Abstract
In this work, a novel 1-benzyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([BeMIM][Tf2N]) ionic liquid (IL) was used for extracting three organophosphorus pesticides (OPPs) (ethoprophos, fenitrothion, and phoxim) from tea samples by dispersive liquid–liquid microextraction (DLLME). The OPPs were detected by high-performance liquid chromatography (HPLC) with UV detector. Four [Tf2N]-based ILs were compared during the extraction process, and [BeMIM][Tf2N] showed the best performance. Acetonitrile was used as an extracting solvent in the extracting process and as a dispersant solvent in the DLLME process. Several parameters affecting the performance of the method including the selection of extraction solvent type, dispersant type, pH, extraction time, extraction temperature, salt addition, and centrifugation time were optimized. Under the optimal conditions, the enrichment factors of the analytes were in the range of 242.8–266.0, and the limit of detection was 0.1 μg/kg. The optimized method was also used for testing tea samples from different brands. The recoveries of the analytes were between 78.8 and 102.2%, with the RSDs were in the range of 0.9–4.5. The developed method is low-cost, time-saving, and reagent-saving; it is a promising and efficient sample technique for solid-sample preparation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tea is becoming a popular drink worldwide due to its unique flavor and the benefit to human health. During the process of tea cultivation and storage, to ensure the normal growth and storage of it, inevitably, tea farmers need to use various pesticides to reduce the harm of pests, weeds, and viruses to tea [1].
Organophosphorus pesticides (OPPs) are a class of broad-spectrum insecticides extensively used in agricultural activities including tea cultivation for pest inhibition. The phosphoryl group in OPPs could covalently bond to the active part of the acetylcholine enzyme to form a phosphorylated cholinesterase, which inhibits the activity of the acetylcholinesterase and makes it lose its ability to decompose acetylcholine, resulting in a large accumulation of acetylcholine in the organism, and ultimately damages the central nervous system and cholinergic nerves [2]. OPPs are such highly effective pesticides that their use accounts for approximately 50% of the pesticides used worldwide [3]. However, the soil and water pollutions caused by the abuse of OPPs and the difficult degradation of it after extensive use is threatening the environmental security and human life. After entering human body through various pathways, OPPs would cause brain dysfunction [4], neurotoxic [5], respiratory system damage [6], and cancer [7]. Therefore, it is necessary to develop a rapid, simple, and effective method for OPPs’ extraction and detection in tea samples.
For solid and complex matrix samples, the pretreatment of the samples to maximize the extraction of the analytes into a neat solvent is an essential step prior to analysis. Liquid–liquid extraction (LLE) and solid-phase extraction (SPE) are the most basic and widely used extraction methods. However, the LLE procedure usually requires a large volume of organic solvent and is time-consuming and expensive to operate [8]. SPE needs less amounts of solvent, but it is also cumbersome to perform [9]. In the past two decades, due to less reagent consumption, microextraction technology such as solid-phase microextraction (SPME) [10, 11], liquid-phase microextraction (LPME) [12, 13], and dispersive liquid–liquid microextraction (DLLME) [14, 15] has emerged and is quickly accepted and widely used by researchers.
Among them, DLLME is a more popular microextraction technique, which has the advantages such as simplicity of operation, rapid extraction, low time and economic costs, and high enrichment factor [16]. Therefore, DLLME has been successfully used for the extraction and determination of various compounds such as benzophenone [17], metal [18], fungicides [19], phosphodiesterase [20], acrylamide [21], nitrosamines [8], and so on. Chloroform and dichloromethane are the mostly used extractant in the previous reports on DLLME, but they are volatile, highly toxic, and flammable. Since zhou et al. [22] developed the DLLME using ionic liquid (IL) as extractant, in the past 10 years, ILs have been frequently used as extraction solvent in DLLME procedure instead of conventional organic solvent due to the unique properties of ILs including high extraction efficiency, low vapour pressure, high thermal stability, and the controllable synthesis [23, 24]. Thus, IL-based dispersive liquid–liquid microextraction (IL-DLLME) combines both the superiority of ILs and DLLME and has been successfully applied for the extraction of glucocorticoid [25], heavy metal iron [26], estrogens [27], benzodiazepine [28], triazines and sulfonamides [29], and so on.
