Abstract
An analytical method for the simultaneous determination of residues of five insecticides and two fungicides in cabbage was developed and validated. Pesticide residues were extracted from the samples using QuEChERS extraction and the analysis was performed by liquid chromatography triple quadrupole mass spectrometry (LC-MS/MS). The performance of the method was investigated in terms of accuracy, precision, linearity, limit of detection (LOD), and limit of quantification (LOQ). The average recoveries ranged from 80 to 110 % with RSDr ≤ 16 % for all the analytes at four fortification levels of 0.01, 0.05, 0.10, and 0.20 mg/kg. The LOD values ranged from 0.001 to 0.003 mg/kg, and the LOQ for all the analytes was set at 0.01 mg/kg. The linearity was very good and coefficient of determination was ≥ 0.997 for all the analytes when matrix-matched calibration standards were used. Matrix effect was estimated and it was found prominent for cabbage with cypermethrin and deltamethrin (+49 and +97 %, respectively). The method was applied very satisfactorily for the residue analysis of 132 fresh cabbage samples collected from different market places in Thessaloniki, Greece. Among the analyzed samples, 41 (31 % of the total no. of samples) had pesticide residues, of which, two had multiple pesticide residues and 39 had a single pesticide residue. Only one sample was found contaminated with deltamethrin at a level above the European Union maximum residue levels (EU-MRLs). Variability of pesticide residues originated from the samples of cabbage purchased from different market places of Thessaloniki in Greece were also estimated in this study. The estimated variability factors ranged from 1.00 to 6.75 and the average VFs was 5.00.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cabbage is a member of the Brassica family, also known as cruciferous vegetables. Cabbage is an abundant source of vitamin C and very rich in fiber. One of their most important celebrated benefits to health is their powerful antioxidant quality. It also helps to prevent constipation, stomach ulcers, headaches, obesity, skin disorders, eczema, jaundice, scurvy, rheumatism, arthritis, gout, eye disorders, heart diseases, aging, and Alzheimer’s disease (Organic Facts 2015). However, it can be a prominent source of different harmful substances like pesticides resulting from the indiscriminate use, overuse, and misuse of pesticides. On the other hand, pesticides play a key role to control the insect pests and diseases and to increase the production.
The presence of pesticide residues in fruits and vegetables and especially in cabbage is a major concern for the consumer’s and particularly for the children as they consume a higher portion of fruits and vegetables in relation to their body weight and more susceptible to chemicals since they are in the early development stage. In order to protect the consumer’s health and also fulfill the consumer’s demand of safe food, pesticides should be used following good agricultural practice (GAP) and it is also necessary to consider the issue of variability of pesticide residues present in individual food items because it is very difficult to apply pesticides uniformly in the field and even though pesticides might have been applied following GAP. The distribution of pesticide residue levels in individual crop items might be very wide with some food items containing residue levels much higher than others. This is because, during application, some field areas are receiving higher amounts of pesticides than others. So, the variability of pesticide residue levels is inevitable. Variability is defined as the variation in concentrations of pesticide residues among individual units of any commodity (Hill and Reynolds 2002).
Variability factor is defined as the ratio between the 97.5th percentile of the residue levels and the mean of residue level of that lot of a certain commodity (Pesticides Safety Directorate 1998; Harris et al. 2000). Variability factors are used in the acute dietary intake assessment of pesticides to account for the unit-to-unit residue variability in the composite samples, i.e., to consider the situation, where the estimated total residue in the composite sample may come from only one single unit, while all other units in the composite sample contained no residues at all. So, it is necessary to consider this critical situation in risk assessment.
Until now, a very few research were conducted with large-sized commodities (Ambrus 2006; Andersson 2000; Caldas et al. 2006; European Food Safety Authority 2005; Fujita et al. 2012a, b; Kaethner 2002; Pesticides Safety Directorate 1998; Hill and Reynolds 2002; Harris and Davis 1998; Yu-feng et al. 2011). However, VFs estimated for cabbage derived from the market places are limited. Only one experiment was conducted till to date so far with the marketed samples of cabbage (Yu-feng et al. 2011). Based on the short literature review presented above, it appears that internationally there is a lack of residue data to estimate residue variability on/in large food items. Residue variability among food items is very important for consumer food safety evaluation and risk assessment, and that is why it was decided to explore the issue of pesticide residue variability in/on certain large food items of vegetable crops like cabbage. The most commonly used and registered pesticides for the control of insect pests and diseases were selected (Ministry of Rural Development and Food of Hellenic Republic 2012).
