Abstract
In this work, microwave-assisted digestion using the diluted nitric acid procedure for trace elements determination in rice samples by inductively coupled plasma optical emission spectrometry (ICP OES) and inductively coupled plasma mass spectrometry (ICP-MS) was investigated in a multivariate way. The response surface methodology (RSM), based on the Box–Behnken design, was employed to obtain the optimum conditions of the significant parameters. The optimal conditions, for 250 mg of sample, could be obtained at a temperature of 200 °C, digestion time of 20 min, and 4.5 mol L−1 for nitric acid concentration. The accuracy of the optimized method was evaluated by analysis of the rice flour certified reference materials (NIST SRM 1568a) and spike recoveries. The results showed good agreement with the reference values of the CRM samples. The analytical method was applied to the determination of Al, As, Ca, Cd, Cu, Fe, K, Mg, Mn, Mo, Se, and Zn in rice samples. Considering some critical parameters such as cost, green chemistry, and analyst safety, the proposed method has advantages compared to classical microwave-assisted digestion procedures based on concentrated acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa) is used as an energy source for human consumption due to its high concentration of starch. It also provides proteins, vitamins, and minerals. Because of the importance of rice in the diet of much of the world’s population, its nutritional quality directly affects human health (Walter et al. 2008; Vieira et al. 2008). In this sense, the determination of chemical species in rice is important for the assessment of its nutritional capacity and to identify the presence of species that are toxic in the human diet (Erdemi and Gucer 2014; Antoine et al. 2012; Batista et al. 2011; Teixeira et al. 2012; Freschi et al. 2012).
Solid samples require pretreatment before they can be used in spectroscopy techniques with nebulization systems. The most common pretreatment before determination of inorganic species in food is acid digestion of the samples (Gonzalez et al. 2009). Acid digestion assisted by microwave radiation has been widely used in sample preparation, especially by employing the use of high temperatures and pressures in closed systems. These procedures allow the use of small volumes of reagents with minimal loss of volatiles, and the time taken for decomposition is reduced compared with decomposition by conventional heating (Nobrega et al. 2002; Gonzalez et al. 2009; Arruda and Santelli 1997). These advantages make it possible to reduce the acid concentration while keeping the efficiency of the digestion, decreasing the dilution factor prior to analysis, and generating less waste (Krug 2008; Bizzi et al. 2010; Santos et al. 2013), in accordance with the green analytical chemistry (Gałuszka et al. 2013; Lenardão 2003). However, optimization of the digestion is required in order to obtain satisfactory decomposition, when dilute acid solutions are employed (Castro et al. 2009).
The minerals are widespread in nature in different forms. Ingestion is the primary way to enter in the human body. The chemical elements are classified according to their importance in human health: essential species such as Ca, K, Na, and Mg (needed in large quantities) or Fe, Co, Mn, and Zn (necessary for physiological functions, but at high concentrations can become toxic); and non-essential or toxic species such as Cd and Pb (no function known) (Leal et al. 2009; Naseri et al. 2015). Thus, the determination of chemical elements content in foods is important for the knowledge of their nutritional quality.
In recent years, multivariate optimization techniques have been widely used in the development of analytical methods which require the optimization of several variables simultaneously (Bezerra et al. 2008; Ferreira et al. 2007; Lopes et al. 2009; Khajeh et al. 2010). Among these techniques, the Box–Behnken design has been widely employed (Ferreira et al. 2007; Macedo et al. 2009; Khajeh and Sanchooli 2010; Almeida et al. 2015).
In this work, a microwave-assisted digestion procedure using the diluted nitric acid was optimized for trace elements determination in rice samples by inductively coupled plasma optical emission spectrometry (ICP OES) and inductively coupled plasma mass spectrometry (ICP-MS). The Box–Behnken design was employed to obtain the optimum conditions of the significant parameters: temperature and time for the final stage of digestion, and nitric acid concentration. The developed method was employed in the determination of 12 elements (Al, As, Ca, Cd, Cu, Fe, K, Mg, Mn, Mo, Se, and Zn) in rice samples.
Material and Methods
Apparatus
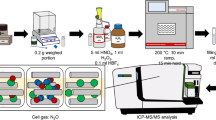
Acid digestion of the samples was performed using a commercial high-pressure laboratory microwave oven (Milestone Ethos 1600 Microwave Labstation, Sorisole, Italy) equipped with 10 vessels made of perfluoroalkoxy polymer (PFA) with a volume of 100 mL.