ILs’ family is large, and different ILs have different solubilities and extraction properties. The type of the anion and the length of the alkyl chain of the cation are two factors influencing the solubility and miscibility of an IL. In recent years, bis[(trifluoromethyl)sulfonyl]imide ([Tf2N])-anionic-based ILs have attracted great attention of researchers. [Tf2N]-based ILs with different cations have been applied for the extraction of organic compound [30], recovery of metal ions [31, 32], absorbing water from the air [33], CO2 separation [34,35,36], and lubricant [37]. Since the size of [Tf2N] anion is bigger than [PF6] and [BF4], [Tf2N] anion-based ILs exhibit higher hydrophobicity and capacity [38], which may lead to different extraction efficiencies.
ILs have been used in the extraction process of organic matters from tea samples, for instance, Yang et al. applied IL of 1-dodecyl-3-methylimidazolium bromide–attapulgite (C12MIM-ATP) and SPE method for extracting pyrethroid residues in tea drinks, and good linearity (2–500 μg/L) and limits of detection (LODs) of 0.6 μg/L were obtained [39]. Hu et al. employed the dispersive solid-phase extraction (DSPE) method with calixarene IL for the extraction of flavonoids in green tea, and the LOD of 0.15–0.75 μg/L as well as the recoveries of 92.5–104.8% was obtained [40]. Yang et al. used ionic liquid (IL)-modified beta-cyclodextrin/attapulgite (beta-CD/ATP) combined DSPE method to extract benzoylurea insecticides from tea beverages, the enrichment factors (EF) was 112–150, linearity range was 5–500 μg/L, and LOD was 0.12–0.21 μg/L [41].
There are also some studies applied IL-DLLME for extraction organics and heavy metal ions from tea samples. Wang et al. used [N8881][PF6] IL coupled with DLLME for benzoylurea insecticides extraction from water and tea beverage samples, the linearity range 2–500 μg/L and the LOD varied between 0.29 and 0.59 μg/L [42]. Werner et al. proposed trioctylmethylammonium thiosalicylate (TOMATS) IL-based DLLME method to extract cadmium (II), cobalt (II), and lead (II) ions in tea samples with the recovery was 90–104% and the LOD was 2–13 μg/L [43]. In recent years, IL based on [Tf2N] anion has also been used as extractant. Yang et al. reported the usage of 1-octyl-2,3-dimethylimidazolium bis(trifluoromethanesulfonimide [OMMIM][Tf2N] IL-DLLME for the extraction of acaricides from tea infusions, the LOD was 0.44–1.0 μg/L, and the recovery was 83–104.4% [44]. It can be seen that [Tf2N]-based ILs are promising extractants in the application of DLLME method. As discussed above, with the same [Tf2N] anion, ILs would exhibit different extraction properties when the cation is different. Therefore, the research on the extraction ability of organics from tea samples based on [Tf2N]-ILs with different cations is expected.
In this paper, four ILs of 1-benzyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([BeMIM][Tf2N]), 1,3-dibutylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([BBIM][Tf2N]), 1,3-dihexylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([HHIM][Tf2N]), and 1-octyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([OMIM][Tf2N]) were used as extractant and combined with DLLME to extract three OPPs (ethoprophos, fenitrothion, and phoxim) from tea samples. The structures of ILs and OPPs are shown in Figs. 1 and S2, respectively. Several parameters affecting the performance of the method were optimized including the selection of extraction solvent type, dispersant type, pH, extraction time, extraction temperature, salt addition, and centrifugation time.
