in the last 10 years (Barnett et al. 2016; Fleming et al. 2015, 2017; McIntosh et al. 2016, Rouillon and Taylor 2016; Shehab et al. 2016). We verified that the benchtop and handheld EDXRF techniques were suitable for the quantification of Se in biofortified samples. Both techniques are more expeditious and present lower costs of analysis compared with ICP-MS.
Materials and Methods
Plant Material
Broccoli seeds of the Avenger variety (Sakata Seed Sudamerica) were provided by IBS MUDAS (Piracicaba, São Paulo, Brazil). The broccoli seedlings were cultivated in black polyethylene trays containing 200 cells filled with commercial substrate (coconut fiber). After sowing, the trays were transferred to an arc-type agricultural greenhouse (8 m wide, 103 m long, and 4.5 m height) right foot, covered with 150-μm-thick anti-UV polyethylene film and closed on the sides with anti-insect screens. After 15 days of germination, broccoli seedlings were randomly separated and received an application in each cell of either 2 mL of distilled water (control treatments; BS0Se) or 50 μM sodium selenate (Sigma-Aldrich) solution (BS50Se) and remained for another 15 days in the greenhouse (Bachiega et al. 2016). In the 30th day after sowing, broccoli seedlings were collected, and only the aerial part of the plant was selected for analysis. Subsequently, the plant tissue was sanitized (0.5% sodium dichloroisocyanurate for 10 min), freeze-dried (Modelo E-C–Modulyo), and stored at − 20 °C until use. Samples of broccoli seedlings were cultivated in August–October 2014 (BS0Se14 and BS50Se14) and August–October 2016 (BS0Se16 and BS50Se16).
Sample Preparation for ICP-MS Analysis
Initially, 100 mg of each the broccoli seedling treatments (BS0Se14, BS0Se16, BS50Se14, and BS50Se16) and certified reference material of plankton (BCR 414) and sea lettuce (Ulva lactuca) (BCR 527) were weighed and transferred to poly(tetrafluoroethylene) flasks in a microwave oven (DGT 100 Plus). The decomposition of such samples was carried out using a mixture of 4 mL HNO3 with 200 μL H2O2 for 7 min at 400 W. After the microwave decomposition, the samples were transferred to poly(propylene) tubes and volume made up to 50 mL with deionized water. For sea lettuce sample preparation, the volume was completed to 14 mL.
ICP-MS Analysis
The operational conditions of ICP-MS quadrupole (model ELAN® DRC-e, PerkinElmer) are shown in Table 1, and the analytical calibration curve ranged from 0.5 to 25 μg L−1. The selenium concentration was expressed in mg kg−1 dry basis units.
Sample and Standard Preparation for EDXRF Analysis
The standard addition calibration curve of Se was prepared by adding known volumes of a 100-μg Se mL−1 working standard solution of Se in a broccoli seedling sample (control treatment). This standard was provided from the dilution (1:10) of 1000 μg mL−1 Se stock standard (Acros Organics, 1 mg mL−1). Subsequently, the standard samples were dried in a laboratory oven at 50 °C and then thoroughly homogenized in an agate mortar. The Se calibration curve concentration ranged from 0 to 250 μg g−1.
Samples and standards (1 g) were weighed in a poly(ethylene) sample cup (cat. no. 1530, Chemplex) with a 23.9-mm aperture and sealed with 6-μm-thick Mylar® film (no. 3517, Spex). The loose powder sample was gently manually pressed with an acrylic piston in order to prevent void in the powder sample. The typical sample thickness was 7 mm.
Benchtop EDXRF Analysis
The benchtop EDXRF measurements were carried out using a Shimadzu EDX-720 spectrometer equipped with a 50 W Rh target X-ray tube using 50-kV voltage and auto-tunable current for a maximum 30% detector dead time. A Si (Li) detector was used for sample X-ray spectrum acquisition. To optimize the EDXRF analysis for Se quantification, six filter conditions were tested: no filter, silver (Ag), molybdenum (Mo), molybdenum-nickel (MoNi), aluminum (Al), and titanium (Ti). The Ag filter was chosen for the analyses. The acquisition time used was 300 s, and all three trials were taken and analyzed in no vacuum.
