Abstract
Grape juice is rich in bioactive compounds that are known for beneficial effects, such as prevention of cancer and diabetes. The determination of the content of these compounds has been carried out following several protocols which generated toxic waste. In this context, infrared spectroscopy in near (NIR) and mid- (MIR) regions were applied for the determination of total phenolic and anthocyanin content (TPC and TAC, respectively) in grape juice for being a rapid green methodology. Calibration models were built using partial least squares regression (PLSR) to predict TPC and TAC in grape juice. MIR and NIR had a similar satisfactory performance to predict TAC presenting low RMSEP (4.22 mg/100 mL and 4.44 mg/100 mL). In TPC prediction, MIR presented a RMSEP (0.21 mg GAE/mL) slightly better in comparison to the one presented by NIR (0.37 mg GAE/mL). Errors can be considered acceptable for the determination of TPC and TAC in grape juice, qualifying both infrared techniques to replace traditional wet methodologies, with the advantage of being environmentally friendly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to its resistance to many fungal diseases and a high productivity, the main cultivar applied in grape juice processing in Brazil is cv. Isabel (Vitis vinifera × Vitis labrusca). Nevertheless, Isabel grapes present light color, requiring blending with other varieties of grape, as the hybrids BRS Cora and BRS Violet (Lima et al. 2015; Yamamoto et al. 2015). Grapes, as well as the juices produced from them, are known for their significant concentration of bioactive compounds, such as phenolic acids (e.g., gallic acid), flavonoids (e.g., proanthocyanidins), and anthocyanins (Ribeiro et al. 2015; Eshghi, Salehi and Karami 2014; Lima et al. 2015).
Grape juice and derivatives are very important commodities in the international juice trade. In the USA, wine, grape, and derivatives generate $162 billion dollars a year; in Brazil, the consumption of grape juice has grown by 400% in 5 years (from 10 million liters in 2007 to 50 million liters in 2012). Grape juice is used not only as beverage but also as ingredient for other foodstuffs. Furthermore, grape juice meets the growing consumer demand for a product without preservatives but with color, flavor, high nutritional qualities, high concentration of minerals, vitamins, and phenolic compounds (Hui 2008; Dani et al. 2016; Santos 2014).
Phenolic compound content in grape juice is affected by culture and post-harvest conditions, or according to the grape species used in juice production. The mean compounds found in grapes, the anthocyanins, are related to a reduction in oxidative stress; they can help in the prevention of diseases as cancer, arteriosclerosis, diabetes, and neurological diseases. In addition, anthocyanins have antihyperglycemic and anti-inflammatory effects; besides that, they are extremely important in sensory attributes as color (Dal Magro et al. 2016; Eshghi, Salehi and Karami 2014; Xu et al. 2010; Moreno-Montoro et al. 2015; Lambri et al. 2015).
The determination of bioactive compounds as phenolic compounds and anthocyanins has been performed by many different methodologies. Spectrophotometric methods are widely applied in total phenolic compound quantification; an estimation by measurement of absorption at 280 nm and the Folin-Ciocalteu method are the most used (Lorrain et al. 2013). Liquid chromatography, coupled or not with mass spectrometry, was also used to quantify these compounds in many researches (Díaz-de-Cerio, Gómez-Caravaca, Verardo, Fernández-Gutiérrez and Segura-Carretero 2016; Moreno-Montoro et al. 2015; Dal Magro et al. 2016). The main problem, when such methodologies are used in the determination, is the extraction of compounds, which is mainly performed by liquid-liquid or solid-liquid extraction (Lorrain et al. 2013), resulting in the generation of wastes which are toxic for both environment and operator. Moreover, the fact that phenolic compounds are photosensitive leads to the need of using amber glassware and/or manipulation under reduced brightness to maintain the compound stability during chemical analysis.