Experimental
Reagents, Standards, and Materials
Organophosphorus pesticides (OPPs) of ethoprophos, fenitrothion, and phoxim standards were purchased from Cerilliant Corporation (Texas, USA). Acetonitrile and methanol (HPLC grade) were obtained from Tianjin Siyou Fine Chemicals Co., Ltd. (Tianjin, China). ILs of [BeMIM][Tf2N], [BBIM][Tf2N], [HHIM][Tf2N] [OMIM][Tf2N] used in this work were prepared in the laboratory. The detailed synthesis process was shown in the Support Information, and the 1H-NMR spectrum of [BeMIM][Tf2N] is shown in Fig. S1. Double-distilled water was prepared for the use of aqueous solution. Tea samples were purchased from the local market (Wuhan, China). Standard solutions of the OPPs with the concentration of 1 g/L were prepared by dissolving each OPP standard (0.0100 g) into 10.0 mL acetonitrile and stored at 4 °C. The working solution was prepared by diluting standard solutions to 0.02 g/L with acetonitrile.
Apparatus
HPLC analysis was carried out by Shimadzu HPLC system which equipped with an LC-10AT pump (Shimadzu, Japan), an SPD-10A UV–Vis detector (Shimadzu, Japan), a Shimadzu VP-ODS column (150 mm × 4.6 mm i.d., 5 μm), and a Rheodyne 7725i six-way valve injector with 20 μL sample loop (Rheodyne, Rohnert Park, CA, USA). The mobile phase was the mixture of methanol and water (70/30, v/v) with the flow rate of 1 mL/min. The wavelength was set at 254 nm. An 80-1 centrifuge (Huafeng Instrument Co. Ltd., Jintan, China) was used for centrifuging.
Extraction Procedure
Tea sample extraction (step 1):
Tea sample was to grinded into fine powder and 1 g tea sample was put in a 50 mL beaker and sprayed with 10 μL working solution of OPPs, then 5 mL of acetonitrile containing 155 μL [BeMIM][Tf2N] IL was added in the spiked tea sample. After that, the mixed solution was sonicated for 10 min. During this process, OPPs were extracted from tea powder into the acetonitrile phase. Next, the above mixed solution was centrifuged for 5 min to separate residue tea powder and acetonitrile solution. Then, a clear acetonitrile solution was obtained.
DLLME procedure (step 2):
1.0 mL acetonitrile solution containing OPPs and [BeMIM][Tf2N] was taken and rapidly injected into a 10 ml centrifuge tube containing 5 mL double-distilled water. Then, a cloudy solution was formed in the centrifuge tube. Next, the above solution was immediately centrifuged for 5 min. Finally, 5 μL of sedimentary facies formed at the bottom of the centrifuge tube was taken and injected to HPLC system for analysis.
During the process, acetonitrile plays dual function, which acts as an extraction solvent in step 1 and as a dispersant solvent in step 2.
Enrichment Factor and Extraction Recovery
Enrichment factor (EF) and extraction recovery (ER) were the two evaluating indicators of this developed method. They were calculated by the equations as follows:
where \(C_{0}\) and \(C_{\text{sed}}\) present the original concentration and the concentration in sedimentary facies of the OPPs, respectively. \(V_{\text{aq}}\) and \(V_{\text{sed}}\) are the volumes of aqueous solution and IL sedimentary facies obtained after centrifugation. The relative recovery (RR) was used in tea beverage samples analysis and it was calculated by the equation as follows:
where Cdetected, Creal, and Cadd represent the detected concentration of the OPPs after a known amount of standard was added into the sample, the real concentration of OPPs in the sample, and the concentration of the spiked known amount of standard in the sample, respectively. All experimental data obtained were the average of three repetitions in each turn.