The X-ray spectrum processing was carried out utilizing the EDX-720 software package provided by Shimadzu.
Handheld XRF Analysis
A handheld Bruker AXS, Tracer III-SD model (2 kg, 30 cm long × 10 cm wide × 28 cm height) was also employed. The samples were excited with a 4 W Rh target X-ray tube at 40 kV and 30 μA and X-ray spectra recorded by a 10 mm2 X-Flash® Peltier-cooled Silicon Drift Detector (SDD).
First, the conditions of the Se determination using the handheld XRF were optimized. Five primary filters with distinct thickness and composition were tested: no filter, filter Al Ti Cu 1 (304.8 μm Al + 25.4 μm Ti + 152.4 μm Cu), filter Al Ti Cu 2 (304.8 μm Al + 25.4 μm Ti + 25.4 μm Cu), filter Al Ti (304.8 μm Al + 25.4 μm Ti), and filter Ti (25.4 μm Ti) to optimize the Se evaluation. The acquisition time used was 300 s; three trials were taken and analyzed without a vacuum and selecting the filter Al Ti Cu 2. The sample spectra were processed by the Artax software. For both EDXRF systems, only the Se Kα net peak was considered as the Se X-ray intensity.
Limits of Detection and Quantification
ICP-MS limits of detection (LOD) and quantification (LOQ) (mg kg−1 dry basis) were calculated based on the standard deviation (σ, counts) of 10 readings of the standard solution blank, per Eq. 1:
where slope is the sensitivity (counts μg−1 L) and k is the dilution factor (g−1 L).
The benchtop and handheld EDXRF LOD and LOQ (mg kg−1 dry basis) were calculated according to Eq. 2 (Inczédy et al. 2000):
where S is the sensitivity (counts mg kg−1) and BG is the background intensity under Se Kα characteristic X-ray (counts).
Statistical Analysis
Statistical analyses were performed using SAS version 9.0 (SAS Inst. Inc., Cary, NC). After verifying the normality of data variance using the Shapiro-Wilk test and the homogeneity by Levene’s test, the difference between the means of the methods was tested using Student’s t test. Significance was defined as p < 0.05, and the variables are presented as the mean ± standard deviation (SD).
Results and Discussion
Previously, our research group observed that Se-enriched seedlings presented higher content of phenolic compounds, antioxidant activity, and antiproliferative activity in vitro against tumoral strains when compared with inflorescences and sprouts (Bachiega et al. 2016). These results are corroborated by other recent studies that demonstrated how biofortification with selenium alters the bioactive compounds of different maturation stages of broccoli (sprouts, inflorescences, or young leaves) (Mahn 2017; McKenzie et al. 2017; Tian et al. 2018). The broccoli maturation stage of 30 days after germination is a rapid alternative for biofortification by providing samples with important amounts of Se and bioactive compounds. The work presented herein focuses on Se determination in broccoli seedlings.
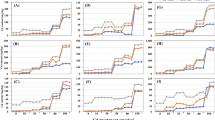
Data shown in Fig. 1 a and b demonstrates the broccoli seedling fluorescence X-ray spectra from 10.5 to 13.75 keV for both the X-ray fluorescence units; the Se Kα and Kβ peaks do not have any spectral interference from the broccoli seedling elemental composition. It also shows an impressive Se Kα net intensity and noise ratio for the 73 mg kg−1 concentration range (tens of mg kg−1 level) for both EDXRF systems, in spite of the handheld XRF’s remarkably low X-ray tube power (4 W).
First, to optimize the determination of Se by benchtop and handheld EDXRF systems, the figure of merit of its Kα X-ray characteristic intensity and background under the X-ray peak (noise) square root ratio (Guerra et al. 2014; Ernst et al. 2014) was evaluated for different primary filters in both EDXRF systems. Indeed, different primary filters absorb the X-ray tube’s polychromatic X-ray excitation beam differently, thus changing its photon energy distribution, which may improve the analyte X-ray characteristic intensity-to-noise ratio. The choice of the proper filter depends on the X-ray tube and its operational condition, sample matrix, analyte and filter thickness, and composition. As selecting the best filter option is not straightforward, the analyst must carry out a preliminary study that will lead to the best choice. Figure 2 a and b show the effect of several primary filters on the ratio of Se Kα X-ray intensity-to-background square root for the benchtop and handheld EDXRF units.
