Abstract
In this article, an accurate mass multiresidue screening method has been developed for the determination of over 630 multiclass food contaminants in different matrices using ultra-high performance liquid chromatography/(quadrupole)-time-of-flight mass spectrometry. The compounds included in the study were 426 pesticides, 117 veterinary drugs, 42 food-packaging contaminants, 21 mycotoxins, 10 perfluorinated compounds, 9 nitrosamines, and 5 sweeteners. The separation was carried out by liquid chromatography using a C18 column (50 mm × 2.1 mm, 1.8 μm particle size). The identification of the targeted species was accomplished using accurate masses of the targeted ions (protonated or deprotonated molecule) along with retention time data and characteristic fragment ion for reliable identification, using specific software for automated data mining and exploitation. The performance of the screening method was validated in terms of linearity, matrix effect, and limits of quantification for three representative food matrices (tomato, orange, and baby food) using a generic sample treatment based on liquid partitioning with acetonitrile (QuEChERS). The overall method performance was satisfactory with limits of quantification lower than 10 μg kg −1 for the 44 % of studied compounds. In some cases (ca. 10–15 % of the pesticides depending on the matrix tested, maximum residue levels were not fulfilled). In orange, 15 % of the compounds displayed LOQs above the maximum residue levels (MRLs) set for the studied pesticides, which can be partially attributed to matrix effects. Moderate signal suppression was observed in the three matrices tested in most cases, being orange the matrix which produced the highest matrix effect and baby food the lowest one.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food quality and safety have become of increasing concern for consumers, governments, and producers in such globalized market, where commodities are produced and distributed throughout the world (Malik et al. 2010; Di Stefano et al. 2012). Food chemical contaminants have been defined as “any chemical not intentionally added to food but present from many potential sources,” including residues from the application of pesticides and veterinary medicines, those entering the food chain from the environment, and those formed during the processing of food, natural toxins, accidental contamination, or adulteration (Hird et al. 2014).
To protect the health of consumers, stringent regulations enforced with diligent monitoring of foods have been recently established. The need of methods covering multiclass contaminants such as pesticides, veterinary drugs, and mycotoxins is illustrated by selected recent examples in the literature (Zhan et al. 2012; Mol et al. 2008; Garrido Frenich et al. 2014; Ferrer-Amate et al. 2010; Pérez-Ortega et al. 2012; Gómez-Pérez et al. 2015). For instance, derivate food products such as baby food combine different matrices: cereal-based food, meat-based food, powdered milk-based infant formulae, and fruit- and vegetable-based food (European commission 2006a). Consequently, they should be tested keeping in mind the potential simultaneous presence of both pesticides and veterinary drugs. Other contaminants such as parabens, human pharmaceuticals and antibiotics, and veterinary drugs have been recently reported in processed food (Fussell et al. 2014) due to contamination either during farming/crop production—as the use of reclaimed water is becoming more common (Matamoros et al. 2012)—or in the food-producing scenarios. Furthermore, contaminants can also enter the food chain through adulteration of food (international contamination, e.g., melamine in milk formulae) (Langman 2009). To cope with these outstanding numbers of contaminants/commodity combinations, laboratories must use multiresidue strategies.
The monitoring of residues from either pesticides or other contaminants in products of both plant and animal origin is of great interest for the protection of human health. It is currently addressed by means of a plethora of regulations worldwide (EPA 2016; European Commission 2005; US Department of Agriculture 2014; European Commission 2010; US Food Drug Administration 2011; European Commission 2006b; Canadian Food Inspection Agency 2016; European Commission 2016; Health Canada 2014; National Standard GB-2763 2014; Codex Alimentarius 2016). Laboratories monitoring these chemicals must have cost-effective, rapid, and comprehensive methods for detecting their presence. Current food safety methods are aimed at the simultaneous determination of several families of contaminants and/or residues. These methods increase sample throughput and the capabilities of routine laboratories (Malik et al. 2010; Di Stefano et al. 2012; Hird et al. 2014; Picó et al. 2015).
The standard method for determining pesticides, veterinary drugs, and other relevant contaminants, namely multiresidue method, is a targeted approach based on multiple reaction monitoring (MRM) acquisition, using liquid chromatography/tandem mass spectrometry (LC-MS/MS) or/and gas chromatography/tandem mass spectrometry (GC-MS/MS) (Gilbert-López et al. 2010). However, the main flaw of the approach is the previous knowledge required to set up the acquisition method (retention time and optimized MS/MS transitions for each analyte sought). Consequently, LC-MS/MS multiresidue methods are blind to compounds not defined in the MRM method, so that none or scarce information on possible non-target or unknown pesticides or their degradation products are available when using these techniques. Multiresidue methods also require dedicated validation and quality control (QC) (due to the large number of species). In quantitative multiresidue methods, valuable time and effort are wasted in generating ongoing QC data for many compounds that are not frequently detected. Therefore, screening methods skipping such reference materials and all ongoing QC measurements associated are desirable.
Liquid chromatography combined with high-resolution mass spectrometry (LC-HRMS) has shown to be an effective approach to screen food samples for the presence of high number of analytes. In contrast to low-resolution MS/MS acquisition, LC full-scan HRMS enables a fully untargeted measurement with the ability to retrospectively detect additional compounds in the raw data, which were not anticipated to be of interest at the time of sample analysis (Gómez-Ramos et al. 2013; Mezcua et al. 2009; Polgar et al. 2012; Díaz et al. 2011; Pérez-Ortega et al. 2016). Although the interrogation of the data is performed against the list of compounds included in the database or library, retrospective evaluation is always possible as data for all compounds that have given sufficient detector response is acquired (Gómez-Ramos et al. 2013; Mezcua et al. 2009; Polgar et al. 2012; Díaz et al. 2011).