A quadrupole ICP-MS X series II (Thermo, Germany) equipped with a hexapole collision cell (CCT) was used for all determinations. In order to obtain optimal accuracy and precision, the internal standards were chosen as close in mass number as possible to that of the analyte element. Thus, 74Ge and 103Rh isotopes were selected to be used as internal standards. The CRM NIST 1568a was analyzed twice. Firstly, the standard mode (XT), normal mode of operation of the equipment, was tested and, then, hexapole collision cell operation mode (CCT) was tried, to eliminate possible polyatomic interference. Taking into account the agreement with the certified values, the XT mode was selected for the determination of all the elements. The equipment was configured with a Peltier cooled, quartz impact bead spray chamber, glass concentric nebulizer, and single piece, 1.5-mm i.d. injector torch. This spray chamber is usually used with conventional or low-flow pneumatic nebulizers to reduce the amount of solvent entering the plasma. The daily optimization procedure involved xyz alignment of the torch, and determination of the optimum nebulizer gas flow rate and the ion lens voltage to maximize the 115In+ signal and obtain low oxide (140Ce16O+/140Ce+) and double charged ions (137Ba++/137Ba+). The detector was operated in dual mode and cross-calibration was performed weekly. Samples were introduced via a peristaltic pump with an uptake rate of 0.8 mL min−1. Table 1 presents the ICP-MS operational conditions.
A Varian Vista (Mulgrave, Australia) simultaneous ICP OES with axial viewing and a charge coupled device (CCD) detector was used for Al, Ca, Cu, Fe, K, Mg, Mn, and Zn determinations. The ICP OES was calibrated with a multi-element stock solution, and the optical alignment was adjusted using a solution containing 5.0 mg L−1 Mn. Emission lines were selected according to the absence of spectral interferences and adequate sensitivity for the determination of the elements at low and high concentrations. The optimal values for the instrumental parameters were radio frequency power of 1.3 kW, plasma gas flow rate of 15 L min−1, auxiliary gas flow rate of 1.5 L min−1, nebulizer gas flow rate of 0.7 L min−1, replicate reading time of 1 s, instrument stabilization delay of 15 s, replicates (n = 3), and pump rate of 15 rpm. The analytical wavelengths (nm) employed were Al (I) 237.312, Ca (II) 396.847, C (I) 193.024, Cu (I) 327.395, Fe (II) 238.204, K (I) 766.491, Mg (I) 285.213, Mn (II) 257.610, and Zn (II) 206.200.
Reagents, Solutions, and Samples
All labware was soaked in a HNO3 acid bath (65 % w/w, diluted 1/10 with high-purity water) for 24 h and rinsed with high-purity water. Subsequently, all materials were dried under clean-air conditions at ambient temperature. All solvents and reagents were of the highest commercially available purity.
High-purity deionized water (resistivity 18.2 MΩ cm), from a Milli-Q water purified system (Millipore, Bedford, MA, USA), was employed to prepare all standard and sample solutions. Suprapur grade 65 % HNO3 (w/w) and 30 % H2O2 (w/w) (Merck, Darmstadt, Germany) were used for sample digestion. The plasma torch argon purity was higher than 99.99 %. Mono-elemental, high-purity grade 1 g L−1 stock solutions of 75As, 111Cd, 95Mo, and 82Se and a 100 mg L−1 multi-element solution of Bi, Ge, In, Tl, Rh, and Sc were purchased from Merck (Darmstadt, Germany). Element determination by ICP-MS was performed using external calibration. The reference analytical solutions and the samples were adjusted to an acid concentration 0.288 mol L−1 HNO3, as recommended by the manufacturer.
The optimized procedure was used in the analysis of 23 rice samples (10 parboiled, 8 white, and 5 brown rice) purchased in markets in Salvador City, Brazil. Dried samples were ground in a ball mill with a tungsten carbide vial set (Model 8000 M, Spex Sample Prep, USA) and stored in clean polyethylene vials inside a desiccator at room temperature.
Optimization of Sample Digestion
Sample masses of 250 mg of each dried and ground rice sample were weighed in a 100-mL microwave closed vessel and 7.0 mL nitric acid solution at different concentrations (2.0; 4.5, and 7.0 mol L−1) and 1.0 mL of 30 % (w/w) H2O2 were also added to each vessel. The heating program was performed in four successive steps. In the first step, the temperature was increased linearly to 90 °C over a period of 4 min. In the second step, the temperature was kept at 90 °C for 2 min. In the third step, the temperature was increased linearly to 200 °C over a period of 10 min. For the fourth step, the temperature and time were optimized. After digestion and cooling, the digests were transferred to plastic flasks and made up to a final volume of 15.0 mL with deionized water. Blanks were prepared in each batch of samples.