In recent years, a number of new analytical approaches are being used to monitor food quality, such as vibrational spectroscopy, including infrared spectroscopy (near and mid), Raman spectroscopy, and hyperspectral/multispectral imaging. These techniques present some advantages such as the following: non-invasive and non-destructive, require a small amount of sample, do not require extraction, do not use toxic reagents, and can be used online (Lohumi et al. 2015; Ropodi, Panagou and Nychas 2016). Near infrared (NIR) spectra (10,000–4000 cm−1) provide information from overtones and their combinations, while mid-infrared (MIR) spectra (4000–400 cm−1) give information from fundamental molecular vibrational frequencies. Therefore, the spectra obtained by MIR are straightforward (de Oliveira et al. 2014; Shiroma and Rodriguez-Saona 2009). Despite the differences between the analysis of NIR and MIR spectra, both require the application of chemometrics to develop models and to obtain other data (Hell et al. 2016).
Several works have been developed applying NIR and MIR spectroscopies to evaluate quality and to quantify different chemical compounds in food products, food authenticity, and adulterations. Infrared analysis has been successfully used to quantify total phenolic compounds and condensed tannins contained in grape seed, to evaluate ripeness of white grape, and to a qualitative and quantitative evaluation of grape berries at various stages of development (de Oliveira et al. 2014; Bag, Srivastav and Mishra 2011; Shiroma and Rodriguez-Saona 2009; Hell et al. 2016; Martelo-Vidal and Vázquez 2014; Mendes et al. 2015; Li et al. 2013; Ignat et al. 2012; Wang and Xie 2014; Kyraleou et al. 2015; Giovenzana et al. 2015; Musingarabwi et al. 2016).
This article reports a study that aimed at applying the techniques NIR and MIR for the determination of total phenolic compounds and anthocyanin contents in grape juices consumed in Brazil as alternatives for routine analysis, thus reducing the risk of accidents, the use of toxic reagents, work in sample preparation, and time spent on analysis.
Materials and Methods
Materials
A total of 65 commercial samples of grape juice were acquired in a local market and analyzed during this study. Sixty-two samples were products of different brands and lots, and three of them were obtained by lyophilization (Terroni Equipamentos Científicos, Brazil, Série LS). The commercial sample of grape juice with the highest phenolic content was concentrated by lyophilization during 30, 60, and 90 min. This process was carried out in order to ensure greater variability of the results to construct a calibration model with large values.
Total Anthocyanin Content Analysis
The total content of anthocyanins in 65 samples of grape juice was analyzed. The extraction was carried out mixing the grape juice with a solution of HCl 0.1 mol/L at room temperature (25 °C) for 30 min in a dark room. The samples were centrifuged at 3600 rpm/10 min to remove the solid particles, and the supernatant was collected according to the reference method (AOAC method 2005-02) with modifications (AOAC 2006; Giusti and Wrolstad 2001). The absorbance was recorded with a spectrophotometer (Hitachi High-Technologies, Japan, U-2900) in wavelengths of 520 and 700 nm, for both solutions, pH 1.0 and pH 4.5.
Total Phenolic Compound Content
The total phenolic content (TPC) was determined in the 65 samples of grape juice according to Singleton and Rossi (1965). The extract of the samples was obtained according to Paz et al. (2015). A volume of 500 μL of sample extract, 75 μL of water, 250 μL of Folin-Ciocalteu, 1 mol/L reagent, and 1250 μL of sodium carbonate was placed in the cuvette for 30 min in a dark room. The absorbance was determined at 700 nm in a spectrophotometer (Hitachi High-Technologies, Japan, U-2900). To calculate the phenolic concentration in samples, a calibration curve was done using gallic acid (GA) as standard antioxidant; the results were expressed in gallic acid equivalents (GAEs).
Spectra Acquisition
About 2 g of each sample was placed in a petri dish, and the liquid reflector (PerkinElmer, Waltham, USA, part number L118-0503) was placed on top of the sample and carefully pushed against the glass before scanning. Transflectance measurements were conducted using near infrared reflectance accessory (PerkinElmer, Waltham, USA, model NIRA) in spectrometer FT-NIR (PerkinElmer, Waltham, USA, model Spectrum 100N) in the range of 10,000–4000 cm−1, with 4 cm−1 of resolution. All data were obtained in triplicate, and the mean was used in subsequent calculations.
All spectrum data of Fourier transformation mid-infrared (MIR) were determined using the attenuated total reflectance (ATR-FTIR) technique (Cary 630 FTIR Spectrometer, Agilent Technologies, USA). They were collected with 64 scans, 4 cm−1 of resolution, in a wavenumber range of 4000 to 400 cm−1.