Regarding the benchtop system (Fig. 2a), the Se Kα X-ray intensity (counts) to BG (counts) square root ratio using the Ag filter was roughly four times higher than the no-filter condition. The Ag Kα X-ray absorption edge energy (25.517 keV) is higher than Rh–K lines anode energies (Kα = 20.170 and Kβ = 22.725 keV), therefore absorbing weakly and only partly the high-intensity anode K lines. The latter lines from the X-ray tube anode excite the Se K shell efficiently, and the Ag filter absorbs the background of the Se Kα line energy region (16.209 keV) from the X-ray tube more effectively, thus increasing significantly the Se Kα X-ray intensity (counts) to BG (counts) square root ratio. Although the Mo filter did not present a ratio value as high as the Ag filter, it shows a meaningful improvement over the other filters. The Mo filter has the K X-ray absorption edge energy just below the Rh anode K lines, thus efficiently absorbing the anode K lines and consequently reducing the sensitivity; conversely, this filter also dramatically diminishes the background of the Se Kα line energy region, resulting in the second-best option for Se evaluation. Unfortunately, the thicknesses of these filters were not disclosed by the manufacturer.
Similarly, for the handheld system, as shown in Fig. 2b, the filter Al Ti Cu 2 figure of merit gain was approximately twice compared with the no-filter condition. Although this filter reduces the X-ray excitation intensity, it decreases the background to a larger extent and is therefore the best filter option of the available handheld XRF filters. Figure 2 a and b highlight the importance of adequate filter selection for improving EDXRF analysis performance.
There are different strategies for the quantification in EDXRF, such as fundamental parameters, external calibration, standard addition, and sample dilution. In our study, we selected the standard addition method. For this, the absorption and enhancement matrix effects are corrected, since the calibration is performed using the sample itself. Although this method may be somewhat time-consuming and labor-intensive, including the careful pipetting-drying-homogenizing step, once the standards have been prepared, they can be used indefinitely because of the non-destructive EDXRF feature. Figure 3 a and b show the calibration curve for Se Kα characteristic X-ray intensity (counts) versus concentration (mg kg−1) for the benchtop and handheld XRF units. The dashed lines indicate 95% confidence interval, and the error bar represents one standard deviation. Comparing the two techniques, it is possible to observe that the handheld XRF sensitivity for Se was 22% higher than that of the benchtop. By way of information, considering the benchtop equipment was set for auto-tuneable X-ray tube current, a slight current difference may occur among standards and samples; then, for all benchtop quantification steps, the peak heights were normalized by the current. However, for handheld and benchtop X-ray spectra comparison purpose, the unit counts in the y-axis was used in Fig. 1 a and b.
Calibration curves for Se Kα intensity (counts) versus concentration (mg kg−1) for the benchtop (a) and handheld XRF (B) units. The y-axis errors are indicated in Fig. 3 a and b
The results of the concentration of Se (mg kg−1) in broccoli by both EDXRF systems and ICP-MS are summarized in Table 2. The concentration of Se in the BS50Se samples determined by benchtop and handheld EDXRF units was close to the corresponding values quantified by ICP-MS, taking into account the SD of measurements. It must be considered that EDXRF SD is due to only instrumental error presenting a low SD value, whereas ICP-MS SD encompasses the whole analytical process (including digestion and instrumental reading replicates for each sample). Statistically, the Se concentration in the sample BS50Se14 by both EDXRF systems did not differ significantly from the ICP-MS and presented similar recovery values. For BS50Se16, the concentration of Se determined by the benchtop and handheld methods was significantly higher than ICP-MS (p < 0.05). In addition, the recovery value for the former sample was 96%, and for the latter sample, 117 and 119% for the benchtop and handheld, respectively.