A full two-level factorial design (23) was used in order to verify the influence and interactions of the variables. At this stage, a rice sample was prepared in large quantities, with the aim of using a single sample in all experiments. Three variables were regarded as factors in the optimization study: the time (15, 20 e 25 min) and temperature (190, 200 e 210 °C) of digestion in the fourth step of the sample digestion and the concentration of HNO3 (2.0; 8.0, and 14 mol L−1). Table 2 shows the experimental design with the minimum, maximum, and central point levels for each factor (coded and real values). The factorial design totalized 12 experiments (experiments 01–12), including four replicates at the central point.
Additional experiments employing a Box–Behnken design were applied to determine the optimum conditions for the sample digestion (Table 3). The Box–Behnken design included 15 experiments (experiments 13–27), including three replicates at the central point. All digestion procedures were also evaluated by comparing the residual carbon content of the digests and the recoveries of the analytes. Multiple responses (MR) were employed in both designs for elements quantified, using a mathematical tool based on the Eq. 1(Santos et al. 2009):
where R Mn is the recovery of each analyte in the experiment and Rmax Mn is the maximum recovery of the analyte in the design.
Determination of the Acidity and Residual Carbon Content of the Digests
To determine the final acidity, acid-base titrations of the digests were performed. The titrations were carried out with a standard solution of sodium hydroxide (0.0997 mol L−1) and phenolphthalein (1.0 % m v−1 in ethanol). The residual carbon content (RCC) was measured in the ICP OES with axial configuration, using the line emission for C(I) at 193.025 nm (Bizzi et al. 2010). Carbon reference solutions (25–500 mg L−1) used for external calibration were prepared by dissolution of citric acid (Merck) in water.
Quality Assurance and Quality Control
All of the samples were digested in triplicate and all measurements were run in triplicate for each sample. Element concentrations were calculated and expressed in μg g−1 dry weight. The accuracy of the analytical method was verified by analyzing a certified reference material (Rice Flour—NIST SRM 1568a—National Institute of Standard and Technology, USA). At least one sample of the SRM 1568a and three blanks (reagents and digestion blanks) were included in each analytical batch. The precision of the method, defined as the closeness of agreement between mutually independent test results, was expressed in terms of relative standard deviation (RSD%).
Results and Discussion
The efficiency of decomposition in the microwave oven was evaluated by three variables: acid concentration, digestion time, and temperature. Firstly, a full two-level factorial design experiment (23) was carried out in order to verify the influence and interactions of these variables on the digestion of rice samples. The results obtained from this design are shown in Table 2 and the Pareto chart (Fig. 1). The temperature and time factors (negative effect) and the interaction of temperature and concentration of nitric acid (positive effect) were significant in the experimental domain studied.
A Box–Behnken design was then used in order to adjust the digestion procedure, using a quadratic model to identify the optimum conditions. Considering that good recoveries were obtained with diluted nitric acid solution, lower concentrations of acid were used in Box–Behnken design. The Box–Behnken design was performed employing the MR. The residual carbon concentrations and residual acidity were also determined in all the samples in the experiment. According to Nobrega et al. (2002), a residual carbon content of up to 13 % did not cause interference in the determination of metals by ICP OES. The residual carbon concentrations in the present study were between 2.21 and 18.4 % (m/m), and only experiments 2 and 3 had concentrations greater than 13 %. Low residual acidity solutions are recommended for elemental determination by spectrometric techniques, because acidity affects the sample introduction system and low residual acidity solutions result in better detection (Todolí and Mermet 1999). The residual acidity obtained after the digestions, as expected, was highest in the samples digested with highly concentrated solutions. The residual acidity of the diluted digests varied from 0.9 to 3.2 mol L−1.
The Box–Behnken design confirmed that the temperature and concentration of HNO3 had a positive effect on digestion, as can be seen in the response surface (Fig. 2). As can be seen in Table 3, the highest MR values were obtained at the central point. These experiments were performed with an acid concentration of 4.5 mol L−1, which correspond to a volume of 2 mL of concentrated HNO3 per 250 mg of sample. This condition conforms to the recommendation of Krug (2008) to use between 0.4 and 2.0 mL acid per 200 mg of sample. Thus, the central point conditions proved to be suitable for the digestion of rice samples, confirmed by the values calculated by the software Statistica 6.0 for the critical points: an acid concentration of 4.8 mol L−1 HNO3, a temperature of 208 °C, and a digestion time of 21 min.