Results and Discussion
Optimization of the Proposed Method
Selection of Ionic Liquid Type
IL acts as an extractant in the extraction process. Thus, the type of IL is a significant factor that will influence the final extraction results because of their different extraction abilities. Therefore, the choice of IL is very important. In this work, four different kinds of ILs based on the same [Tf2N] anion and different cations were selected for the enrichment of OPPs in tea sample. The ILs were [BeMIM][Tf2N], [BBIM][Tf2N], [HHIM][Tf2N], and [OMIM][Tf2N], respectively. In addition, the structures of them are shown in Fig. S2. To select the most appropriate ionic liquid, the same volume (155 μL) of these ILs was used in extracting process. The results are shown in Fig. 2. It is clear that when using [BeMim][Tf2N], the EF reached about 260, which is the highest compared with other ILs. The EF of [BBIM][Tf2N] showed a little lower than [BeMim][Tf2N]. Both [HHIM][Tf2N] and [OMIM][Tf2N] showed very low EF values, while the ER of them were higher than [BeMim][Tf2N] and [BBIM][Tf2N], but the gap was not significant. The different results may due to the different structures of them. As shown in Fig. S2, The cation of [BeMIM][Tf2N] is an imidazole functional group substituted with a benzyl group, which is different from other ILs. In addition, the bulkier side benzyl substituent group reduces the rotational freedom of the molecules, which leads to high viscosity and hydrophobicity for benzyl-substituted imidazole-Tf2N IL, resulting in stronger extraction performance. On one hand, the high hydrophobicity made the volume of [BeMIM][Tf2N] IL in sedimentary facies larger than the other ILs when using the same volume of IL, resulting in a higher ER value. On the other hand, the unique conjugated system of the benzene ring in [BeMIM][Tf2N] IL could interact with the target molecule to enhance its extraction ability. Therefore, a satisfactory and superior extraction effect could be obtained using [BeMIM][Tf2N] IL. Considering that the EF of [BeMIM][Tf2N] was highest and the ER of it was also good, thus, [BeMim][Tf2N] was chosen as extractant in the DLLME process in this work.
Selection of the Volume of [BeMIM][Tf2N]
The amount of IL is a key factor that can affect the extraction efficiency. To select the optimum amount of it for using to extract OPPs, different volumes of [BeMIM][Tf2N] from 125 to 175 μL in 5 mL acetonitrile for ultrasonic extraction, namely, 25–37 μL of it in 1.0 mL acetonitrile in DLLME, were investigated, and the results are shown in Fig. 3. It can be seen from Fig. 4 that extraction recovery (ER) of all OPPs increased with the increase of the volume of IL extraction, while enrichment factor (EF) showed an opposite trend. That can be explained that on one hand, when the IL volume is large, it cannot be effectively dispersed during the DLLME process, resulting in the decrease in the efficiency of the distribution of OPPs from the acetonitrile phase to the IL phase, thereby the EF value was lower, on the other hand, a large volume of IL in turn increases the volume of the sedimentary phase, resulting in the increasing value of ER. Considering both the EF and ER results together, volume of 31 μL [BeMIM][Tf2N] was selected for the DLLME procedure, that is, 155 μL of it was used for ultrasonic extraction.
Selection of Extraction Solvent
In this proposed method, the selection of extraction solvent in the first stage is important. Not only is it required to adequately extract organic matter from the solid matrix, but also it is required to be miscible in both IL and aqueous phase. Acetonitrile is a good choice which can avoid extracting other matters from the tea sample, and the high boiling point of acetonitrile makes it stable during the ultrasonic extraction process; therefore, acetonitrile was selected as an extraction solvent in the first extracting stage. The volume of acetonitrile would affect the dispersion of IL in the mixture, and ultimately would affect the values of both EF and ER; therefore, it is necessary to optimize the volume of acetonitrile. Thus, the volumes of acetonitrile from 2 to 6 mL which contained 155 μL [BeMIM][Tf2N] (namely, 0.4–1.2 mL of it which contained 31 μL [BeMIM][Tf2N] in DLLME procedure) were investigated for OPPs’ extraction from tea sample. The results are shown in Fig. 4. It can be seen that EF value enhanced with the volume of acetonitrile increased; on the contrary, ER decreased. The reason was that even though [BeMIM][Tf2N] IL was highly hydrophobic, it could be completely dissolved in acetonitrile. When the volume of acetonitrile was increased, the dispersion effect is enhanced during DLLME process. [BeMIM][Tf2N] IL was present in finer droplets, and its solubility was increased in acetonitrile/water solution. As a result, the sedimentary facies volume of [BeMIM][Tf2N] was reduced, and the EF value was increased, but the ER value was decreased. To ensure that both EF and ER values were high, 1.0 mL acetonitrile was selected for the DLLME process, namely, 5 mL of it was used for the extracting process.