Multi-residue methods for the determination of pesticide residues in fruits and vegetables are essential and cost effective tools. A few multi-residue methods based on LC-MS/MS were developed for cabbage (Takatori et al. 2013; Heimstra and Kok 2007; Jansson et al. 2004; Hans et al. 2003; Pang et al. 2006). To analyze pesticide residues, effective extraction and cleanup techniques are also essential. Nowadays, the quick, easy, cheap, effective, rugged, and safe (QuEChERS) technique, which was first introduced by Anastassiades et al. (Anastassiades et al. 2003), is widely used for the extraction and cleanup of fruit and vegetable matrices (Prodhan et al. 2015; Gilbert-Lopez et al. 2010; Lehotay et al. 2005; Lehotay et al. 2010; Singh et al. 2012; Romero-Gonzalez et al. 2008; Fernández Moreno et al. 2008). Therefore, the QuEChERS extraction technique followed by LC-MS/MS is a valuable tool for pesticide residue analysis. Taking this into consideration, the present study was initiated to develop and validate a multi-residue analytical method for cabbage using LC-MS/MS, to monitor pesticide residues in cabbage and to estimate the variability factors for cypermethrin, deltamethrin, chlorpyrifos, indoxacarb, propamocarb hydrochloride, pirimicarb, and fluopicolide within cabbage units collected from different market places in Greece.
Materials and Methods
Sampling and Sample Preparation
132 samples of cabbage were collected from different market places such as super markets, open markets, and retail vegetable shops in Thessaloniki, Greece. The collected samples were placed in clean polyethylene bags to reduce cross contamination and were labeled properly. All collected samples were carried to the pesticide Science Laboratory, Aristotle University of Thessaloniki (AUTH), Greece on the same sampling day. Unit weight of each individual sample was recorded. The outer leaves were removed then the whole unit of each sample cut into small pieces and mixed properly was stored at −20 °C until homogenization. Homogenization was done by the use of a fruit blender.
Chemicals and Reagents
Reference standards of chlorpyrifos, deltamethrin, indoxacarb, propamocarb hydrochloride, and fluopicolide were obtained from Sigma-Aldrich (St Louis, MO, USA), pirimicarb was from Neochema (Bodenheim, Germany), and cypermethrin was from LGC (Middlesex, UK). LC-MS grade methanol, gradient-grade acetonitrile, and chromatography-grade water were from Merck (Darmstadt, Germany). Sodium chloride (NaCl) was purchased from Chem-Lab (Zedelgem, Belgium), anhydrous magnesium sulfate (MgSO4) was from Panreac (Barcelona, Spain), and primary secondary amine (PSA) was from Agilent (Santa Clara, CA, USA). Ammonium acetate of mass spectrometry grade was purchased from Fluka (Buchs, Switzerland).
Preparation of Pesticide Standard Solution
Pesticide standard stock solutions of chlorpyrifos, cypermethrin, deltamethrin, indoxacarb, pirimicarb, propamocarb hydrochloride, and fluopicolide were prepared separately in methanol at a concentration of 1000 mg/L and stored at −20 °C until use. A mixed standard solution of 50 mg/L in methanol containing all the aforementioned pesticides was prepared by adding the appropriate volume of each individual stock solution in a 50-mL volumetric flask and made to volume by addition of methanol. An intermediate mixed standard solution of 10 mg/L in methanol was prepared from the mixed standard solution of 50 mg/L. Then, working standard solutions of 0.05, 0.1, 0.2, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 5.0 mg/L in methanol were prepared by transferring the appropriate amount from 10 mg/L intermediate mixed standard solution into ten separate 10-mL volumetric flasks.
Matrix matched calibration standards were prepared by adding 100 μL of the mixed pesticide standards working solutions of 0.05, 0.1, 0.2, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 5.0 mg/L and 900 μL of the mobile phase mixture (80 % mobile phase A and 20 % mobile phase B) used for LC-MS/MS analysis in the blank extract to reach the final concentrations of 0.005, 0.01, 0.02, 0.05, 0.10, 0.15, 0.2, 0.3, 0.4, and 0.5 mg/L, respectively. Calibration standards in methanol having the same concentrations as in the matrix-matched calibration standards were also prepared. All the standard solutions were kept in a freezer at −20 °C until use.
Extraction and Cleanup
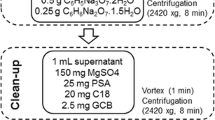
Ten grams of thoroughly homogenized sample was accurately weighted in a 50-mL polypropylene centrifuge tube, and 10 mL acetonitrile (MeCN) was added into the centrifuge tube. The centrifuge tube was closed properly and shaken vigorously for 30 s by vortex mixer. Then, 4 g anhydrous MgSO4 and 1 g NaCl were added into the centrifuge tube, and it was shaken immediately by vortex for 1 min to prevent the formation of magnesium sulfate aggregates. Afterward, the extract was centrifuged for 5 min at 5000 rpm. An aliquot of 3 mL of the MeCN layer was transferred into a 15-mL microcentrifuge tube containing 600 mg anhydrous MgSO4 and 75 mg PSA. Then, it was thoroughly mixed by vortex for 30 s and centrifuged for 5 min at 4000 rpm. After that, a 1-mL supernatant was taken into a clean test tube, and one drop of toluene was added into the test tube. Then, it was concentrated to dryness under a gentle stream of nitrogen (15 psi) by using the Turbovap LV (Caliper Life Sciences, Germany) evaporator at 30 °C. The test tube containing the extract was stored at −20 °C until analysis. The extract was reconstituted by adding 100 μL LC-MS-grade methanol and 900 μL of the mobile phase mixture (80 % mobile phase A and 20 % mobile phase B) used for the LC-MS/MS analysis. The amount of the sample in the final extract was equivalent to 1 g/mL.