Analytical Performance
The limits of detection (LOD) and quantification (LOQ) of each analyte were calculated as the analyte concentration corresponding to three and ten times, respectively, the standard deviation of ten independent measurements of the blank, divided by the slope of the calibration curve employing sample mass of 250 mg and final volume of 15.0 mL after digestion. The LODs for Al, Ca, Cu, Fe, K, Mg, Mn, and Zn (elements determined by ICP OES) were 0.8, 1.5, 0.1, 0.1, 0.7, 2.3, 0.1 and 0.2 μg g−1, respectively. When ICP-MS was employed, the LODs for As, Cd, Mo, and Se determinations were 0.05, 0.002, 0.02, and 0.02 μg g−1, respectively.
In order to evaluate the accuracy of the proposed procedure, a CRM sample (NIST 1568a, Rice Flour) was digested by the proposed procedure and the analytes were determined by ICP OES and ICP-MS. The results are presented in Table 4 and show that the obtained values were in agreement with certified values (80–102 %). The worst results were for Ca, K, As, Cu, Se, and Zn (between 80 and 85 %); however, these results are acceptable for mg g−1 or μg g−1concentration levels as reported in previous works concerning the determination of metals in food samples (AOAC SMPR 2012; Mohd-Taufek et al. 2016; Leme et al. 2014; Ni et al. 2016). Additionally, the evaluation of the analytical performance of procedure was also made by a spike recovery study at two concentration levels: 0.25 and 3.0 mg L−1. The recovery results ranged from 92 to 115 %. The recoveries obtained confirmed that the proposed procedure showed good analytical performance. Table 5 shows the recovery ranges obtained by previous methods for the determination of different elements in rice samples. Moreover, the procedure resulted in solutions with low residual acidity after dilution (2.0 mol L−1) and carbon (7.2 ± 2.5 %), which are important prerequisites for multi-element determination using ICP-MS and ICP OES.
Analytical Application in Rice Samples
The proposed method was applied to the determination of trace elements in 23 rice samples purchased from different local markets in Salvador, Bahia, Brazil (10 parboiled, 8 white, and 5 brown rice). The concentration ranges, i.e., the minimum and maximum values, for each type of rice are shown in Table 6. The average concentration of the analytes differed according to the type of rice, with brown rice showing the highest levels of the nutrients Ca, Mg, Fe, Mn, and Zn. This difference in concentration is related to differences in the processing of the rice. Brown rice is only subjected to a stripping step which does not lead to removal of these species from the grains. The determined concentrations of the analytes were also compared with values published in the literature, and the results were in good agreement with them (Silva et al. 2013; Teixeira et al. 2012; Walter et al. 2008; Oliveira et al. 2012; Okada et al. 2007).
Comparing the range of concentration of the analytes in three types of rice studied with the concentration regulated by Brazilian Agency for Sanitary Surveillance (Brazil 2005), it can be seen that all the element contents were below the maximum tolerated.
Conclusions
The microwave-assisted digestion procedure using dilute nitric acid was successfully applied for multi-element determination in rice samples by ICP OES and ICP-MS. The optimal conditions for analyte extraction from rice samples were obtained using a response surface methodology based on Box–Behnken experimental design, resulting in a fast and efficient method of optimization. Considering some critical parameters such as cost, minimization of residues, and analyst safety, the proposed procedure has advantages compared to classical digestion procedures.