The development of accurate mass LC-HRMS screening methods has been addressed by different authors, using either time-of-flight (Mezcua et al. 2009; Polgar et al. 2012; Díaz et al. 2011; García-López et al. 2014; Lacina et al. 2010; Wang et al. 2014) or Orbitrap mass spectrometers (Gómez-Pérez et al. 2015; Alder et al. 2011). These methodologies include typically between 200 and 450 pesticides, although there are also a few examples covering other contaminants such as veterinary drugs (Masía et al. 2016, Picó et al. 2015, Romero-González et al. 2011). Hybrid mass spectrometers performing MS/MS acquisition of product ions at high resolution provide additional structural information for identification purposes, enabling the discrimination among isobaric or isomeric species and the discovering of metabolites or degradation pathways. In this article, an accurate mass multiresidue screening method using ultra-high performance liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC-Q-TOFMS) has been developed and its performance evaluated for over 600 multiclass food contaminants (pesticides, veterinary drugs, mycotoxins, nitrosamines, perfluorinated compounds, sweeteners, and food-packaging contaminants) in food, using tomato, orange, and baby food as model matrices.
Experimental Section
Chemicals and Reagents
Pesticides, veterinary drugs, food-packaging contaminants, perfluorinated compounds, mycotoxins, nitrosamines, and sweeteners of analytical grade standards were purchased from Fluka (Pestanal quality) (Madrid, Spain), Sigma-Aldrich (Madrid, Spain), or Dr. Ehrenstorfer (Augsburg, Germany). Individual stock solutions (ca. 500 mg L−1 each) were prepared in different solvents depending on compound solubility and stability (acetonitrile, methanol (MeOH), and/or water in basic or acidic media) and were stored at −20 °C. Working solutions containing ca. 30 compounds each were prepared by appropriate dilution of the stock solutions with MeOH at 10 mg L−1. HPLC-grade acetonitrile and MeOH were obtained from Merck (Darmstadt, Germany). Formic acid was obtained from Fluka (Buchs, Switzerland). Primary-secondary amine (PSA) Bond Elut was obtained from Varian, Inc. (Palo Alto, CA, USA). Acetic acid was from Panreac (Barcelona, Spain). Anhydrous magnesium sulfate anhydrous (MgSO4) and sodium acetate (NaCOOCH3) were from Sigma-Aldrich (Madrid, Spain). A Milli-Q-Plus ultrapure water system from Millipore (Milford, MA, USA) was used throughout the study to obtain the HPLC-grade water used during the analyses.
Selection of the Studied Compounds
The 630 compounds included in the screening method were carefully selected considering different lists established by official bodies from the European Union and the USA, previous relevant literature, and thus their potential presence in different types of foodstuffs and water. Up to 426 pesticides, 117 veterinary drugs and pharmaceuticals, 42 food-packaging contaminants, 10 perfluorinated compounds, 21 mycotoxins, 9 nitrosamines, and 5 sweeteners were included. From the 426 pesticides included, most of them are covered in Annex 1 of Directive 396/2005 for several commodities (European Commission 2005). A significant number (over 130 species), of priority pesticides (according to Annex I of Commission Implementing Regulation 788/2012 due to their usage and frequency of detection), were also included in the targeted list (Gallart-Ayala et al. 2013). Most of the selected food-packaging contaminants and perfluorinated compounds are regulated by different documents (European Commission 2012; Gallart-Ayala et al. 2013; European Commission 2011; FDA 2016; European Commission 2006b; EPA 2009, 2015; European Commission 2015). With regards to the veterinary drugs and pharmaceuticals, most of the selected substances are US FDA-approved veterinary drugs for animal use (FDA 2016) or authorized products in the European Union. It should be noted that some of the species are included in Table 1 as pesticides, although they can be also classified as veterinary drugs such as albendazole, fenbendazole, fenthion, ivermectin, lufenuron, spinosad, sulfaquinoxaline, thiabendazole, and trichlorfon, all of them included in US FDA-approved list for animal use. Along with the veterinary drugs, other human pharmaceuticals were included due to their ubiquitous presence in the environment. Besides, all the main mycotoxins including those regulated in Commission Regulation EC 1881/2006 (European Commission 2006b) are among those 21 substances selected. The nine nitrosamines selected are included in US EPA final Drinking Water Contaminant Candidate lists (CCL-3) (EPA 2009, 2015). Finally, all the sweeteners included are SANTE-authorized food additives (European Commission 2015).
Sample Treatment
Different baby food samples from different local markets containing meat and vegetables were pooled and used as model matrix, along with tomato and orange. Extraction was accomplished using QuEChERS approach (Lehotay 2011). A representative 10-g portion of homogenized sample was weighed in a 50-mL plastic centrifuge tube and mixed with 10 mL of 0.1 % acetic acid in acetonitrile, being the tube vigorously shaken for 1 min. Then, 1 g of NaCOOCH3 and 4 g of MgSO4 anhydrous were added, and the tube was shaken again to prevent coagulation of MgSO4. The extract was centrifuged (1464 rcf) for 3 min. A 5-mL aliquot of supernatant (acetonitrile phase) was taken with a pipette and transferred to a 15-mL centrifuge tube containing 250 mg of PSA and 750 mg of MgSO4 anhydrous that was energetically shaken for 20 s. The extract was centrifuged again (1464 rcf) for 3 min. Three milliliters of supernatant were taken and evaporated to near dryness and reconstituted to 3 mL of 20 % MeOH. Prior UHPLC-MS analysis, the extract was filtered through a 0.45-μm PTFE filter and transferred into a vial. These extracts were used for method performance evaluation by appropriate spiking with the compound mixtures.