Chemometrics
Spectrum data were processed using the PLS toolbox for MatLab version 5.2 (The MathWorks Inc. 2009; Eigenvector Research Inc. 2010). For noise reduction and scattering correction, preprocessing was applied to it. The method of data smoothing based on the Savitzky-Golay algorithm, mean center, and standard normal variate (SNV) was used in this work. Cross-validation was used for optimization of the number of latent variables (LVs) in each model, and the number of LVs chosen was four and seven, for TAC and TPC, respectively.
Outliers were identified and excluded based on Student residual values and leverage in order to improve the quality of the calibration model.
The samples were divided into calibration and validation sets to perform external validation, where 75% of samples were used in the calibration set and 25% represent the validation set.
The partial least squares regression (PLSR) was the methodology chosen for building the calibration model. To evaluate the quality of the obtained models, the coefficient of determination (R 2) and the relative mean square errors of calibration (RMSEC) and prediction (RMSEP) were calculated.
Results and Discussion
Chemical Analysis
A summary of the total phenolic content (TPC) and total anthocyanin content (TAC) in grape juice samples evaluated is presented in Table 1. Range, mean, and coefficient of variation are given for each analysis. A phenolic content study of red grapes from the south of Brazil (V. labrusca L.) determined values of 2015.00 mg GAE/L for TPC and 420.01 mg/L for TAC, using conventional wet methodology. Those values were superior to the ones founded in this work, and these differences may occur since phenolic compound content is dependent on various factors as culture conditions or post-harvest conditions, or according to the grape species used in juice production as well as the methodology applied to determine TPC and TAC. Other studies with anthocyanin and phenolic contents in grape were done, most of them in grapes V. vinifera or hybrids used in wine production (Fraige et al. 2014; Lambri et al. 2015; Toaldo et al. 2015). Grape juice showed higher mean content of TPC when compared to apple juice (1.295 mg GAE/mL and 0.098 mg GAE/mL) and similar TAC content to cranberry juice (13.64 mg/100 mL). Moreover, some studies suggest that the anthocyanins of grape juice present higher antioxidant activity than some vitamins as E and C, a feature that enhances the nutritional quality of the product (Knockaert, Pulissery, Colle, Van Buggenhout, Hendrickx and Loey 2012; Sharma et al. 2015; Fraige et al. 2014; Islam et al. 2016; Bazinet, Brianceau, Dubé and Desjardins 2012).
Overview and Pretreatment of Spectrum Data
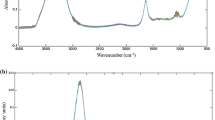
The raw spectrum data obtained in MIR and NIR are represented in Fig. 1a, b, respectively. In MIR, larger peaks at 3200 and at 1600 cm−1 associated with O-H stretch are observed, appearing as a result of the amount of water present in the sample. The fingerprint region about 1200–900 cm−1 was determinant in the posterior PLS calibration model. This region is usually associated with C-O and C-C stretching and C-O-C and C-O-H, which could be related with esters, carbohydrates, and organic acids (Shiroma and Rodriguez-Saona 2009; Musingarabwi et al. 2016).
Analyzing the NIR spectrum data, two maximum peaks at ≅ 6900 and ≅5200 cm−1 are observed as notorious. These peaks refer to O-H first overtone and in combination, and their higher position in the spectrum is related to the huge amount of water present in grape juice (Shiroma and Rodriguez-Saona 2009; Xie et al. 2009).
The preprocessing performed in all NIR and MIR spectrum data is detailed in Table 2. As a consequence of the light scattering presented by NIR spectrum data, it was necessary to apply the SNV algorithm for baseline correction. In order to remove the noise presented in the MIR spectrum data, the Savitzky-Golay method of data smoothing was used. The spectral region used in MIR calibration is near the fingerprint region, and qualitative and quantitative evaluation of grape berries was previously used. The rest of the spectral data were hindered because of the huge water amount (Musingarabwi et al. 2016).