References
Almeida JS, Anunciação TA, Brandão GC, Dantas AF, Lemos VA, Teixeira LSG (2015) Ultrasound-assisted single-drop microextraction for the determination of cadmium in vegetable oils using high-resolution continuum source electrothermal atomic absorption spectrometry. Spectrochim Acta Part B 107:159–163
Antoine JMR, Fung LAH, Grant CN, Dennis HT, Lalor GC (2012) Dietary intake of minerals and trace elements in rice on the Jamaican market. J Food Compos Anal 26:111–121
Arruda MAS, Santelli RE (1997) Mecanização no preparo de amostras por micro-ondas: o estado da arte. Quím Nov. 20:638–643
Batista BL, Souza JMO, Souza SS, Barbosa F Jr (2011) Speciation of arsenic in rice and estimation of daily intake of different arsenic species by Brazilians through rice consumption. J Hazard Mater 191:342–348
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Bizzi CA, Flores EMM, Picoloto RS, Barin JS, Nobrega JA (2010) Microwave-assisted digestion in closed vessels: effect of pressurization with oxygen on digestion process with diluted nitric acid. Anal Methods 2:734–738
Brazil (2005) ANVISA—Brazilian Agency for Sanitary Surveillance, Resolution # 269/2005. Technical Regulation on recommended daily ingestion of protein, vitamins and minerals. http://portal.anvisa.gov.br/documents/33880/2568070/RDC_269_2005.pdf/36ef25bc-80d4-465a-ac85-340587a7b850. Accessed 26 July 2016
Castro JT, Santos EC, Santos WPC, Costa LM, Korn M, Nóbrega JA, Korn MGA (2009) A critical evaluation of digestion procedures for coffee samples using diluted nitric acid in closed vessels for inductively coupled plasma optical emission spectrometry. Talanta 78:1378–1382
Erdemi US, Gucer S (2014) Assessment of copper bioavailability in spinach (Spinaciaoleracea L) leaves by chemical fractionation. Food Anal Method 7:994–1001
Fang Y, Pan Y, Li P, Xue M, Pei F, Yang W, Ma N, Hu Q (2016) Simultaneous determination of arsenic and mercury species in rice by ion-pairing reversed phase chromatography with inductively coupled plasma mass spectrometry. Food Chem 213:609–615
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC, Silva EGP, Portugal LA, Reis PS, Souza AS, Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186
Freschi GPG, Fortunato FM, Freschi CD, Gomes Neto JA (2012) Simultaneous and direct determination of As, Bi, Pb, Sb, and Se and Co, Cr, Cu, Fe, and Mn in milk by electrothermal atomic absorption spectrometry. Food Anal Method 5:861–866
Gałuszka A, Migaszewski Z, Namiesnik J (2013) The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TRAC-Trend Anal Chem 50:78–84
Gonzalez MH, Souza GB, Oliveira RV, Forato LA, Nóbrega JA, Nogueira ARA (2009) Microwave-assisted digestion procedures for biological samples with diluted nitric acid: identification of reaction products. Talanta 79:396–401
Gunduz S, Akman S (2013) Determination of lead in rice grains by solid sampling HR-CS GFAAS. Food Chem 141:2634–2638
Huang S, Jiang S, Sahayam AC (2014) Ultrasonic slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry for the determination of Cr, Fe, Cu, Zn and Se in cereals. Spetrochim Acta B 101:46–50
Khajeh M, Sanchooli E (2010) Optimization of microwave-assisted extraction procedure for zinc and iron determination in celery by Box–Behnken design. Food Anal Method 3:75–79
Khajeh M, Moghaddam ARA, Sanchooli E (2010) Application of Doehlert Design in the optimization of microwave-assisted extraction for determination of zinc and copper in cereal samples using FAAS. Food Anal Method 3:133–137
Krug FJ (2008) Métodos de preparo de amostras. Fundamentos sobre o preparo de amostras orgânicas e inorgânicas para análise elementar. CENA-USP, Piracicaba
Leal F, Catarino R, Pimenta A, Souto R (2009) Importância da especiação de metais na avaliação do seu impacto na saúde humana—desenvolvimento de metodologias voltamétricas com eletrodos de filme de bismuto. Revista de Faculdade de Ciências e Saúde 6:220–230
Leme ABP, Bianchi SR, Carneiro RL, Nogueira ARA (2014) Optimization of sample preparation in the determination of minerals and trace elements in honey by ICP-MS. Food Anal Method 7:1009–1015
Lenardão EJ (2003) “Green chemistry”—Os 12 princípios da química verde e sua inserção nas atividades de ensino e pesquisa. Quim Nov. 26:123–129
Lopes WL, Santelli RE, Oliveira EP, Carvalho MFB, Bezerra MA (2009) Application of multivariate techniques in the optimization of a procedure for the determination of bioavailable concentrations of Se and As in estuarine sediments by ICP OES using a concomitant metals analyzer as a hydride generator. Talanta 79:1276–1282