Ultra-High Performance Liquid Chromatography-Electrospray-Quadrupole-Time-of-Flight Mass Spectrometry
The separation and identification of the analytical standards were carried out using a reversed phase C18 column (50 mm × 2.1 mm and 1.8 μm particle size, Zorbax Rapid Resolution High Definition (RRHD) Eclipse-Plus C18) by means of an Agilent UHPLC system (Agilent 1260, Agilent Technologies, Santa Clara, CA, USA), consisting of a vacuum degasser, an autosampler, and a binary pump. Mobile phases A and B were water and acetonitrile, respectively, both with 0.1 % formic acid. The flow rate used was 0.5 mL min−1. The chromatographic method held the initial mobile phase composition (5 % B) constant for 2 min, followed by a linear gradient to 100 % B at 8 min and held constant for a 2 min at 100 % B. Twenty microliters of extract was injected in each study. A 5-min post-time was used for each analysis.
The UHPLC system was connected to a quadrupole-time-of-flight mass spectrometer Agilent Q-TOF 6530 (Agilent Technologies, Santa Clara, CA, USA) equipped with an electrospray interface operated in either positive or negative ionization mode, using the following operation parameters: capillary voltage, 4000 V; nebulizer pressure, 40 psig; drying gas, 9 L min−1; gas temperature, 325 °C; and fragmentor voltage, 90 V. LC-MS accurate mass spectra were recorded across the range m/z 50–1000. Two different experiments were conducted, full-scan acquisition and all-ion mode MS/MS, in order to perform CID experiments in a dedicated collision cell with no precursor ion isolation along with high-resolution full-scan acquisition. All-ion mode full-scan acquisition was used at two different collision energy conditions (0 (full scan with no fragmentation) and 20 V), using 400 ms for each experiment (1.25 spectra/acquisition points per second). Accurate mass measurements of each peak from the total ion chromatograms were obtained by means of an automated calibrant delivery system using a dual-nebulizer electrospray source that introduces the flow from the outlet of the chromatograph together with a low flow of a calibrating solution (calibrant solution A, Agilent Technologies), which contains the internal reference masses (purine (C5H4N4 at m/z 121.050873 and HP-0921 [hexakis-(1H,1H,3H-tetrafluoropentoxy)-phosphazene] (C18H18O6N3P3F24) at m/z 922.009798). All data was recorded with Agilent MassHunter Data Acquisition software (version B.04.00) and processed with Agilent MassHunter Qualitative Analysis software (version B.04.00), which included both Molecular Feature Extractor and Find by Formula applications used.
Development of an Accurate Mass Database of 630 Multiclass Food Contaminant Pollutants
Mixtures containing ca. 30–50 compounds, at individual concentrations of 200 μg L−1 each, were injected in the UHPLC-QTOFMS system to collect retention time (t R) data and the accurate masses of target ions together with the elemental composition. For confirmatory purposes, the mass spectra acquired using all-ion mode acquisition were carefully investigated to identify characteristic fragment ions. In some cases, individual standards of target compounds were required for further confirmation of diagnostic fragment ions. For the screening method step, an Excel spreadsheet was constructed containing for each analyte the compound name, molecular formula, theoretical exact mass, fragment ions, and retention time. This file was converted into csv format for use by the Agilent MassHunter Data Acquisition software (version B.04.00). When a sample run is completed and the corresponding raw data acquired, its components are automatically matched against the csv file (Find by formula application) by the MassHunter software taking into account a defined tolerance for mass and retention time deviations (t R ± 0.25 min and ion exact mass ± 10 ppm), and a report is generated with the compounds tentatively found in the analyzed sample data file.
Results and Discussion
Screening Method Development and General Acquisition Method Considerations
Selection of UHPLC Gradient
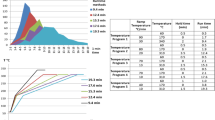
Before developing the screening method, different elution gradients were assayed using matrix-matched standards in representative matrices (such as tomato and orange) in order to obtain appropriate separation of analytes and matrix components within the shortest time period while displaying relatively low or moderate signal suppression effects. Three methods (A, B, and C) were assayed by varying the total gradient time, using the same flow rate (0.5 mL min−1) and mobile phases (total time of 5, 10, and 15 min, respectively). Mobile phases were 0.1 % HCOOH in water (A) and 0.1 % HCOOH in acetonitrile (B). The details of the different gradient elution programs are shown in Table S1 (Electronic Supplementary Material (ESM)). An example on the analysis of a mixture of selected pesticides in orange and some representative extracted ion chromatograms (EICs) (100 μg kg−1) are shown in Fig. 1. To develop the screening method, different criteria were employed to select the most appropriate elution gradient. The comparison of the total ion chromatograms (TICs) revealed that the matrix components and analytes were not separated properly with the shortest method. The number of coelutions and thus the possibility of interferences and quantitation issues due to matrix effects would be clearly increased under these conditions (method A). It must be taken into account the large number of components from a matrix (typically with 5000–10,000 (Gómez-Ramos et al. 2013; Gómez-Ramos et al. 2016) at relevant concentrations which must be separated. For this reason, the shortest method (method A) was discarded. Given the differences of run time, it would be expected that coelutions with method B were more frequent than with method C. This fact was further examined using matrix effects.
