Abstract
Tungsten permanent modifier with coinjection of Pd(NO3)2 and W–Ru permanent modifiers are proposed for the direct and simultaneous determination of As, Bi, Pb, Sb, and Se (group 1) and Co, Cr, Cu, Fe, and Mn (group 2), respectively, in milk by graphite furnace atomic absorption spectrometry. The performance of modifiers was evaluated by means of thermal behavior of analytes, sensitivity, atomic signal profile, repeatability, graphite tube lifetime, and background intensity. An air-assisted pyrolysis step was necessary to quantitative elimination of the organic matter. After methods optimization, 14 commercial milk samples were analyzed. The found concentrations of As, Bi, Pb, Sb, Se, Co, and Cr were lower than their limit of detection (2.13, 2.21, 1.49, 1.63, 2.05, 1.0, and 1.2 μg L−1, respectively). Concentrations of Cu, Fe, and Mn were in the 1.58–5.74 μg L−1, 9.79–49.3 μg L−1, and 2.25–4.08 μg L−1 intervals, respectively. The limits of detection for Cu, Fe, and Mn were 1.7, 5.3, and 2.0 μg L−1, respectively. The accuracy of methods was checked after analysis of two milk standard materials. Results for Cr, Cu, Fe, Mn, Pb, and Mn were in agreement with certified values of SRMs at the 95% confidence level. Accuracy was also evaluated by addition–recovery tests and recoveries in the 86–127% range were obtained for all elements. The use of pretreat platform of graphite tubes with W or W–Ru allowed enlarging the lifetime of atomizer in 750 heating cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of trace heavy metals and nutrient elements in environmental, biological, and food samples is relevant for toxicology and healthy (Soylak et al. 2004, 2005). Inorganic contaminants are usually present in foodstuffs at trace levels, requiring then sensitive analytical techniques for their accurate determination. Among main techniques for elemental analysis are atomic fluorescence, electrothermal atomic absorption spectrometry, inductively coupled plasma mass spectrometry, inductively coupled plasma optical emission spectrometry, voltammetry, or neutron activation analysis (García et al. 2005; Rademeyer et al. 1995). Electrothermal atomic absorption spectrometry (ET AAS) is a suitable and widely used technique for elemental determination at trace levels due to its selectivity, relative simplicity, high sensitivity, and, mainly, its capability for direct determination of several analytes in a sort of complex matrices. The applicability of ET AAS has been further extended with the possibility of simultaneous and multielement determination. The direct and simultaneous determination is particularly advantageous for laboratories dealing with large-scale analysis of routine due to the minimum sample preparation and saved time (Volynsky and Wennrich 2001; Kopysc et al. 2003)
The ET AAS is a useful and powerful analytical technique for trace and ultratrace analysis of different samples such as urine and blood (Campillo et al. 2000; Luna and Campos 1999; Zhang et al. 2007). Conventional and permanent modifications for simultaneous ET AAS are similar to those occurring in monoelementar mode. However, the use of permanent modifier plus coinjection for real samples is mostly employed for monoelementar determination (Ortner et al. 2002; Correia et al. 2002; Welz et al. 1992) Simultaneous multielement determination is particularly challenging due to the necessity of careful optimization of the compromise conditions to be established, since the modifier and atomizer heating program are common for all analytes (Lima et al. 2004, Pereira et al. 2006; Freschi et al. 2006; Harnly and Radziuk 1995). This is not an easy task and makes the selection of the modifiers a critical step and, sometimes, a difficult job (Nomura et al. 2004). The use of a graphite platform coated with permanent modifier may improve the thermal behavior of analyte or lifetime of atomizer (Volynsky 2004). Coinjection of conventional modifier with samples may improve the performance of a permanent modifier. Tungsten or Zr-coated platform with coinjection of Rh solution was exploited for direct analysis of milk by graphite furnace atomic absorption spectrometry (Zanão et al. 2002). This strategy is interesting for analysis of complex samples that require better interaction between modifier and analyte and/or accelerate deabrasion of graphite tube (Freschi et al. 2006, 2008). This study reports on new methods for the direct and simultaneous determination of As, Bi, Pb, Sb, and Se (group 1) and Co, Cr, Cu, Fe, and Mn (group 2) in milk by ET AAS employing, respectively, W permanent modifier with coinjection of Pd(NO3)2 and W–Ru permanent modifiers, and transversely heated graphite atomizer and longitudinal Zeeman effect background correction.