Liquid Chromatography-Triple Quadrupole Mass Spectrometry
A liquid chromatography-mass spectrometry system consisting of a Surveyor LC pump and autosampler, and a TSQ Quantum Discovery Max triple quadrupole mass spectrometer (Thermo Electron Corporation, Waltham, MA, USA) was used for the identification and quantification of the selected pesticides. The LC pump gradient program was 0–5 min, 20 % mobile phase B; 5–16 min, 100 % mobile phase B; 16–27.5 min, 20 % mobile phase B. The mobile phase flow rate was 0–18.5 min: 0.2 mL/min; 18.5–25.5 min: 0.5 mL/min and 25.5–27.5 min: 0.2 mL/min. Mobile phase A consisted of a 90:10, water: methanol mixture containing 5 mM of ammonium acetate, whereas mobile phase B was a 10:90, water: methanol mixture containing 5 mM of ammonium acetate. A HyPurity C18 analytical column (50 mm × 2.1 mm i.d., 3-μm particle size) was used for the chromatographic separation (Thermo Scientific). The column oven temperature was 40 °C, the injection volume was 20 μL, and the total run time was 27.50 min.
The mass spectrometry system was a triple quadrupole which was equipped with an electrospray ionization (ESI) source operated at the positive ion mode. The operating conditions of ESI were as follows: sheath gas (nitrogen) pressure was 30 arbitrary units; Auxiliary gas (nitrogen) pressure was 10 arbitrary units; spray voltage was 4000 V; capillary temperature was 325 °C. The collision gas pressure was 1.5 mTorr. The acquisition was made in the selected reaction monitoring (SRM) mode. The parent ion, quantification and confirmation ions are presented in Table 1. All of the data were acquired and processed by the Trace Finder software (Thermo Scientific).
Method Validation
The analytical method was validated by evaluating the linearity, accuracy, precision, limit of detection, and the limit of quantification.
Accuracy and Precision
The accuracy of the method was calculated as percent recovery of pesticides from spiked samples. Certain quantities of pesticide-free cabbage were purchased from a retail market of fresh products of organic farming. The purchased samples were cut into small pieces and stored at −20 °C until homogenization. Homogenization was performed by fruit blender. A 10-g homogenized sample was spiked prior to the extraction procedure by the addition of a mixed pesticide standard working solution to reach the final fortification levels of 0.01, 0.05, 0.10, and 0.20 mg/Kg. For each level of fortification, five replicates were analyzed. After fortification, the sample was equilibrated by shaking and then allowed to settle for 30 min prior to extraction in order to ensure the sufficient contact of the analytes with the whole matrix. Then, the samples were prepared according to the method described earlier.
Precision in case of repeatability (RSDr) was determined at four fortification levels of 0.01, 0.05, 0.10, and 0.20 mg/Kg with five replicates on the same day. Precision in case of reproducibility (RSDR) was evaluated during a period of 2 months by analyzing fortified samples at two fortification levels of 0.01 and 0.10 mg/Kg with five replicates.
Limit of Detection and Limit of Quantification
The limit of detection (LOD) was calculated according to EURACHEM guidelines (EURACHEM 1998). In order to determine the LOD of each analyte, ten independent blank samples fortified at the lowest acceptable concentration of 0.01 mg/Kg were processed and the LOD was expressed as the analyte concentration corresponding to three times the standard deviation. LOQ was determined according to the European Commission (EC) document no. SANCO/12571/2013 (European commission 2013). LOQ was set as the lowest fortification level for each pesticide giving an acceptable accuracy (mean recoveries for individual pesticides being in the range of 70–120 %) and precision (RSDr ≤20 %).
Results and Discussion
Optimization of Cleanup
Sample cleanup is one of the important steps to reduce the matrix effect. The QuEChERS cleanup method used for cabbage with the selected pesticides was slightly modified is described below.