a Total ion chromatograms (TICs) of a pesticide mixture (100 μg kg−1) in orange using elution gradients A (a1), B (a2), and C (a3). b Extracted ion chromatograms (EICs) of some database compounds (imazalil, diphenylamine, tebuconazole, difenoconazole, prometon, and prometryn (100 μg L−1) in orange with elution gradients A (b1), B (b2), and C (b3)
Matrix effects were evaluated using 15 representative analytes (including pesticides, veterinary drugs, and mycotoxins) in tomato and orange matrices with the two remaining methods (B and C). Matrix effects were calculated as follows: [(calibration curve slope in matrix / calibration curve slope in solvent) − 1] × 100. Positive values indicate signal enhancement while negative signal involves values suppression—the more common phenomenon. Depending on this percentage, matrix effect was classified in different categories, according to previous literature (Ferrer-Amate et al. 2010; González-Antuña et al. 2013). A percentage between −20 and 20 % was considered as mild matrix effect, as the slope ratios matrix/solvent would be approaching the unit. A medium matrix effect occurred when this percentage was from −50 to −20 % or from +20 to +50 %. Strong matrix effect would be produced when this percentage was below −50 % or above +50 %.
As shown in Fig. 2a, all the selected compounds showed signal suppression in tomato with both elution gradients B and C, with the exception of aflatoxin B1, thiabendazole, and azoxystrobin. The extent of matrix effects was not significantly different between the two gradients (B and C). In the case of orange (Fig. 2b), all tested compounds showed signal suppression with both elution gradients assayed, although, in general, the matrix effects are slightly less intense in the case of the longer method. This is consistent with the fact that there is more time to separate species, thus minimizing the potential coelutions and the associated ionization competition and subsequent matrix suppression. As the main objective was to develop a screening method which separate and identify the most number of compounds in a single run in the shortest time possible, elution gradient B was selected.
a Percentages of signal suppression or enhancement for selected compounds in tomato (A aflatoxin B1; B azoxystrobin; C buprofezin; D carbendazim; E cyromazine; F DEET; G diuron; H imazalil; I imidacloprid; J prochloraz; K sarafloxacin; L sulfamethoxazole; M tebuconazole; N tetracycline; O thiabendazole; R thiacloprid). b Percentages of signal suppression or enhancement for selected compounds in orange (A aflatoxin B1; B azoxystrobin; C buprofezin; D carbendazim; E cyromazine; F DEET; G diuron; H imidacloprid; I prochloraz; J sarafloxacin; K sulfamethoxazole; L tebuconazole; M tetracycline; N thiabendazole; O thiacloprid)
Identification of the Targeted Species by UHPLC-Q-TOFMS
A generic full-scan acquisition method with default source parameters was used for the mass spectrometric detection of the studied species. Default values were set for drying and nebulizer flow rates and pressures and drying gas temperatures considering the LC flow rate and mobile phase composition.
The identification of the target species was carried out using retention time values and accurate mass measurements of the (de)protonated molecules in most cases. Exceptionally, either sodium or ammonium adducts were identified as the most abundant ion for a few compounds (4 %). In general, 90 % of compounds were detected in positive ion mode whereas only 10 % of targeted compounds were identified in negative ionization mode. Additionally, for ca. 20 % of the species, it was found that fragments generated—from in-source CID during ion transportation—were more abundant than the corresponding (de)protonated molecules. The detailed information including detected ion, elemental composition, retention time, theoretical m/z (exact mass), and experimental measured accurate masses with the relative mass error (expressed in ppm) are shown in Table 1, where compounds are grouped according to their class (pesticides, veterinary drugs, mycotoxins, perfluorinated compounds, food-packaging contaminants, nitrosamines, and sweeteners). For confirmation of the species, acquisition with the UHPLC-Q-TOFMS instrument was undertaken in the “all-ion mode” acquisition mode. This consists on the use of CID fragmentation in a collision cell without previous precursor isolation, so that all ions entering the mass spectrometer are subjected to thorough fragmentation, thus avoiding restrictions on the number of coeluting compounds subjected to MS/MS and also previous information required information to conduct the MS/MS experiments such as retention time windows or precursor ion masses. The acquisition method proposed consisted on two full-scan experiments with the collision cell different collision energies: 0 V (no fragmentation) and 20 V (fragmentation), using an acquisition time of 400 ms for each experiment. With such experiments, at least two ions were obtained for identification/purposes in most cases with the exception of few low molecular weight molecules, difficult to fragment.
Study of Searching Parameters for Automated Screening
A snapshot of the software application used (Find by Formula tool, Agilent MassHunter Qualitative Analysis (version B.04.00)) is shown in Fig. 3 along with the main information included in the database. The main search parameters (accurate mass tolerance and retention time (t R) window) affecting the performance of the automated search using Find by Formula tool were carefully examined. Different experiments were assayed varying t R windows (±0.05, ±0.1, ±0.25, ±0.5 min) with two fixed accurate mass tolerances (±5 or ±10 ppm). Default settings of peak filtering were used to remove background and mobile phase ion contribution. In these experiments, the database (630 compounds) was applied to 16 synthetic mixtures of pesticides (100 μg L−1) with 30 compounds each. The number of false positives and negatives, average score (%), and success rate (%) were evaluated for each mixture. The term false positive meant that a compound was reported, but it was not present in the actual sample. On the other hand, a compound which was present in the synthetic mixture but not reported by the software after the automated search was a false negative.
a Csv file with relevant information (elemental composition, retention time, exact mass, compound name for the main ion of each compound) for the automatic search of compounds with Agilent MassHunter Qualitative Analysis software. b Selection of mass error tolerance and retention time window for the automatic search using the specific software
In most cases, false negatives were due to two main reasons: (i) detector saturation occurring with high sensitive compounds or compounds at high concentrations and (ii) low sensitivity compounds with very low response factors—those which do not really perform properly mainly because of poor electrospray ionization. In the first scenario, spectra obtained displayed low score values due to accurate mass drifting with saturated detector and also due to the spectra collected which was not statistically representative of the sample. In the latter case, the concentration tested (100 μg L−1) was not high enough to detect the compounds, and thus, they were reported as false negatives.