Macedo SM, Jesus RM, Garcia KS, Hatje V, Queiroz AFS, Ferreira SLC (2009) Determination of total arsenic and arsenic (III) in phosphate fertilizers and phosphate rocks by HG-AAS after multivariate optimization based on Box-Behnken design. Talanta 80:974–979
Mohd-Taufek N, Cartwright D, Davies M, Sumner R, Lee E, Whitfield K, Hewavitharana AK, Koorts P, Shaw PN (2016) The simultaneous analysis of eight essential elements in human milk by ICP-MS. Food Anal Method 9:2068–2075
Naseri M, Vazirzadeh A, Kazemi R, Zaheri F (2015) Concentration of some heavy metals in rice types available in Shiraz market and human health risk assessment. Food Chem 175:243–248
Ni Z, Tang F, Yu Q, Wang Z (2016) Determination of trace elements in camellia oil by vortex-assisted extraction followed by inductively coupled plasma mass spectrometry. Food Anal. Method 9:1134–1141
Nobrega JA, Araujo GCL, Gonzalez MH, Ferreira AG, Nogueira ARA (2002) Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. Spectrochim Acta Part B 57:2121–2132
Okada IA, Duran MC, Buzzo ML, Dovidauskas S, Sakuma AM, Zenebon O (2007) Validação e aplicação de metodologia analítica na determinação de nutrientes inorgânicos em arroz polido. Ciênc Tecnol Aliment 27:492–497
Oliveira A, Baccan N, Cadore S (2012) Evaluation of metal ions in rice samples: extraction and direct determination by ICP OES. J Braz Chem Soc 23:838–845
Oliveira RM, Antunes ACN, Vieira MA, Medina AL, Ribeiro AS (2016) Evaluation of sample preparation methods for the determination of As, Cd, Pb, and Se in rice samples by GF AAS. Microchem J 124:402–409
Santos WPC, Castro JT, Bezerra MA, Fernandes AP, Ferreira SLC, Korn MGA (2009) Application of multivariate optimization in the development of an ultrasound-assisted extraction procedure for multielemental determination in bean seeds samples using ICP OES. Microchem J 91:153–158
Santos WPC, Santos DCMB, Castro JT, Fernandes AP, Korn MGA (2013) Geographical characterization of beans based on trace elements after microwave-assisted digestion using diluted nitric acid. Food Anal Methods 6:1133–1114
Silva MJ, Paim APS, Pimentel MF, Cervera ML, Guardia M (2010) Determination of mercury in rice by cold vapor atomic fluorescence spectrometry after microwave-assisted digestion. Anal Chim Acta 667:43–48
Silva DG, Sacrminio IS, Anunciação DS, Souza AS, Silva EGP, Ferreira SLC (2013) Determination of the mineral composition of Brazilian rice and evaluation using chemometric techniques. Anal Methods 5:998–1003
AOAC SMPR (2012) 007: standard method performance requirements for determination of heavy metals in a variety of foods and beverages (2013. J AOAC Int 96
Teixeira AP, Quintella CM, Korn MGA, Fernandes AP, Castro MTPO (2012) Determinação de Mn e Zn em arroz empregando espectrometria de fluorescência de raios X de energia dispersiva. Quim Nov. 35:1133–1136
Todolí JL, Mermet JM (1999) Acid interferences in atomic spectrometry: analyte signal effects and subsequent reduction. Spetrochim Acta B 54:895–929
Vieira CR, Lopes CO, Ramos CS, Capobiango M, Silvestre MPC (2008) Extração enzimática das proteínas da farinha de arroz. Ciênc Tecnol Aliment 28:599–606
Walter M, Marchezan E, Avila LA (2008) Arroz: composição e características nutricionais. Ciência Rural 38:1184–1192
Wei YH, Zhang JY, Zhang DW, Luo LG, TH T (2014) Simultaneous determination of Se, trace elements and major elements in Se-rich rice by dynamic reaction cell inductively coupled plasma mass spectrometry (DRC-ICP-MS) after microwave digestion. Food Chem 159:507–511
Acknowledgments
The authors are grateful to the Fundação de Amparo a Pesquisa do Estado da Bahia (FAPESB, Brazil), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Petróleo Brasileiro S. A. (PETROBRAS, Brazil), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for providing grants, fellowships, and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Taiana B. Tarantino declares that he has no conflict of interest. Isa S. Barbosa declares that he has no conflict of interest. Daniel de C. Lima declares that she has no conflict of interest. Madson de G. Pereira declares that he has no conflict of interest. Leonardo S. G. Teixeira declares that he has no conflict of interest. Maria Graças A. Korn declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Tarantino, T.B., Barbosa, I.S., de C. Lima, D. et al. Microwave-Assisted Digestion Using Diluted Nitric Acid for Multi-element Determination in Rice by ICP OES and ICP-MS. Food Anal. Methods 10, 1007–1015 (2017). https://doi.org/10.1007/s12161-016-0658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0658-4