The Se contents in samples BS0Se14 and BS0Se16 were not able to be evaluated by EDXRF systems because their concentrations were lower than the LOQ of these techniques. The analytical superiority of the ICP-MS technique compared with both EDXRF systems is clear in terms of LOD, reaching ng g−1 levels. One serious EDXRF limitation for Se quantification in biological samples is its LOD at mg kg−1, taking account that Se is seemingly found in biological samples at concentrations < 1 mg kg−1 (Paltridge et al. 2012). Nevertheless, both EDXRF instruments were appropriate for Se high-content biological samples. Thus, they can be employed in the analysis of Se-rich samples (Brazil nuts, Bertholletia excels) or Se-fortified food.
As a comparison, Li and Yu (2016) used a high-energy polarized beam EDXRF unit for Se determination in biological samples, obtaining a Se LOD of 0.1 μg g−1 for 1000 s of acquisition time. Similarly, the LOD values of our benchtop and handheld approaches (considering the latter’s acquisition time, insofar as the LOD is inversely proportional to the square root of the acquisition time) present at the same order of magnitude as the high-energy polarized EDXRF system mentioned above. Additionally, Paltridge et al. (2012) reported the Se limit of quantification (LOQ) of 2 mg kg−1 for whole-grain wheat using a benchtop EDXRF for an acquisition time of 60 s. Hanley et al. (2017) found the Se LOD of 4 μg g−1 for a Se-enriched dietary supplement with a benchtop method using an acquisition time of 100 s.
Concerning the overall cost of analysis, either benchtop or handheld EDXRF is cheaper than ICP-MS equipment, requiring a less-trained operator and the running cost is much smaller, due to the purge of carrier-purified gas, which is required by ICP-MS. Paltridge et al. (2012) evaluated the costs of running Se determination by EDXRF and ICP-OES. The former technique shows the consumable cost per sample is 67 to 100 times lower than outsourced ICP-OES analysis. For the sake of information, the acquisition and operational cost of the ICP-OES are expected to be lower than ICP-MS.
Regarding ICP-MS, including the sample preparation step and instrumental analysis, we can predict an analysis time of 180 min; in both EDXRF systems, approximately 40 min per sample is required to weigh, prepare, and run the analysis in triplicate. Hanley et al. (2017) reported that the XRF, combined with direct analysis in real-time high-resolution accurate mass-mass spectrometry (DART-HRAM-MS) for Se speciation, resulted in a sevenfold throughput gain.
Comparing the benchtop and handheld EDXRF, both systems presented similar analytical performance for Se determination in broccoli seedlings. Guerra et al. (2014) evaluated P, K, Ca, S, Fe, Mn, and Si in pressed-pellet sugar cane leaf utilizing identical benchtop and handheld XRF systems. Both systems showed comparable figures of merit regarding these analytes; the latter work also discussed the advantages and drawbacks of each system. Besides Guerra et al.’s (2014) viewpoint, we highlight (a) the advantage of a handheld Si drift detector, which does not require liquid nitrogen for its cooling, as with the Si(Li) benchtop detector (however, most benchtop systems are currently equipped with Si drift); (b) the Shimadzu benchtop allows the use of a 1-mm collimator, which ultimately permits a heterogeneity study of the sample; (c) although the filter is manually changed in the handheld unit, it allows for a homemade filter, which permits selecting a proper filter composition and thickness for a particular analyte and matrix; and (d) the XRF handheld has wide-open opportunities for in situ analysis, which may even be coupled to global positioning system technology (Weindorf et al. 2012), affording real-time results, including Se-enriched plant evaluation.
Conclusions
The obtained results show that the EDXRF systems (benchtop and handheld) are a promising tool for the determination of Se in biofortified samples. The choice of a proper filter is an important step in optimizing Se determination in broccoli seedlings with these systems. The benchtop and handheld units present similar Se LOD at 0.6–0.9 mg kg−1 range for this matrix. Neither system presented a problem with spectral interference for Se evaluation and offered a fast, accurate, and low-cost alternative for Se determination in Se-enriched broccoli seedlings, which can be extended to other foods with Se at the mg kg−1 range.