Subtle differences were found in terms of the number of false negatives, average score, and success rate for each mixture using the experiments at different retention time and mass bias. In contrast, significant differences were found in the number of false positives, when different retention time windows were employed with ±5 and ±10 ppm as mass error tolerance. The results in terms of false positive rates obtained for each of the synthetic mixture tested (using ±5 and ±10 ppm as mass error tolerance) are shown in Fig. 4. The results are expressed in number of false positives (out of 630) related to the number of compounds expected (30 in each experiment).
The data collected concluded that wider retention time windows yielded a higher value of false positives. The highest number of compounds was reported when ±0.5 min was used and the lowest with ±0.05 min. Those results did not depend heavily on the mass error tolerance employed. In this sense, it should be kept in mind that complex food extracts may shift the retention times so that these results may be affected, particularly in the case of early eluting compounds (more affected by matrix, pH, and/or composition). For this reason, the narrowest retention time tolerance (± 0.05 min) was discarded. On the other hand, the results obtained using ±0.1 and ±0.25 min tolerances were relatively similar with minor differences in the number of false positives. Both tolerances could be adopted for the final method, although, in order to prevent false negatives due to retention time shifts particularly for polar compounds, and also due to relatively high mass errors obtained for small molecules with m/z lower than 150, ±0.25 min and ±10 ppm were finally selected as the most appropriate retention time window and mass error tolerance for screening step. Eventually, a final additional step would involve confirmation of the findings and accurate mass measurements of ions and fragments for each tentative compound detected, which would be within the widely accepted standard 5-ppm relative mass error threshold (or 1 mDa for molecules below 200 Da) (European Commission 2015)).
Analytical Performance
Three representative food matrices (tomato, orange, and baby food) were employed to evaluate the performance of the proposed screening method in terms of linearity, matrix effects, and limits of quantification (LOQs). In order to avoid coelutions between analytes that could shift the actual performance in terms of matrix effects, mixtures containing ca. 30 compounds (each) were used to prepare the calibration curves in the concentration range from 1 to 1000 μg kg−1 (1, 10, 50, 100, 200, 500, and 1000) in solvent standards (20 % methanol), tomato, orange, and baby food extracts. LOQs were estimated as the minimum concentration of analyte corresponding to a signal-to-noise ratio (S/N) = 10:1. This was experimentally calculated from the injection of matrix-matched standards at low concentration levels, using the more abundant ion for each extracted ion chromatograms with narrow mass windows (±10 ppm relative mass error). In the case of pesticides, sample extracts were prepared by spiking samples before sample extraction step so that recovery percentages were considered. Results obtained for pesticides are detailed in Table S-2 (ESM), along with the maximum residue level (MRLs) established for the pesticide/commodity combinations tested. The data for the rest of classes studied is included in Table S-3 (ESM). They are also summarized in Fig. 5, and the overall data of LOQs for each individual group of compounds is included as Supplementary material (Figs. S1–S2). Most of pesticides and veterinary drugs showed limits of quantification from 1 to 10 μg kg−1 in tomato, orange, and baby food. The percentage of those compounds with LOQs < 1 μg kg−1 was higher in baby food and tomato than in orange. On the other hand, 65 % of food-packaging contaminants displayed LOQs <10 μg kg−1 in baby food. In tomato and orange, most of those compounds exhibited LOQs from 10 to 100 μg kg −1. For the rest of compound classes tested (food-packaging contaminants, mycotoxins, and perfluorinated compounds), the highest percentage of compounds with LOQs > 10 μg kg −1 was obtained in orange extracts. This can be attributed to the complexity of the orange matrix (and the extent of matrix effects therein) compared to both tomato and baby food matrices, as clearly illustrated in Fig. 6. Examples of compounds detected in incurred food samples are shown in Fig. 7, where the extracted ion chromatograms and mass spectra of tebuconazole and imazalil detected in peach jam and oranges, respectively, are shown.
a Overlapped total ion chromatograms (TICs) of a pesticide mixture (100 μg L−1) in solvent, tomato, orange, and baby food. b Overlapped extracted ion chromatograms (EICs) of metribuzin (100 μg kg−1) in solvent, tomato, orange, and baby food. c Overlapped extracted ion chromatograms (EICs) of fluquinconazole (200 μg kg−1) in solvent and tomato
a Extracted ion chromatogram (EIC) of tebuconazole in solvent (20 % methanol) (a1), mass spectrum of tebuconazole in solvent (20 % methanol) (a2), extracted ion chromatogram (EIC) of tebuconazole in peach jam (a3), and mass spectrum of tebuconazole in peach jam (a4). b Extracted ion chromatogram (EIC) of imazalil in solvent (20 % methanol) (b1) and mass spectrum of imazalil in solvent (20 % methanol) (b2). Extracted ion chromatogram (EIC) of imazalil in orange (b3) and mass spectrum of imazalil in orange (b4)
In the case of the pesticides, the LOQs obtained were contrasted with the MRLs for the pesticide/commodity combinations available. Considering the default MRLs for pesticides in baby food set at 10 μg kg −1, over 90 % of the compounds fulfilled this threshold, being 56 above the value set, either because they were not recovered (e.g., highly polar compounds requiring dedicated sample treatment) or because of lower response factors. In the latter case, the compounds are low sensitive due to poor ionization with electrospray. As has been reported by other authors, there is always a percentage in the range of 10 %, which does not yield good response factors due to its features not compatible to electrospray (Alder et al. 2011; García-López et al. 2014), even despite using state-of-the-art instrumentation in some of the studies. In the case of tomato, despite 75 compounds (18 % out of 411 pesticides included) did not achieve the 10-μg kg −1 sensitivity, only 47 were above the MRL value set (10 %), 24 not recovered, and 23 with LOQs above MRLs. Finally, in the case of orange, around 30 % was above the 10-μg kg −1 threshold, with 85 compounds (20 %) not fulfilling the MRL requirements. These results evidence one of the limitations of this type of screening approaches. The sensitivity is yet an issue, and this is more evident as the matrix complexity increases. With the use of state-of-the-art instrumentation, using heated electrospray source providing a remarkable sensitivity increase, there will always be a percentage of “difficult to ionize” compounds that would not fulfill the sensitivity requirements.