MIR × NIR
The statistical data for MIR and NIR calibration models are shown in Table 3 and Fig. 2. TAC provided a satisfactory coefficient of determination (R 2 c) for NIR (0.84) and MIR (0.81). Observing the values of RMSEP, RMSEC, MIR, and NIR, they were quite similar, with RMSEP = 4.22 mg/100 mL and RMSEC = 4.31 mg/100 mL for MIR and RMSEP = 4.44 mg/100 mL and RMSEC = 4.09 mg/100 mL for NIR. However, in the model, seven latent variables (LV) for NIR prediction and only five LV for the MIR model were necessary.
Chen et al. (2015) performed the prediction of TAC in grapes using NIR hyperspectral imaging and presented a more relevant RMSEP (1.29 mg/100 g) using 16 LVs, which is a number twice as large as the one used in this paper to build the NIR model. However, the use of too many LVs may result in an overfitting model. On that account, the use of less LVs is more suitable. The value of RMSEP found in this article was better when compared to Xiaowei et al. (2014) who developed a prediction model of TAC in flowering tea using NIR combined with ant colony optimization models with RMSEP of 12.25 mg/100 g; nevertheless, they used only three LVs.
Martelo-Vidal and Vázquez (2014) determined values of RMSEC between 54.88 and 35.94 mg/L in calibration models constructed to quantify the anthocyanins including malvidin in red wines; as reference method, the authors used HPLC that has lower errors compared to spectrophotometric analysis, therefore contributing to a final calibration model with a RMSEC lower when compared to those achieved in this paper.
TPC was determined with adequate accuracy. Comparing NIR and MIR models, a slightly better RMSEC was obtained by the NIR model (0.171 mg GAE/mL) and a better RMSEP in the MIR model (0.21 mg GAE/mL). Both techniques presented favorable coefficients of determination (R c 2 ≥ 0.90) and comparable number of LVs. Therefore, both techniques can be defined as efficient in determining TPC.
Hu et al. (2016) performed the determination of phenolic content in chocolate by ATR-FTIR and presented satisfactory RMSEP (5.08 mg GAE/g DFW) and RMSEC (3.21 mg GAE/g DFW). Kyraleou et al. (2015) used FT-IR to determine total phenolics contained in grape seeds and reported values of 0.97 for R 2, RMSEC of 4.03 mg catechin/g DW, and RMSEP of 6.49 mg catechin/g DW; all errors presented by the authors were superior than those found in this work.
The content of total phenolic was determined by Frizon et al. (2015), using PLS regression and NIR in yerba mate (Ilex paraguariensis) with RMSEC of 16.07 mg 5CQA/g and RMSEP of 12.12 mg 5CQA/g; the errors were superior to the ones found in the NIR calibration model in this work. TPC was also determined by Páscoa et al. (2014) in milled red grape pomace using NIR and a PLS calibration model, presenting a RMSEC of 1.77 mg GAE/g sample and RMSEP of 2.12 mg GAE/g sample, which were superior than those achieved with grape juice.
Viegas et al. (2015) obtained a RMSEP = 0.022 mg/mL analyzing TPC in wax jambu fruit using NIRS and PLS; this result presented a lower predicting error when compared to the ones determined in this paper. However, to achieve this RMSEP, the authors used nine LVs, while the model constructed to analyze grape juice used only four LVs.
Considering that the models used to predict TPC and TAC in grape juice presented good performance, with low values of RMSEP and RMSEC, other parameters commonly used to evaluate the quality of this product, as soluble solids, total acidity, total sugars, and insoluble solids, could also be calibrated by NIR or MIR.
Conclusion
Both techniques, NIR and MIR, presented quite similar results in the prediction of total phenolic and anthocyanin contents in grape juice. However, the MIR spectra are more accessible to interpret than the NIR spectra, making it possible to obtain a chemical knowledge of the system. The MIR spectra needed less pretreatments than the NIR spectra to achieve the best performance possible. It is important to consider that both spectroscopy techniques presented adequate performance, revealing a possible replacement of wet chemical analyses with the advantage of producing no toxic waste, little time consumption, no danger to the operator, and without any trouble in maintaining the stability of the phenolic compounds during the analytical process.