Experimental
Instrumentation
A PerkinElmer SIMAA™ 6000 simultaneous multielement atomic absorption spectrometer, equipped with longitudinal Zeeman effect background corrector and AS-72 autosampler, was used (PerkinElmer Life and Analytical Sciences, Shelton, CT, USA). The end-capped PerkinElmer transversely heated graphite furnace atomizer (THGA™) with integrated platform was used throughout. PerkinElmer electrodeless discharge lamps were employed for As (193.7 nm), Se (196.0 nm), and Sb/Bi (217.6 nm/223.1 nm). A PerkinElmer Lumina™ hollow cathode lamp was used for Pb (283.3 nm), Co (242.5 nm), Cr (357.9 nm), Cu (324.8 nm), Fe (248.3 nm), and Mn (279.5 nm). Lamps were operated at following currents: 380 mA (As), 290 mA (Se), 380 mA (Sb/Bi), 10 mA (Pb), 30 mA (Co, Fe, Mn), and 25 mA (Cr, Cu). High-purity argon (99.999%, White Martins, São Paulo, Brazil) was used as the purge gas at a flow rate of 250 mL min−1. A PerkinElmer USS-100™ ultrasonic slurry sampler with titanium tip was used to guarantee the homogeneity of diluted milk samples during sampling.
Reagents and Analytical Solutions
High-purity water was obtained using a Millipore RiOs™ 5 reverse osmosis system. A Millipore Milli-Q™ academic deionizer system (resistivity 18.2 MΩ cm; Millipore Corporation, Bedford, MA, USA) and Suprapur® nitric acid (Merck, Darmstadt, Germany) were used to prepare all solutions.
Permanent modifier solutions (1.00 g L−1) were those used as stock solutions for W (H2WO4·2H2O; Merck) and Ru (RuCl3·3H2O; Fluka Gmbh, Buchs, Switzerland). Chemical modifier solution containing 0.05% (w/v) Pd + 0.03% (w/v) Mg(NO3)2 was prepared by appropriate dilution of 10 g L−1 Pd and 10 g L−1 Mg(NO3)2 stock solutions (Merck, Darmstadt, Germany).
Arsenic, Bi, Co, Cr, Cu, Fe, Mn, Pb, Sb, and Se stock standard solutions were prepared from 1 g L−1 solutions by diluting the Normex™ standards (Carlo Erba, Milan, Italy) in 1,000 mL water. Antimony(III) stock standard solution (1 g L−1) was prepared by dissolving 2.7385 g K(SbO)C4H4O6·1/2 H2O (Aldrich, St. Louis, MO, USA) in 1 M HCl. Multielement working standard solutions in the 5.0–50.0-mg L−1 concentration range were prepared daily by appropriate dilution of respective stock solutions.
All solutions were stored in high-density polypropylene bottles (Nalgene®, Rochester, NY, USA). Plastic bottles, autosampler cups, and glassware were cleaned by soaking in 10% (v/v) HNO3. Before using, all materials were rinsed thoroughly in deionized water.
Preparation of Platforms of Graphite Tube with Permanent Modifiers
Graphite tube platforms were coated with W and W–Ru by sequential deposition of 50 μL of 1.00 g L−1 Ru and W solutions onto platform and applying the program published elsewhere (Freschi et al. 2008). For Ru and W coating, the injection and program were run 10 and five times, respectively.
Sample Preparation
Milk samples were purchased at a local market in Araraquara city, São Paulo state, Brazil, and were diluted 1 + 9 (v/v) in 1% (v/v) nitric acid before analysis. This dilution was selected after taking into consideration the need for quantitative removal of carbonaceous residue from atomizer and required sensitivity for all analytes.