Effect of Ultrasonic Time
Ultrasonic time decides the amount of OPPs transferring from tea sample to acetonitrile solution thus affects the EF and ER. Therefore, ultrasonic time from 1 to 20 min was studied. The results are displayed in Fig. 5. It can be seen from Fig. 5 that with the ultrasonic time increased from 1 to 10 min, EF decreased and ER increased. This is because short ultrasonic time would lead to the insufficient extraction of OPPs, while the long ultrasonic time would cause higher temperature of the extract solvent, causing the volatilization of acetonitrile and reducing the extraction efficiency. The changes in EF and ER values were not obvious when ultrasonic time increased from 10 to 20 min. Thus, the ultrasonic time was chosen to be 10 min.
Effect of pH of the Water in DLLME Process
In the DLLME stage, acetonitrile solution containing [BeMIM][Tf2N] ionic liquid was added to 5 mL double-distilled water, and water phase played a part in separating ionic liquid and dispersant through centrifugation process. To investigate the influence of pH of the redistilled water on the EF and ER, different pH values of the water from 3 to 8 were tuned and studied. The results are displayed in Fig. 6. The pH value of redistilled water was 6.1, and at that point, both the EF and ER were highest. That may because different pH values have an effect on the form of the analytes. It can be clearly seen that EF and ER were optimum when the pH of redistilled water was not tuned.
Effect of the Water Temperature in DLLME Process
The temperature of the water may have an influence on the solubility of analytes, extractant, and dispersant, thereby affecting the final EF and ER. Therefore, during the DLLME process, the water temperature in the range of 15–50 °C was investigated. The results are presented in Fig. 7. It can be observed that the temperature changes in the range of 15–50 °C did not have dramatic effect on EF and ER. This indicated that [BeMIM][Tf2N] had stable extraction performance over a wide temperature range. At 25 °C, IL showed the most stable and highest extraction capacity for OPPs; therefore, the water temperature was set at 25 °C.
Effect of Saline Concentration of the Water in DLLME Process
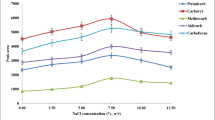
Salt concentration can change the ionic strength in aqueous solution, thereby affecting the extraction efficiency. In this work, the effects of different concentrations of NaCl (1–20%, w/v) in water during the DLLME process on EF and ER were investigated. The results are shown in Fig. 8. It can be seen that as the salt concentration increased from 0 to 1%, EF increased and ER decreased. With the salt concentration increased from 3 to 20%, EF decreased sustainedly. While, ER increased when salt concentration changed from 1 to 3%, and then decreased when salt concentration changed from 3 to 20%. It can be observed that low concentration of salt may reduce the volume of the sedimentary facies and enhance the EF; high concentration of it may reduce both EF and ER. Because the addition of salt affects solubility of ionic liquid in water, and changes the volume of the sedimentary facies, causing changes in EF and ER. To obtain both high EF and ER, salt was not added in water during the DLLME process.
Effect of Centrifugation Time in DLLME Procedure
Centrifugation was used to separate ionic liquid phased from dispersant (acetonitrile) and water phase. Centrifugation time will influence the volume of sedimentary facies and then affect the extraction efficiency. Thus, centrifugation time in the range of 2–15 min was investigated. The results are displayed in Fig. 9. It can be observed from Fig. 9 that with the centrifugation time increased from 2 to 5 min, EF decreased and ER increased. Continue to increase the centrifugation time from 5 to 15 min, both EF and ER changed little. That may because the long centrifugation time increased the temperature of the mixed solution and the solubility of ionic liquid enhanced, causing the volume of sedimentary facies decreased. To obtain both high EF and ER, centrifugation time was set to be 5 min.