References
AOAC (2006) Association of Official analytical chemists: official methods of analysis
Bazinet L, Brianceau S, Dubé P, Desjardins Y (2012) Evolution of cranberry juice physico-chemical parameters during phenolic antioxidant enrichment by electrodialysis with filtration membrane. Sep Purif Technol 87:31–39 http://doi.org/10.1016/j.seppur.2011.11.017
Bag SK, Srivastav PP, Mishra HN (2011) FT-NIR spectroscopy: a rapid method for estimation of moisture content in bael pulp. Br Food J 113(4):494–504 http://doi.org/10.1108/00070701111123970
Chen S, Zhang F, Ning J, Liu X, Zhang Z, Yang S (2015) Predicting the anthocyanin content of wine grapes by NIR hyperspectral imaging. Food Chem 172:788–793 http://doi.org/10.1016/j.foodchem.2014.09.119
Dal Magro L, Goetze D, Ribeiro CT, Paludo N, Rodrigues E, Hertz PF, Rodrigues RC (2016) Identification of bioactive compounds from Vitis labrusca L. variety concord grape juice treated with commercial enzymes: improved yield and quality parameters. Food Bioprocess Technol 9(2):365–377 http://doi.org/10.1007/s11947-015-1634-5
Dani C, Oliboni LS, Henriques JAP, Salvador M. (2016) Suco de uva: componentes e benefícios para a saúde. http://www.sucodeuvadobrasil.com.br/publicacoes/Suco_de_Uva__componentes_e_benefcios_para_a_sade.pdf. Acessed 28 March 2016
Eigenvector Research Inc. (2010) PLS Toolbox 5.8. Manson, USA
de Oliveira GA, de Castilhos F, Renard CM-GC, Bureau S (2014) Comparison of NIR and MIR spectroscopic methods for determination of individual sugars, organic acids and carotenoids in passion fruit. Food Res Int 60:154–162 http://doi.org/10.1016/j.foodres.2013.10.051
Díaz-de-Cerio E, Gómez-Caravaca AM, Verardo V, Fernández-Gutiérrez A, Segura-Carretero A (2016) Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J Funct Foods 22:376–388 http://doi.org/10.1016/j.jff.2016.01.040
Eshghi S, Salehi L, Karami MJ (2014) Antioxidant activity, total phenolic compounds and anthocyanin contents in 35 different grapevine (Vitis vinifera L.) cultivars grown in Fars Province. International Journal of Horticultural Science and Technology 1(2):151–161
Fraige K, Pereira-Filho ER, Carrilho E (2014) Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chem 145:395–403 http://doi.org/10.1016/j.foodchem.2013.08.066
Frizon CNT, Oliveira GA, Perussello CA, Peralta-Zamora PG, Camlofski AMO, Rossa ÜB, Hoffmann-Ribani R (2015) Determination of total phenolic compounds in yerba mate (Ilex paraguariensis) combining near infrared spectroscopy (NIR) and multivariate analysis. LWT Food Sci Technol 60(2, Part 1):795–801 http://doi.org/10.1016/j.lwt.2014.10.030
Giovenzana V, Civelli R, Beghi R et al (2015) Testing of a simplified LED based vis/NIR system for rapid ripeness evaluation of white grape (Vitis vinifera L.) for Franciacorta wine. Talanta 144:584–591. doi:10.1016/j.talanta.2015.06.055
Giusti MM, Wrolstad RE (2001) In: Wrolstad RE (ed) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Current protocols in food analytical chemistry. Wiley, New York
Hell J, Prückler M, Danner L, Henniges U, Apprich S, Rosenau T, Böhmdorfer S (2016) A comparison between near-infrared (NIR) and mid-infrared (ATR-FTIR) spectroscopy for the multivariate determination of compositional properties in wheat bran samples. Food Control 60:365–369 http://doi.org/10.1016/j.foodcont.2015.08.003
Hu Y, Pan ZJ, Liao W, Li J, Gruget P, Kitts DD, Lu X (2016) Determination of antioxidant capacity and phenolic content of chocolate by attenuated total reflectance-Fourier transformed-infrared spectroscopy. Food Chem 202:254–261 http://doi.org/10.1016/j.foodchem.2016.01.130
Hui YH (2008) Handbook of fruits and fruit processing. Wiley