Procedure
Simultaneous determinations of As, Bi, Pb, Sb, and Se (group 1) and Co, Cr, Cu, Fe, and Mn (group 2) were carried out at the main line of each element. All measurements were carried out by injecting onto treated platforms 20 μL of samples plus 5 μL of 0.05% (w/v) Pd solution. The optimized heating program of atomizer is listed in Table 1. A first air-assisted pyrolysis step was used to help the oxidation/elimination of organic materials from atomizer. All measurements were carried out using the stabilized temperature platform furnace conditions including Zeeman effect background correction. All blanks, standards and samples were acidified to 1% (v/v) HNO3. Accuracy was checked after analysis of two milk standard reference materials (SRM 11549 and SRM 8435) from the National Institute of Standards and Technology (Gaithersburg, MD, USA). These SRMs were diluted (1:10 v/v) in 1.0% (v/v) HNO3. Accuracy was also evaluated by recovery tests using samples spiked 10 μg L−1 (analytes of group 1) and 25.0 μg L−1 (analytes of group 2). The limit of detection (LOD) and limit of quantification for all analytes were calculated according to the IUPAC recommendation (Currie 1999).
Results and Discussion
The selected analytes (group1: Sb, As, Bi, Pb, and Se; group 2: Co, Cu, Cr, Fe, and Mn) were selected taking into consideration their importance in toxicology and healthy studies. A systematic study for their simultaneous determination may result in a useful database for implementation of the internal standardization in atomic absorption spectrometry (Correia et al. 2004). The studies of electrothermal behaviors and direct and simultaneous determination of As, Bi, Pb, Sb, and Se and Co, Cr, Cu, Fe, and Mn in milk were done with W+Pd (group 1), W coating plus Pd coinjected, and W–Ru (group 2) permanent modifiers (Table 2).
Among the modifiers tested, the lowest characteristic masses for most elements (As, Co, Cu, Mn, and Se) were observed for W-coated platform with coinjection of Pd. Indeed, this modifier furnished the best precision and the narrowest peak profile for all elements. The main figures of merit were evaluated such as integrated absorbance, atomic peak profile (appearance time and restoration time to baseline), repeatability, and the W-coated with coinjection of Pd and W–Ru modifiers were selected because furnished the best sensitivity for As, Co Pb, Se, and Cr.
Evaluation of End-Capped Tubes on the Thermal Behavior of Analytes
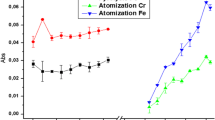
Initial tests with standard THGA showed an important reduction in atomic signals for Pb (50%) and Bi (70%) when the optimum atomization temperature for As and Se (2,200 °C) was used (Fig. 1). This was attributed to Pb and Bi losses by diffusion along the atomizer. The end cap design for THGA atomizers is interesting to improve sensitivity for volatile elements such as Bi and Pb and was evaluated to improve the sensitivity for Pb and Bi. Analysis of Fig. 1 showed the efficiency of the end cap configuration for Bi and Pb in comparison to standard THGA. The thermal behavior of elements in group 2 using end cap and standard configurations is depicted in Fig. 2. For this group, a slight improvement in Mn absorbance at 2,500 °C atomization temperature was observed when the end cap tube was used. For the group 2, both tube configurations could be used with similar analytical performance.
Evaluation of Milk Matrix on the Thermal Behavior of Analytes Using Permanent Modifiers
The air-assisted pyrolysis (step 3, Table 1) of the heating program of the atomizer was essential to eliminate all carbonaceous residues from the tube. It should be commented that the maximum pyrolysis temperature when air is used as auxiliary gas is 600 °C, otherwise the atomizer is ashed. So, the air must be removed previously the next pyrolysis at higher temperature. Two sequential pyrolysis steps (air–argon) were important to avoid the formation of these residues and improve precision and accuracy in the direct analysis of milk by ET AAS.
Preliminary tests involving sample dilution (1 + 4, 1 + 6, and 1 + 9, v/v) suggested that larger dilution favored the elimination of carbonaceous residues from atomizer without significant loss in sensitivity. Then, the dilution 1 + 9 (v/v) was adopted for further experiments.
The matrix effects were studied by building calibration curves in different medium: diluted nitric acid, nonfat milk, low-fat milk, and whole milk. A large difference in slopes between aqueous and milk calibration was observed, suggesting that matrix-matching calibration is mandatory. On the other side, slopes for calibration curves employing milk medium were close. Taking into consideration occurrence of clogging of the autosampler pipette, the matrix-matching calibration was done with nonfat milk for the direct and simultaneous determination of As, Bi, Pb, Sb, and Se and Co, Cr, Cu, Fe, and Mn in milks.