Evaluation of Method Performance
To evaluate the proposed method for determining OPPs pesticides from tea samples, parameters including linearity, repeatability, and limits of detection (LOD) were obtained and investigated through a series of experiments under the optimized conditions. Table 1 shows the results. The linearity range was in the range of 1–1000 μg/kg for all the analytes. The precision of this method was carried out by five time extractions and extracting spiked tea samples at three different concentration levels of 50, 200, and 500 μg/kg. The relative standard deviations (RSDs) were between 2.4 and 4.6% (n = 5). Based on a signal-to-noise ratio (S/N) of 3, the LOD was 0.1 μg/kg for all analytes. These results indicated that this proposed method has high sensitivity and reliability, and can be used for the extraction and detection of OPPs’ pesticides from tea samples.
Analysis of Tea Samples
The method was applied to detect OPPs from different tea samples with different brands. The recoveries of the methods were determined by adding three different concentrations of OPPs (50, 200, and 500 μg/kg) according to the national standards of the People’s Republic of China (GB 2763–2016). The results are shown in Table 2. The experimental results show that the recoveries of the three tea samples are ranged between 76.4 and 102.2%, with the RSD between 0.9 and 4.5, which indicates that this method can be used accurately and reliably for the extraction and determination of the actual tea samples. The chromatograms of the tea sample spiked 200 μg/kg of the three OPPs before and after extraction are shown in Fig. 10. The analytes cannot be detected without the DLLME process, while after the [BeMIM][Tf2N]-DLLME process, OPPs can be effectively detected.
Comparison of this Method with Previous Works
The extraction performance of the [BeMIM][Tf2N] IL-DLLME method proposed in this work was compared with previous works, and is summarized in Table 3. It can be seen that the method in the concentration step proposed in this paper has the advantages of short operation time, high enrichment factor, less volume of IL used in DLLME process and lower LOD, which could support the application of [BeMIM][Tf2N] IL for organics extraction in tea samples.
Conclusions
In this work, [Tf2N] anion-based ionic liquids were applied and compared for extracting OPPs from tea samples through dispersive liquid–liquid microextraction process. In this proposed method, [BeMIM][Tf2N] was first and successfully used to extract OPPs. 5 mL acetonitrile/IL mixed solution was applied to extract OPPs and then was used in the DLLME step. Various factors that may affect the extraction results were investigated, and real tea samples were also analyzed. The results demonstrate that this proposed method has high sensitivity and reliability, good recoveries, wider LR, lower LOD, and RSD. The results were satisfactory, and due to the simplified operating procedures, this proposed method is promising for OPPs analysis in tea samples or other solid matrix. Extraction with less organic solvent is still the focus of further research.
References
Yang M, Gu Y, Wu X, Xi X, Yang X, Zhou W, Zeng H, Zhang S, Lu R, Gao H, Li J (2018) Food Chem 239:797–805. https://doi.org/10.1016/j.foodchem.2017.06.080