Besides the performance in terms of LOQs, matrix effects were also tested. Matrix effects usually occur during ionization step, where the matrix constituents influence the ionization of coeluted analyte(s). Coelution with matrix interferences or with compounds belonged to the same batch could produce signal suppression or enhancement of the target compounds. This fact also could cause mass measurement deviations from theoretical m/z values. As an example, Fig. 6b includes the extracted ion chromatograms of a pesticide (metribuzin, 100 μg kg−1) in solvent, tomato, orange, and baby food. Signal suppression was observed in these three matrixes. Orange was the one that produced the highest signal suppression, followed by tomato and baby food. Figure 6c shows EICs for fluquinconazole in solvent and tomato. For this compound, signal enhancement was observed in matrix (200 μg kg−1), although this is not the standard behavior. The same criterion—described previously—was applied for matrix effect evaluation. Slope ratios of matrix/solvent from 0.8 to 1 were considered as soft signal suppression, from 0.5 to 0.8 medium signal suppression, and lower than 0.5 strong signal suppression. Signal enhancement could also be classified as soft (slope ratios of matrix/solvent from 1 to 1.2), medium (slope ratios of matrix/solvent from 1.2 to 1.5), and strong (slope ratios of matrix/solvent from 1.5 to 2). Figure 8 includes a 2D plot representing the matrix effects obtained for all the tested compounds in the three different matrices tested. Table S4 and Fig. S2 (Supplementary data) include the data from the matrix effects displayed by the different classes of compounds, being signal suppression the most common effect produced in tomato, orange, and baby food. Medium signal suppression was the most common effect produced in tomato for pesticides, mycotoxins, veterinary drugs, food-packaging contaminants, and nitrosamines, with the exception of perfluorinated compounds and sweeteners. Results for orange were distinctly worse than the other two matrices, with average suppression of 30–40 % as illustrated in Fig. 8b. These results are consistent with previous studies (Gómez-Ramos et al. 2016) and may be attributed, perhaps, to the complexity of the orange matrix due to its composition and the presence of waxes and citrus oils. Finally, soft signal suppression for pesticides, veterinary drugs, mycotoxins, food-packaging contaminants, and sweeteners was the most common effect produced in baby food. This is consistent with the complexity of each of the matrix revealed by the TIC profiles shown (Fig. 6). As an alternative, the use of longer column (e.g., 100 mm) and longer gradient may help to reduce matrix effects as it would enable a better separation, at the expense of method throughput though.
Conclusions
A screening method using UHPLC-QTOFMS has been developed for the examination of 630 food contaminants, including pesticides, veterinary drugs, food-packaging contaminants, mycotoxins, nitrosamines, perfluorinated compounds, and sweeteners. The method was based on a database with retention time values and mass accurate measurements of the ions of interest. It was found that software parameters such as retention time window and mass error tolerance have a clear influence on the automatic search results. The proposed methodology was also examined in terms of linearity, matrix effect, and limits of quantification in three different matrixes: tomato, orange, and baby food. For most of compounds, signal suppression was the most common matrix effect produced. In general, baby food and orange produced the lowest and the highest matrix effect, respectively. This clearly had an impact on the sensitivity of the method. Limits of quantification were also calculated for the 630 compounds included, and most of them were <10 μg kg−1 in tomato, orange, and baby food. However, in the particular case of pesticides with relatively low response factors (ca. 10–20 % of the compounds depending on the complexity of the matrix), the detection was not fulfilling the MRL established for the tested pesticide/commodity combination. This is a drawback of the entire approach that may be partially solved with more sensitive and updated instrumentation, except for the case of compounds not really amenable to electrospray ionization.