Simultaneous Determination of Analytes
The accuracy was also checked using two standard reference materials of milk (SRM 1549—nonfat milk powder; SRM 8435 whole-milk powder). Table 3 summarizes the found values of analytes, which are in agreement with certified values at 95% confidence level. SRMs have only certified values for Pb and Se. Accuracy was also evaluated by addition/recovery tests in different milk slurries containing 10 μg L−1 (analytes of group 1) and 25.0 μg L−1 (analytes of group 2). A volume of 20 μL of samples was injected into graphite tube W coating with injected 5 μg L−1 Pd solution for first group determination and W–Rh coating tube for the second group. The results showed recoveries within 86% and 108% for As, 88% and 115% for Bi, 90% and 120% for Pb, 94% and 120% for Sb, 93% and 116% for Se, 93% to 113% for Co, 115% and 127% for Cr, 88% and 107% for Cu, 93% and 116% for Fe, and 87% and 99% for Mn.
The method was then applied to the simultaneous determination of As, Bi, Pb, Sb, and Se and Co, Cr, Cu, Fe, and Mn in 14 different commercial milks was carried out using W+Pd (group 1) and W–Ru (group 2). Samples were prepared directly in the autosampler cups. The found concentrations of As, Bi, Co, Cr, Pb, Sb, and Se in samples were lower than the LOD. The content of Cu, Fe, and Mn varied from 1.58 to 5.74, 9.79 to 49.3, and 2.25–4.08 μg L−1 intervals, respectively. Limits of detection of the method, considering the dilution factor were 21 μg L−1 As, 22 μg L−1 Bi, 15 μg L−1 Pb, 16 μg L−1 Sb, 21 μg L−1 Se, 10 μg L−1 Co, 12 μg L−1 Cr, 17 μg L−1 Cu, 53 μg L−1 Fe, and 20 μg L−1 Mn. However, these LODs were lower than the maximum allowed level by Brazilian legislation. Tungsten and W–Ru permanent modifier provided an excellent lifetime graphite tube (about 750 firings). Relative standard deviations for all analytes using W+Pd and W–Ru were always <6%, indicating the good repeatability of the proposed method.
Conclusion
This work presents a new strategy for direct and simultaneous determination of As, Bi, Pb, Sb, and Se (group 1) and Co, Cr, Cu, Fe, and Mn (group 2) in milks samples by graphite furnace atomic absorption spectrometry. The use of W- or W–Ru-coated platforms allowed to enlarge the lifetime of graphite tubes atomizer in ca. three times in comparison to only conventional modifier. The “dilution and shoot” procedure is attractive for large-scale analysis of milk due to the minimum sample preparation involved.
References
Campillo N, Viñas P, López-García I, Hernández-Córdoba M (2000) Determination of arsenic in biological fluids by electrothermal atomic absorption spectrometry. Analyst 125:313–316
Correia PRM, Oliveira E, Oliveira PV (2002) Simultaneous determination of manganese and selenium in serum by electrothermal atomic absorption spectrometry. Talanta 57:527–535
Correia PRM, Oliveira PV, Neto JAG, Nóbrega JA (2004) Silver as internal standard for simultaneous determination of Cd and Pb in whole blood by electrothermal atomic absorption spectrometry. J Anal At Spectrom 19:917–922
Currie LA (1999) Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Anal Chim Acta 39:105–126
Freschi GPG, Freschi CD, Oliveira SR, Neto JAG (2006) Evaluation of Ir-based modifiers in the simultaneous determination of As, Bi, Pb, Sb, and Se in milk by graphite furnace atomic absorption spectrometry. Atom Spectros 27:179–185
Freschi GPG, Freschi CD, Neto JAG (2008) Evaluation of different rhodium modifiers and coatings on the simultaneous determination of As, Bi, Pb, Sb, Se and of Co, Cr, Cu, Fe Mn in milk by electrothermal atomic absorption spectrometry. Microchim Acta 161:129–135
García JCR, García JB, Latorre CH, Martín SG, Crecente RMP (2005) Comparison of palladium–magnesium nitrate and ammonium dihydrogenphosphate modifiers for lead determination in honey by electrothermal atomic absorption spectrometry. Food Chem 91:435–439