References
Alfthan G, Eurola M, Ekholm P, Venäläinen ER, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A (2015) Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J Trace Elem Med Biol 31:142–147. https://doi.org/10.1016/j.jtemb.2014.04.009
Babaknejad N, Sayehmiri F, Sayehmiri K, Rahimifar P, Bahrami S, Delpesheh A, Hemati F, Alizadeh S (2014) The relationship between selenium levels and breast cancer: a systematic review and meta-analysis. Biol Trace Elem Res 159(1–3):1–7. https://doi.org/10.1007/s12011-014-9998-3
Bachiega P, Salgado JM, De Carvalho JE, Ruiz ALTG, Schwarz K, Tezotto T, Morzelle MC (2016) Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem 190:771–776. https://doi.org/10.1016/j.foodchem.2015.06.024
Bañuelos GS, Arroyo I, Pickering IJ, Yang SI, Freeman JL (2015) Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem 166:603–608. https://doi.org/10.1016/j.foodchem.2014.06.071
Barnett MC, Forster NA, Ray GA, Li L, Guppy CN, Hegarty RS (2016) Using portable x-ray fluorescence (pXRF) to determine fecal concentrations of non-absorbable digesta kinetic and digestibility markers in sheep and cattle. Anim Feed Sci Technol 212:35–41. https://doi.org/10.1016/j.anifeedsci.2015.12.015
Bouis HE, Saltzman A (2017) Improving nutrition through biofortification: a review of evidence from HarvestPlus, 2003 through 2016. Glob Food Sec 12:49–58. https://doi.org/10.1016/j.gfs.2017.01.009
Ernst T, Berman T, Buscaglia J, Eckert-Lumsdon T, Hanlon C, Olsson K, Palenik C, Ryland S, Trejos T, Valadez M, Almirall JR (2014) Signal-to-noise ratios in forensic glass analysis by micro x-ray fluorescence spectrometry. X-Ray Spectrom 43:13–21. https://doi.org/10.1002/xrs.2437
Fleming DB, Foran KA, Kim JS, Guernsey JR (2015) Portable x-ray fluorescence for assessing trace elements in rice and rice products: comparison with inductively coupled plasma-mass spectrometry. Appl Radiat Isot 104:217–223. https://doi.org/10.1016/j.apradiso.2015.07.014
Fleming DEB, Nader MN, Foran KA, Groskopf C, Reno MC, Ware CS, Tehrani M, Guimarães D, Parsons PJ (2017) Assessing arsenic and selenium in a single nail clipping using portable x-ray fluorescence. Appl Radiat Isot 120:1–6. https://doi.org/10.1016/j.apradiso.2016.11.015
Goodlaxson B, Curtzwiler G, Vorst K (2018) X-ray fluorescence analysis of antimony content in extruded polyethylene terephthalate food packaging below the infinite thickness. Food Anal Methods 11:1722–1727. https://doi.org/10.1007/s12161-018-1165-6
Guerra MBB, de Almeida E, Carvalho GGA, Souza PF, Nunes LC, Júnior DS, Krug FJ (2014) Comparison of analytical performance of benchtop and handheld energy dispersive x-ray fluorescence systems for the direct analysis of plant materials. J Anal At Spectrom 29:1667–1674. https://doi.org/10.1039/C4JA00083H
Hanley TA, Kubachka K, Taylor AM, Kern SE (2017) Selenium enriched dietary supplement rapid screening method using XRF and DART-HRAM-MS for label verification. J Anal At Spectrom 32:1196–1202. https://doi.org/10.1039/C7JA00050B
Hart DJ, Fairweather-Tait SJ, Broadley MR, Dickinson SJ, Foot I, Knott P, McGrath SP, Mowat H, Norman K, Scott PR, Stroud JL, Tucker M, White PJ, Zhao FJ, Hurst R (2011) Selenium concentration and speciation in biofortified flour and bread: retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem 126:1771–1778. https://doi.org/10.1016/j.foodchem.2010.12.079
Hawrylak-Nowak B, Dresler S, Rubinowska K, Matraszek-Gawron R, Woch W, Hasanuzzaman M (2018) Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol Biochem 127:446–456. https://doi.org/10.1016/j.plaphy.2018.04.018