Li D, He M, Chen B, Hu B (2019) J Chromatogr A 1583:19–27. https://doi.org/10.1016/j.chroma.2018.11.012
Covantes-Rosales CE, Trujillo-Lepe AM, Díaz-Reséndiz KJG, Toledo-Ibarra GA, Ventura-Ramón GH, Ortiz-Lazareno PC, Girón-Pérez MI (2019) Fish Shellfish Immun 84:189–195. https://doi.org/10.1016/j.fsi.2018.10.002
Sheih P, Jan CR, Liang WZ (2019) Toxicology. DOI. https://doi.org/10.1016/j.tox.2019.02.004
Alfonso M, Duran R, Fajardo D, Justo L, Faro LRF (2019) Neurochem Int 124:130–140. https://doi.org/10.1016/j.neuint.2019.01.001
Liu T, Xu S, Lu S, Qin P, Bi B, Ding H, Liu Y, Guo X, Liu X (2019) Sci Total Environ 651:2247–2268. https://doi.org/10.1016/j.scitotenv.2018.10.087
Martinez-Morcillo S, Perez-Lopez M, Soler-Rodriguez F, Gonzalez A (2019) Toxicol In Vitro 54:89–97. https://doi.org/10.1016/j.tiv.2018.09.011
Lu S, Wu D, Li G, Lv Z, Gong P, Xia L, Sun Z, Chen G, Chen X, You J, Wu Y (2017) Food Chem 234:408–415. https://doi.org/10.1016/j.foodchem.2017.05.032
Asghari A, Fahimi E, Bazregar M, Rajabi M, Boutorabi L (2017) J Chromatogr B 1052:51–59. https://doi.org/10.1016/j.jchromb.2017.03.012
Liu F, Yang X, Wu X, Xi X, Gao H, Zhang S, Zhou W, Lu R (2018) Food Chem 268:485–491. https://doi.org/10.1016/j.foodchem.2018.06.105
Hatamluyi B, Es’haghi Z (2017) J Electroanal Chem 801:439–449. https://doi.org/10.1016/j.jelechem.2017.08.032
Biata NR, Nyaba L, Ramontja J, Mketo N, Nomngongo PN (2017) Food Chem 237:904–911. https://doi.org/10.1016/j.foodchem.2017.06.058
López-López JA, Pirkwieser P, Leyma R, Kandioller W, Krachler R, Keppler BK, Jirsa F, Moreno C (2018) J Clean Prod 201:22–27. https://doi.org/10.1016/j.jclepro.2018.08.008
Altunay N (2018) Lwt 93:9–15. https://doi.org/10.1016/j.lwt.2018.03.021
Cacho JI, Campillo N, Vinas P, Hernandez-Cordoba M (2018) J Chromatogr A 1559:95–101. https://doi.org/10.1016/j.chroma.2017.12.059
Li Y, Hu J, Liu W, Jin L, Zhou P, Zhang Y, Zhang B, Dahlgren RA, Wang X, Zhou Y (2019) Talanta 195:785–795. https://doi.org/10.1016/j.talanta.2018.11.106
Wang H, Hu L, Liu X, Yin S, Lu R, Zhang S, Zhou W, Gao H (2017) J Chromatogr A 1516:1–8. https://doi.org/10.1016/j.chroma.2017.07.073
Peng B, Chen G, Li K, Zhou M, Zhang J, Zhao S (2017) Food Chem 230:667–672. https://doi.org/10.1016/j.foodchem.2017.03.099
Pastor-Belda M, Garrido I, Campillo N, Vinas P, Hellin P, Flores P, Fenoll J (2017) Food Chem 233:69–76. https://doi.org/10.1016/j.foodchem.2017.04.094
Campillo N, Marin J, Fenoll J, Garrido I, Lopez-Garcia I, Hernandez-Cordoba M, Vinas P (2017) Talanta 174:638–644. https://doi.org/10.1016/j.talanta.2017.06.076
Zokaei M, Abedi AS, Kamankesh M, Shojaee-Aliababadi S, Mohammadi A (2017) Food Chem 234:55–61. https://doi.org/10.1016/j.foodchem.2017.04.141
Zhou Q, Bai H, Xie G, Xiao J (2008) J Chromatogr A 1188:148–153. https://doi.org/10.1016/j.chroma.2008.02.094
Wang H, Liu C, Huang X, Jia C, Cao Y, Hu L, Lu R, Zhang S, Gao H, Zhou W, Xu D (2018) New J Chem 42:8791–8799. https://doi.org/10.1039/c7nj04356b
Amado Alviz PL, Alvarez AJ (2017) J Clean Prod 168:1614–1624. https://doi.org/10.1016/j.jclepro.2017.02.107
Abujaber F, Corps Ricardo AI, Rios A, Guzman Bernardo FJ, Rodriguez Martin-Doimeadios RC (2019) J Pharmaceut Biomed 165:141–146. https://doi.org/10.1016/j.jpba.2018.12.001