Abbreviations
- CCL-3:
-
Contaminant candidate lists
- CID:
-
Collision-induced dissociation
- GC-MS/MS:
-
Gas chromatography/tandem mass spectrometry
- HRMS:
-
High-resolution mass spectrometry
- LC-MS/MS:
-
Liquid chromatography/tandem mass spectrometry
- MeOH:
-
Methanol
- QC:
-
Quality control
- QuEChERS:
-
Quick, easy, cheap, effective, rugged, and safe
References
Alder L, Steinborn A, Bergelt S (2011) Suitability of an Orbitrap mass spectrometer for the screening of pesticide residues in extracts of fruits and vegetables. J AOAC Int 94:1661–1673
Canadian Food Inspection Agency (CFIA) (2016) Available on: http://www.inspection.gc.ca/
Codex Alimentarius (2016) Pesticide residues in food, maximum residue limits. Available on: http://www.fao.org/fao-who-codexalimentarius/standards/pestres/en/
Di Stefano V, Avellone G, Bongiorno D, Cunsolo V, Muccilli V, Sforza S, Dossena A, Drahos L, Vekey K (2012) Applications of liquid chromatography-mass spectrometry for food analysis. J Chromatogr A 1259:74–85
Díaz R, Ibañez M, Sancho JV, Hernández F (2011) Building an empirical mass spectra library for screening of organic pollutants by ultra-high pressure liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Sp 25:355–369
European Commission (2005) EC Regulation No. 396/2005, of 23 February 2005, On maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC, Off J Eur Commun L70: 1–16. Available on http://ec.europa.eu/food/plant/pesticides/max_residue_levels/eu_rules/index_en.htm
European Commission (2006a) Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC
European Commission (2006b) Commission Regulation (EC) no. 1881/2006 of 19 December 2006, setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364(2006):5–24
European Commission (2010) Council Regulation (EEC) No. 37/2010 of the 22 December 2009, On pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin, Off J Eur Union L15, (2009) 1–76. Available on: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2010R0037:20110714:ES:PDF
European Commission (2011) Commission Regulation (EU) 10/2011 EC of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off J Eur Union L 12:1
European Commission (2012). Commission Implementing Regulation (EU) No. 788/2012 of 31 August 2012 concerning a coordinated multiannual control programme of the Union for 2013, 2014 and 2015 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin. Off J Eur Union, L235/8-L235/27, 01.09.2012
European Commission (2015) Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. SANTE/11945/2015. Available on: http://ec.europa.eu/food/plant/docs/pesticides_mrl_guidelines_wrkdoc_11945.pdf
European Commission (2016) EU pesticides MRLs; maximum pesticide levels for food products for human consumption and animal feeding stuffs. Available on: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN
Ferrer-Amate C, Unterluggauer H, Fischer RJ, Fernández-Alba AR, Masselter S (2010) Development and validation of a LC-MS/MS method for simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal Bioanal Chem 3971:93–107
Fussell RJ, García-López M, Mortimer DN, Wright S, Sehnalova M, Sinclair CJ, Fernandes A, Sharman M (2014) Investigation into the occurrence in food and veterinary medicines, pharmaceuticals and chemicals used in personal care products. J Agr Food Chem 62:3561–3659
Gallart-Ayala H, Nuñez O, Lucci P (2013) Recent advances in LC-MS analysis of food-packaging contaminants. Trac-Trend Anal Chem 42:99–124
García-López M, Fussell RJ, Stead SL, Roberts D, McCullagh M, Rao R (2014) Evaluation and validation of an accurate mass screening method for the analysis of pesticides in fruits and vegetables using liquid chromatography-quadrupole-time-of-flight mass spectrometry with automated detection. J Chromatogr A 1373:40–50
Garrido Frenich A, Romero-González R, Aguilera-Ruiz MM (2014) Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. Trac-Trend Anal Chem 63:158–169
Gilbert-López B, García-Reyes JF, Lozano A, Fernández-Alba A, Molina-Díaz A (2010) Large-scale pesticide testing in olives by liquid chromatography–electrospray tandem mass spectrometry using two sample preparation methods based on matrix solid-phase dispersion and QuEChERS. J Chromatogr A 1217:6022–6035
Gómez-Pérez ML, Romero-González R, Martínez-Vidal JL, Garrido-Frenich A (2015) Analysis of pesticide and veterinary drug residues in baby food by liquid chromatography coupled to Orbitrap high resolution mass spectrometry. Talanta 131:1–7
Gómez-Ramos MM, Ferrer C, Malato O, Agüera A, Fernández-Alba AR (2013) Liquid chromatography high resolution mass spectrometry for pesticide residue analysis in fruit and vegetables: screening and quantitative studies. J Chromatogr A 1287:24–37
Gómez-Ramos MM, Rajski L, Lozano A, Fernandez Alba AR (2016) The evaluation of matrix effects in pesticide multi-residue methods via matrix fingerprinting using liquid chromatography electrospray high-resolution mass spectrometry. Anal Methods 8:4664–4673
González-Antuña A, Domínguez-Romero JC, García-Reyes JF, Rodríguez-González P, Centineo G, García-Alonso JI, Molina-Díaz A (2013) Overcoming matrix-effects in electrospray: quantitation of ß-agonists in complex matrices by isotope dilution liquid chromatography-mass spectrometry using singly 13C-labeled analogues. J Chromatogr A 1288:40–47
Health Canada (2014) Maximum residue limits for pesticides. Available on: http://www.hc-sc.gc.ca/cps-spc/pest/part/protect-proteger/food-nourriture/mrl-lmr-eng.php
Hird SJ, Lau BPY, Schuhmacher R, Krska R (2014) Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. Trac-Trend Anal Chem 59:59–72
Lacina O, Urbanova J, Poustka J, Hajslova J (2010) Identification/quantification of multiple pesticide residues in food plants by ultra-high performance liquid chromatography-time-of-flight mass spectrometry. J Chromatogr A 1217:648–659
Langman CB (2009) Melamine, powdered milk, and nephrolithiasis in Chinese infants. New Engl J Med 360:1139–1141