Ignat T, Schmilovitch Z, Fefoldi J, Steiner B, Alkalai-Tuvia S (2012) Non-destructive measurement of ascorbic acid content in bell peppers by VIS-NIR and SWIR spectrometry. Postharvest Biol Technol 74:91–99 http://doi.org/10.1016/j.postharvbio.2012.06.010
Islam MS, Patras A, Pokharel B, Wu Y, Vergne MJ, Shade L, Sasges M (2016) UV-C irradiation as an alternative disinfection technique: study of its effect on polyphenols and antioxidant activity of apple juice. Innovative Food Sci Emerg Technol 34:344–351 http://doi.org/10.1016/j.ifset.2016.02.009
Knockaert G, Pulissery SK, Colle I, Van Buggenhout S, Hendrickx M, Loey AV (2012) Lycopene degradation, isomerization and in vitro bioaccessibility in high pressure homogenized tomato puree containing oil: effect of additional thermal and high pressure processing. Food Chem 135(3):1290–1297 http://doi.org/10.1016/j.foodchem.2012.05.065
Kyraleou M, Pappas C, Voskidi E, Kotseridis Y, Basalekou M, Tarantilis PA, Kallithraka S (2015) Diffuse reflectance Fourier transform infrared spectroscopy for simultaneous quantification of total phenolics and condensed tannins contained in grape seeds. Ind Crop Prod 74:784–791 http://doi.org/10.1016/j.indcrop.2015.06.016
Lambri M, Torchio F, Colangelo D, Río Segade S, Giacosa S, De Faveri DM, Rolle L (2015) Influence of different berry thermal treatment conditions, grape anthocyanin profile, and skin hardness on the extraction of anthocyanin compounds in the colored grape juice production. Food Research International, 77. Part 3:584–590 http://doi.org/10.1016/j.foodres.2015.08.027
Li J, Huang W, Zhao C, Zhang B (2013) A comparative study for the quantitative determination of soluble solids content, pH and firmness of pears by Vis/NIR spectroscopy. J Food Eng 116(2):324–332 http://doi.org/10.1016/j.jfoodeng.2012.11.007
Lima M d S, da Conceição Prudêncio Dutra M, Toaldo IM, Corrêa LC, Pereira GE, de Oliveira D, Ninow JL (2015) Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem 188:384–392 http://doi.org/10.1016/j.foodchem.2015.04.014
Lohumi S, Lee S, Lee H, Cho B-K (2015) A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci Technol 46(1):85–98 http://doi.org/10.1016/j.tifs.2015.08.003
Lorrain B, Ky I, Pechamat L, Teissedre P-L (2013) Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules (Basel, Switzerland) 18(1):1076–1100 http://doi.org/10.3390/molecules18011076
Martelo-Vidal MJ, Vázquez M (2014) Determination of polyphenolic compounds of red wines by UV–VIS–NIR spectroscopy and chemometrics tools. Food Chem 158:28–34 http://doi.org/10.1016/j.foodchem.2014.02.080
Mendes TO, da Rocha RA, Porto BLS, de Oliveira MAL, de C. dos Anjos V, Bell MJV (2015) Quantification of extra-virgin olive oil adulteration with soybean oil: a comparative study of NIR, MIR, and Raman spectroscopy associated with chemometric approaches. Food Anal Methods 8(9):2339–2346 http://doi.org/10.1007/s12161-015-0121-y
Moreno-Montoro M, Olalla-Herrera M, Gimenez-Martinez R, Navarro-Alarcon M, Rufián-Henares JA (2015) Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J Food Compos Anal 38:19–26 http://doi.org/10.1016/j.jfca.2014.10.001
Musingarabwi DM, Nieuwoudt HH, Young PR, Eyéghè-Bickong HA, Vivier MA (2016) A rapid qualitative and quantitative evaluation of grape berries at various stages of development using Fourier-transform infrared spectroscopy and multivariate data analysis. Food Chem 190:253–262 http://doi.org/10.1016/j.foodchem.2015.05.080
Páscoa RNMJ, Machado S, Magalhães LM, Lopes JA (2014) Value adding to red grape pomace exploiting eco-friendly FT-NIR spectroscopy technique. Food Bioprocess Technol 8(4):865–874 http://doi.org/10.1007/s11947-014-1454-z
Paz M, Gúllon P, Barroso MF, Carvalho AP, Domingues VF, Gomes AM, Delerue-Matos C (2015) Brazilian fruit pulps as functional foods and additives: evaluation of bioactive compounds. Food Chem 172:462–468 http://doi.org/10.1016/j.foodchem.2014.09.102