Harnly JM, Radziuk B (1995) Effect of furnace atomization temperatures on simultaneous multielement atomic absorption measurement using a transversely heated graphite atomizer. J Anal At Spectrom 10:197–206
Kopysc E, Bulska E, Wennrich R (2003) On the use of noble metals modifiers for simultaneous determination of As Sb and Se by electrothermal atomic absorption spectrometry. Spectrochim Acta B Atom Spectros 58:1515–1523
Lima EC, Brasil JL, Santos AHDP (2004) Determination of antimony in environmental samples by ETAAS using different permanent modifier. Microchim Acta 146:21–29
Luna AS, Campos RC (1999) Determination of Mn in whole blood and urine by graphite furnace AAS using different modifiers. Atom Spectros 20:108–112
Nomura CS, Correia PRM, Oliveira PV, Oliveira E (2004) W+Rh as permanent chemical modifier in simultaneous atomic absorption spectrometry: interference studies on As, Cd, Pb and Se determination. J Braz Chem Soc 15:75–82
Ortner HM, Bulska E, Rohr U, Schlemmer G, Weinbruch S, Welz B (2002) Modifiers and coatings in graphite furnace atomic absorption spectrometry—mechanisms of action (a tutorial review). Spectrochim Acta B Atom Spectros 57:1835–1853
Pereira LA, Amorim IG, Silva JBB (2006) Determination of cadmium, chromium and lead in marine sediment slurry samples by electrothermal atomic absorption spectrometry using permanent modifiers. Talanta 68:771–775
Rademeyer CJ, Radziuk B, Romanova N, Skaugset NP, Skogstad A, Thomassen Y (1995) Permanent iridium modifier for electrothermal atomic absorption spectrometry. J Anal At Spectrom 10:739–745
Soylak M, Tuzen M, Narin I, Sari H (2004) Comparison of microwave dry and wet digestion procedures for the determination of trace metal contents in spice samples produced in Turkey. J Food Drug Anal 12:254–258
Soylak M, Saracoglu S, Tuzen M, Mendil D (2005) Determination of trace metals in mushroom samples from Kayseri, Turkey. Food Chem 92:649–652
Volynsky AB (2004) Comparative efficacy of platinum group metal modifiers in electrothermal atomic absorption spectrometry. Spectrochim Acta B Atom Spectros 59:1799–1821
Volynsky AB, Wennrich R (2001) Comparative efficiency of Pd, Rh and Ru modifiers in electrothermal atomic absorption spectrometry for the simultaneous determination of As, Se and In in a sodium sulfate matrix. J Anal At Spectrom 16:179–187
Welz B, Schlemmer G, Mudakavi JR (1992) Palladium nitrate–magnesium nitrate modifier for electrothermal atomic absorption spectrometry. Part 5. Performance for the determination of 21 elements. J Anal At Spectrom 7:1257–1271
Zanão RA, Barbosa F Jr, Krug FJ, Abdalla AL (2002) Direct determination of selenium in whole blood by ETAAS using W–Rh-coated platform and co-injection of Rh as thermal stabilizer. Spectrochim Acta B Atom Spectros 57:291–301
Zhang L, Morita Y, Yoshikawa K, Isozaki A (2007) Direct simultaneous determination for ultratrace As, Se and Sb in river water with graphite-furnace atomic absorpion spectrometry by TiO2-slurry sampling. Anal Sci 23:365–369
Acknowledgments
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financially supporting this work, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for researchships to JAGN, and the Coordenadoria de Aperfeiçoamento de Pessol de Ensino Superior for fellowships to GPGF and CDF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freschi, G.P.G., Fortunato, F.M., Freschi, C.D. et al. Simultaneous and Direct Determination of As, Bi, Pb, Sb, and Se and Co, Cr, Cu, Fe, and Mn in Milk by Electrothermal Atomic Absorption Spectrometry. Food Anal. Methods 5, 861–866 (2012). https://doi.org/10.1007/s12161-011-9323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9323-0