Lehotay SJ (2011) QuEChERS sample preparation approach for mass spectrometry analysis of pesticide residues in foods. Methods Mol Biol 747:65–91
Malik AK, Blasco C, Picó Y (2010) Liquid chromatography-mass spectrometry in food safety. J Chromatogr A 1217:4018–4040
Masía A, Suarez-Varela MM, Llopis González A, Picó Y (2016) Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: a review. Anal Chim Acta 936:40–61
Matamoros V, Calderón-Preciado D, Domínguez C, Bayona JM (2012) Analytical procedures for the determination of emerging organic contaminants in plant material: a review. Anal Chim Acta 722:8–20
Mezcua M, Malato O, García-Reyes JF, Molina-Díaz A, Fernández-Alba AR (2009) Accurate-mass databases for comprehensive screening of pesticide residues in food by fast liquid chromatography time-of-flight mass spectrometry. Anal Chem 81:913–929
Mol HGJ, Plaza-Bolaños P, Zomer P, de Rijk TC, Stolker AAM, Mulder PPJ (2008) Toward a generic extraction method for simultaneous determination of pesticides, mycotoxins, plant toxins, and veterinary drugs in feed and food matrices. Anal Chem 80:9450–9459
National Standard GB-2763-2014 (2014) Maximum residue limits for pesticides in food, Standardization Administration of the People’s Republic of China
Pérez-Ortega P, Gilbert-López B, García-Reyes JF, Ramos-Martos N, Molina-Díaz A (2012) Generic sample treatment method for simultaneous determination of multiclass pesticides and mycotoxins in wines by liquid chromatography-mass spectrometry. J Chromatogr A 1249:32–40
Pérez-Ortega P, Lara-Ortega FJ, García Reyes JF, Beneito Cambra M, Gilbert López B, Ramos Martos N, Molina Díaz A (2016) Determination of over 350 multiclass pesticides in jams by ultra-high performance liquid chromatography time-of-flight mass spectrometry (UHPLC-TOFMS). Food Anal Methods 9:1939–1957
Picó Y, Farré M, Barceló D (2015) Quantitative profiling of perfluoroalkyl substances by ultrahigh-performance liquid chromatography and hybrid quadrupole time-of-flight mass spectrometry. Anal Bioanal Chem 407:4247–4259
Polgar L, García-Reyes JF, Fodor P, Gyepes A, Dernovics M, Abranko L, Gilbert-López B, Molina-Díaz A (2012) Retrospective screening of relevant pesticide metabolites in food using liquid chromatography high resolution mass spectrometry and accurate-mass databases of parent molecules and diagnostic fragment ions. J Chromatogr A 1249:83–91
Romero-González R, Aguilera-Luiz MM, Plaza-Bolaños P, Garrido Frenich A, Martinez Vidal JL (2011) Food contaminant analysis at high resolution mass spectrometry: application for the determination of veterinary drugs in milk. J Chromatogr A 1218:9353–9365
US Department of Agriculture (2014) Foreign agricultural service, maximum residue limit database, 2014. Available from: http://www.fas.usda.gov/maximum-residue-limits-mrl-database
US Environmental Protection Agency (EPA) (2015) Basic Information- Unregulated Contaminant monitoring Rule-2 Available on : http://water.epa.gov/lawsregs/sdwa/ucmr/ucmr2/basicinformation.cfm
US Environmental Protection Agency (EPA) (2016) Available on https://www.epa.gov/pesticide-registration/about-pesticide-registration#laws
US Environmental Protection Agency (EPA) (2009), Contaminant candidate list 3—final, 2009
US Food Drug Administration (FDA) (2011) Tolerances for residues on new animal drugs in food. Code of Federal Regulations. Available on: http://www.gpo.gov/fdsys/pkg/CFR-2011-title21-vol6/pdf/CFR-2011-title21-vol6.pdf Food and Drugs, Part 556, Title 21; U.S. Food Drug Administration. Office of the Federal Register, National Archives and Records Administration: Washington, DC, April
US Food Drug Administration (FDA) (2016) http://www.fda.gov/AnimalVeterinary/Products/ApprovedAnimal Drug Products/UCM2006464
Wang J, Chow W, Chang J, Wong JW (2014) Ultrahigh-performance liquid chromatography electrospray ionization Q-Orbitrap mass spectrometry for the analysis of 451 pesticide residues in fruits and vegetables: method development and validation. J Agr Food Chem 62:10375–10391
Zhan J, Yu XJ, Zhong YY, Zhang Z, Cui XM, Peng JF, Feng R, Liu XT, Zhu Y (2012) Generic and rapid determination of veterinary drug residues an other contaminants in raw milk by ultra performance liquid chromatography tandem mass spectrometry. J Chromatogr B 906:48–57
Acknowledgments
The authors acknowledge funding from the Spanish Ministerio de Economía y Competitividad (MINECO) (Ref. CTQ-2009-10897). P.P.-O acknowledges a PhD scholarship from the University of Jaén. F.J.L.-O. acknowledges a FPI Program PhD scholarship from MINECO (Ref. BES-2013-064014). D.M.G. thanks the MINECO for a Juan de la Cierva postdoctoral contract. B.G.L. acknowledges MINECO for her Juan de la Cierva postdoctoral research contract (Ref. JCI-2012-12972). The authors acknowledge Servicios Centrales de Apoyo a la Investigación of the University of Jaen (SCAI-UJAEN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Patricia Pérez-Ortega declares that she has no conflict of interest. Felipe J. Lara-Ortega declares that he has no conflict of interest. Bienvenida Gilbert-López declares that he has no conflict of interest. David Moreno-González declares that he has no conflict of interest. Juan F. García-Reyes declares that he has no conflict of interest. Antonio Molina-Díaz declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Electronic Supplementary Material
ESM 1
(DOCX 769 kb)
Rights and permissions
About this article
Cite this article
Pérez-Ortega, P., Lara-Ortega, F.J., Gilbert-López, B. et al. Screening of Over 600 Pesticides, Veterinary Drugs, Food-Packaging Contaminants, Mycotoxins, and Other Chemicals in Food by Ultra-High Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-QTOFMS). Food Anal. Methods 10, 1216–1244 (2017). https://doi.org/10.1007/s12161-016-0678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0678-0