Inczédy J, Lengyel T, Ure AM (2000) Compendium on analytical nomenclature. International Union of Pure and Applied Chemistry. http://media.iupac.org/publications/analytical_compendium/. Accessed 20 Nov 2018
Ivory K, Nicoletti C (2017) Selenium is a source of aliment and ailment: do we need more? Trends Food Sci Technol 62:190–193. https://doi.org/10.1016/j.tifs.2016.11.012
Jayaprakash V, Marshall JR (2011) Selenium and other antioxidants for chemoprevention of gastrointestinal cancers. Best Pract Res Clin Gastroenterol 25(4–5):507–518. https://doi.org/10.1016/j.bpg.2011.09.006
Jolly YN, Iqbal S, Rahman MS, Kabir J, Akter S, Ahmad IV (2017) Energy dispersive x-ray fluorescence detection of heavy metals in Bangladesh cows’ milk. Heliyon 3(9):e00403. https://doi.org/10.1016/j.heliyon.2017.e00403
Khoso PA, Yang Z, Liu C, Li S (2015) Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus. Biol Trace Elem Res 167(1):48–55, 2015. https://doi.org/10.1007/s12011-015-0282-y
Li X, Yu Z (2016) Determination of selenium in biological samples with an energy-dispersive x-ray fluorescence spectrometer. Appl Radiat Isot 111:45–49. https://doi.org/10.1016/j.apradiso.2016.02.010
Liu K, Chen F, Zhao Y, Gu Z, Yang H (2011) Selenium accumulation in protein fractions during germination of Se-enriched brown rice and molecular weights distribution of Se-containing proteins. Food Chem 127:1526–1531. https://doi.org/10.1016/j.foodchem.2011.02.010
Mahn A (2017) Modelling of the effect of selenium fertilization on the content of bioactive compounds in broccoli heads. Food Chem 233:492–499. https://doi.org/10.1016/j.foodchem.2017.04.144
Malagoli M, Schiavon M, Dall'acqua S, Pilon-Smits EA (2015) Effects of selenium biofortification on crop nutritional quality. Front Plant Sci 6(280). https://doi.org/10.3389/fpls.2015.00280
McIntosh KG, Guimarães D, Cusack MJ, Vershinin A, Chen ZW, Yang K, Parsons PJ (2016) Evaluation of portable XRF instrumentation for assessing potential environmental exposure to toxic elements. Int J Environ Anal Chem 96:15–37. https://doi.org/10.1080/03067319.2015.1114104
McKenzie MJ, Chen RKY, Leung S, Joshi S, Rippon PE, Joyce NI, McManus MT (2017) Selenium treatment differentially affects sulfur metabolism in high and low glucosinolate producing cultivars of broccoli (Brassica oleracea L.). Plant Physiol Biochem 121:176–186. https://doi.org/10.1016/j.plaphy.2017.10.027
Mehdi Y, Hornick JL, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18(3):3292–3311. https://doi.org/10.3390/molecules18033292
Noda T, Tsuda S, Mori M, Takigawa T, Matsuura-Endo C, Kim SJ, Hashimoto N, Yamauchi H (2006) Determination of the phosphorus content in potato starch using an energy-dispersive x-ray fluorescence method. Food Chem 95(4):632–637. https://doi.org/10.1016/j.foodchem.2005.02.002
Nunes RGFL, da Luz JMR, Freitas RB, Higuchi A, Kasuya MC, Vanetti MCD (2012) Selenium bioaccumulation in shiitake mushrooms: a nutritional alternative source of this element. J Food Sci 77(9):C983–C986. https://doi.org/10.1111/j.1750-3841.2012.02837.x
Otten JJ, Hellwig IP, Meyers DM (2006) Dietary reference intakes: the essential guide to nutrient requirements. The National Academies Press, Washington
Paltridge NG, Milham PJ, Ortiz-Monasterio JI, Velu G, Yasmin Z, Palmer LJ, Guild GE, Stangoulis JCR (2012) Energy-dispersive x-ray fluorescence spectrometry as a tool for zinc, iron and selenium analysis in whole grain wheat. Plant Soil 361:261–269. https://doi.org/10.1007/s11104-012-1423-0