Ribeiro LF, Ribani RH, Francisco TMG, Soares AA, Pontarolo R, Haminiuk CWI (2015) Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J Chromatogr B 1007:72–80 http://doi.org/10.1016/j.jchromb.2015.11.005
Ropodi AI, Panagou EZ, Nychas G-JE (2016) Data mining derived from food analyses using non-invasive/non-destructive analytical techniques; determination of food authenticity, quality & safety in tandem with computer science disciplines. Trends Food Sci Technol 50:11–25 http://doi.org/10.1016/j.tifs.2016.01.011
Santos N (2014) Consumo do suco de uva integral aumentou 400% | A Vindima. Retrieved from http://www.avindima.com.br/?p=3266. (Accessed 28 Mar 2016)
Sharma K, Ko EY, Assefa AD, Ha S, Nile SH, Lee ET, Park SW (2015) Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J Food Drug Anal 23(2):243–252 http://doi.org/10.1016/j.jfda.2014.10.005
Shiroma C, Rodriguez-Saona L (2009) Application of NIR and MIR spectroscopy in quality control of potato chips. J Food Compos Anal 22(6):596–605 http://doi.org/10.1016/j.jfca.2008.09.003
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic—phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
The Math Works Inc. (2009) Matlab 7.8. Natick, USA
Toaldo IM, Cruz FA, Alves T d L, de Gois JS, Borges DLG, Cunha HP, Bordignon-Luiz MT (2015) Bioactive potential of Vitis labrusca L. grape juices from the southern region of Brazil: phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem 173:527–535 http://doi.org/10.1016/j.foodchem.2014.09.171
Viegas TR, Mata ALML, Duarte MML, Lima KMG (2016) Determination of quality attributes in wax jambu fruit using NIRS and PLS. Food Chem 190:1–4
Wang A, Xie L (2014) Technology using near infrared spectroscopic and multivariate analysis to determine the soluble solids content of citrus fruit. J Food Eng 143:17–24 http://doi.org/10.1016/j.jfoodeng.2014.06.023
Xiaowei H, Xiaobo Z, Jiewen Z, Jiyong S, Xiaolei Z, Holmes M (2014) Measurement of total anthocyanins content in flowering tea using near infrared spectroscopy combined with ant colony optimization models. Food Chem 164:536–543 http://doi.org/10.1016/j.foodchem.2014.05.072
Xie L, Ye X, Liu D, Ying Y (2009) Quantification of glucose, fructose and sucrose in bayberry juice by NIR and PLS. Food Chem 114(3):1135–1140 http://doi.org/10.1016/j.foodchem.2008.10.076
Xu C, Zhang Y, Cao L, Lu J (2010) Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem 119(4):1557–1565 http://doi.org/10.1016/j.foodchem.2009.09.042
Yamamoto LY, de Assis AM, Roberto SR, Bovolenta YR, Nixdorf SL, García-Romero E, Hermosín-Gutiérrez I (2015) Application of abscisic acid (S-ABA) to cv. Isabel grapes (Vitis vinifera × Vitis labrusca) for color improvement: effects on color, phenolic composition and antioxidant capacity of their grape juice. Food research international, 77. Part 3:572–583 http://doi.org/10.1016/j.foodres.2015.10.019
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process 2015/15848-0) and the Brazilian National Counsel of Technological and Scientific Development (CNPq) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 2015/15848-0.
Conflict of Interest
Elem T. S Caramês declares that she has no conflict of interest. Priscila D. Alamar declares that she has no conflict of interest. Ronei J. Poppi declares that he has no conflict of interest. Juliana A. Lima Pallone declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Caramês, E.T.S., Alamar, P.D., Poppi, R.J. et al. Rapid Assessment of Total Phenolic and Anthocyanin Contents in Grape Juice Using Infrared Spectroscopy and Multivariate Calibration. Food Anal. Methods 10, 1609–1615 (2017). https://doi.org/10.1007/s12161-016-0721-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0721-1