Sadeghi S, Sarrafi N (2018) J Iran Chem Soc 15:1913–1920. https://doi.org/10.1007/s13738-018-1388-x
Merib J, Spudeit DA, Corazza G, Carasek E, Anderson JL (2018) Anal Bioanal Chem 410:4689–4699. https://doi.org/10.1007/s00216-017-0823-7
De Boeck M, Dehaen W, Tytgat J, Cuypers E (2018) J Forensic Sci 63:1875–1879. https://doi.org/10.1111/1556-4029.13778
Chatzimitakos TG, Pierson SA, Anderson JL, Stalikas CD (2018) J Chromatogr A 1571:47–54. https://doi.org/10.1016/j.chroma.2018.08.013
Sas OG, Domínguez I, Domínguez Á, González B (2019) J Chem Thermodyn 131:159–167. https://doi.org/10.1016/j.jct.2018.11.002
Sun T, Xu C, Fu J, Chen Q, Chen J, Shen X (2017) Sep Purif Technol 188:386–393. https://doi.org/10.1016/j.seppur.2017.07.055
Onghena B, Borra CR, Van Gerven T, Binnemans K (2017) Sep Purif Technol 176:208–219. https://doi.org/10.1016/j.seppur.2016.12.009
Ramenskaya LM, Grishina EP, Kudryakova NO (2018) J Mol Liq 272:759–765. https://doi.org/10.1016/j.molliq.2018.10.005
Mannan HA, Mohshim DF, Mukhtar H, Murugesan T, Man Z, Bustam MA (2017) J Ind Eng Chem 54:98–106. https://doi.org/10.1016/j.jiec.2017.05.022
Karousos DS, Vangeli OC, Athanasekou CP, Sapalidis AA, Kouvelos EP, Romanos GE, Kanellopoulos NK (2016) Chem Eng J 306:146–154. https://doi.org/10.1016/j.cej.2016.07.040
Wang Y, Liu X, Kraslawski A, Gao J, Cui P (2019) J Clean Prod 213:480–490. https://doi.org/10.1016/j.jclepro.2018.12.180
Battez AH, Ramos D, Blanco D, González R, Fernández-González A, Viesca JL (2017) Tribol. Lett 66:99. https://doi.org/10.1007/s11249-017-0964-z
Booth RS, Annesley CJ, Young JW, Vogelhuber KM, Boatz JA, Stearns JA (2016) Phys Chem Chem Phys 18:17037–17043. https://doi.org/10.1039/c6cp02657e
Yang X, Lin X, Mi Y, Gao H, Li J, Zhang S, Zhou W, Lu R (2018) J Chromatogr B 1089:70–77. https://doi.org/10.1016/j.jchromb.2018.04.043
Hu K, Qiao J, Wu X, Yang H, Huang Y, Zhang S (2018) Microchem J 143:39–46. https://doi.org/10.1016/j.microc.2018.07.029
Yang M, Wu X, Xi X, Zhang P, Yang X, Lu R, Zhou W, Zhang S, Gao H, Li J (2016) Food Chem. 197(Pt B):1064–1072. https://doi.org/10.1016/j.foodchem.2015.11.107
Wang H, Hu L, Li W, Yang X, Lu R, Zhang S, Zhou W, Gao H, Li J (2017) Talanta 162:625–633. https://doi.org/10.1016/j.talanta.2016.10.035
Werner J (2016) J Sep Sci 39:1411–1417. https://doi.org/10.1002/jssc.201501200
Yang M, Zeng H, Wu X, Yang X, Zhou W, Zhang S, Lu R, Li J, Gao H (2016) RSC Adv 6:111982–111992. https://doi.org/10.1039/c6ra22353b
Faraji M, Noorani M, Sahneh BN (2017) Food Anal Method 10:764–772. https://doi.org/10.1007/s12161-016-0635-y
Wang J, Huang S, Wang P, Yang Y (2016) Food Control 67:278–284. https://doi.org/10.1016/j.foodcont.2016.03.015
Zeng H, Yang X, Yang M, Wu X, Zhou W, Zhang S, Lu R, Li J, Gao H (2017) J Sep Sci 40:3513–3521. https://doi.org/10.1002/jssc.201700464
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 71871173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, W., Quan, J. A Novel Ionic Liquid of [BeMIM] [Tf2N] for Extracting Pesticides Residues in Tea Sample by Dispersive Liquid–Liquid Microextraction. Chromatographia 83, 41–51 (2020). https://doi.org/10.1007/s10337-019-03819-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03819-5