Perring L, Monard F (2010) Improvement of energy dispersive x-ray fluorescence throughput: influence of measuring times and number of replicates on validation performance characteristics. Food Anal Methods 3:104–115. https://doi.org/10.1007/s12161-009-9101-4
Rahman MM, Erskine W, Zaman MS, Thavarajah P, Thavarajah D, Siddique KHM (2013) Selenium biofortification in lentil (Lens culinaris Medikus subsp. culinaris): farmers’ field survey and genotype × environment effect. Food Res Int 54:1596–1604. https://doi.org/10.1016/j.foodres.2013.09.008
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241
Reis HPG, Barcelos JPQ, Junior EF, Santos EF, Silva VM, Moraes MF, Putti FF, dos Reis AR (2018) Agronomic biofortification of upland rice with selenium and nitrogen and its relation to grain quality. J Cereal Sci 79:508–515. https://doi.org/10.1016/j.jcs.2018.01.004
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54. https://doi.org/10.1039/C3MT00185G
Rouillon M, Taylor MP (2016) Can field portable x-ray fluorescence (pXRF) produce high quality data for application in environmental contamination research? Environ Pollut 214:255–264. https://doi.org/10.1016/j.envpol.2016.03.055
Shehab H, Desouza ED, O’Meara J, Pejović-Milić A, Chettle DR, Fleming DEB, McNeill FE (2016) Feasibility of measuring arsenic and selenium in human skin using in vivo x-ray fluorescence (XRF)—a comparison of methods. Physiol Meas 37(1):145–161. https://doi.org/10.1088/0967-3334/37/1/145
Smoleń S, Kowalska I, Sady W (2014) Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci Hortic 166:9–16. https://doi.org/10.1016/j.scienta.2013.11.011
Tian M, Yang Y, Avila FW, Fish T, Yuan H, Hui M, Pan S, Thanhauser T, Li L (2018) Effects of selenium supplementation on glucosinolate biosynthesis in broccoli. J Agric Food Chem 66:8036–8044. https://doi.org/10.1021/acs.jafc.8b03396
Weindorf DC, Zhu Y, Chakraborty S, Bakr N, Huang B (2012) Use of portable x-ray fluorescence spectrometry for environmental quality assessment of peri-urban agriculture. Environ Monit Assess 184(1):217–227. https://doi.org/10.1007/s10661-011-1961-6
Wichman J, Winther KH, Bonnema SJ, Hegedüs L (2016) Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta-analysis. Thyroid 26(12):1681–1692. https://doi.org/10.1089/thy.2016.0256
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES, Brasília Brazil, finance Code 001, and 88887.115406/2015), Financiadora de Estudos e Projetos (FINEP, Brasília, Brazil, grant number 264801), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, Brazil, 401170/2016-0), Petrobrás – Petróleos Brasileiros (Rio de Janeiro, Brazil, 5241), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo, Brazil, grant number 2015/05942-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Patricia Bachiega declares that she has no conflict of interest. Eduardo de Almeida declares that he has no conflict of interest. Jocelem Mastrodi Salgado declares that she has no conflict of interest. Marco Aurelio Zezzi Arruda declares that he has no conflict of interest. Eraldo Luiz Lehmann declares that he has no conflict of interest. Maressa Caldeira Morzelle declares that she has no conflict of interest. Hudson Wallace Pereira de Carvalho declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bachiega, P., de Almeida, E., Salgado, J.M. et al. Benchtop and Handheld Energy-Dispersive X-Ray Fluorescence (EDXRF) as Alternative for Selenium Concentration Measurement in Biofortified Broccoli Seedling. Food Anal. Methods 12, 1520–1527 (2019). https://doi.org/10.1007/s12161-019-01489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01489-5