For cleanup of the cabbage samples, a series of trials were conducted to determine the appropriate amounts of anhydrous MgSO4 and PSA (Table 2). At first, the appropriate amount of anhydrous MgSO4 was determined by performing recovery experiments employing 450 and 600 mg of anhydrous MgSO4 per 3 mL extract. The same amount of PSA (75 mg) was used for both experiments. The results revealed that for most of the pesticides there was not a significant difference between 450 and 600 mg of anhydrous MgSO4, except in the case of chlorpyrifos (107 and 92 %, respectively), cypermethrin (129 and 99 %, respectively), and deltamethrin (125 and 105 %, respectively) for cabbage matrix; Thus, 600 mg of anhydrous MgSO4 was selected for further use.
In a second series of experiments, the proper amount of PSA was determined employing 75 and 120 mg of PSA per 3 mL extracts (Table 2). The results revealed that the percent recoveries were within the acceptance limits (70–120 %) for all pesticides except in the case of deltamethrin when 120 mg of PSA was used (126 %). Thus, 75 mg of PSA was selected since for all pesticides, recoveries were in the range of 84–105 %, and moreover, better recoveries were obtained than the 120 mg of PSA treatment for chlorpyrifos (92 and 99 %, respectively), cypermethrin (99 and 120 %, respectively) and deltamethrin (105 and 126 %, respectively. Therefore, 600 mg of anhydrous MgSO4 and 75 mg of PSA for 3 mL extracts were set for the proper cleanup of the cabbage matrix.
Method Validation
Accuracy and Precision
A very good accuracy and precision was found for all the analytes at four fortification levels of 0.01, 0.05, 0.10, and 0.20 mg/Kg. The average recoveries ranged from 80 to 110 % with RSDr ≤16 % for all the analytes (Table 3). Reproducibility (interday accuracy and precision) was evaluated during a period of two months at two fortification levels (0.01 and 0.10 mg/Kg) and it was also found to be very good. Average recoveries ranged from 80 to 109 % with RSDR were ≤12 % (Table 4). The relative percent differences (RPDs) were also calculated for all of the analytes. RPDs were below 7 % for all the analytes (Table 4).
Calibration Curve and Linearity
Calibration curves were prepared by use of matrix-matched standards and also standards in methanol and analyzed in triplicate. Calibration curves were made by plotting the mean peak areas of the selected pesticides versus the respective concentrations. Linearity was evaluated by calculating the correlation coefficients, intercepts, and slopes of the respective regression line at six to ten concentration levels ranging from 0.005 to 0.5 mg/kg. Linearity was very good and coefficients of determination were ≥0.997 for all the selected pesticides with matrix-matched calibration standards. The slopes, intercepts, and correlation coefficients for all the selected pesticides are summarized in Table 5.
Limit of Detection and Limit of Quantification
The limit of detection (LOD) of each analyte is presented in Table 5. The LODs ranged from 0.001 to 0.003 mg/Kg for all the analytes. The limit of quantification (LOQ) for all the selected pesticides was set to 0.01 mg/kg since at this level acceptable accuracy (mean recoveries for individual pesticides in the range of 84 % to 104 %) and precision (RSDr was ≤16 %) were achieved. The LOQ values for all the analytes were lower than the respective European Union maximum residue limits (EU-MRLs).
Matrix Effects
It is well known that the matrix effect is one of the vital problems for pesticide residue analysis using LC-MS/MS (Hajslova and Zrostlikova 2003). Matrix effects are not only dependent on the instrument that is used for analysis but also dependent on the type of matrix, the physicochemical properties of the pesticide, analyte concentration, etc. (Hajslova et al. 1998). Therefore, the matrix effects vary from analyte to analyte and matrix to matrix. The presence of coeluting species of the matrix can interfere in the ionization of the target analytes. Thus, the response of the target analytes may be enhanced or suppressed compared to the solvent-based standards. Hence, sample extraction and cleanup play a key role for the reduction of matrix effects. In this study, matrix effects were estimated as the ratio of the slopes of the calibration curves prepared with matrix-matched standards to those of solvent-based standards (Gilbert-Lopez et al. 2010).
Matrix effects of cabbage for the selected pesticides are presented in Fig. 1. Results revealed that the effect of the matrix was prominent for cabbage with cypermethrin and deltamethrin and it was +49 and +97 %, respectively. To overcome overestimation of concentrations of some analytes and underestimation of others, the use of the matrix-matched standards is recommended as the most appropriate way for quantification.
Application of the Method for Real Sample Analysis
The proposed method was used for the residue analysis of 132 fresh cabbage samples collected from different market places in Thessaloniki, Greece. In Table 6 is shown the data of the contaminated samples (samples found with pesticide residues higher than the LOQ level). Among the analyzed samples, 91 (69 % of the total no. of samples) contained no detectable residues of the pesticides sought and 41 (31 % of the total no. of samples) had pesticide residues, of which two had residues of multiple pesticides and 39 had residues of a single pesticide. The levels of the residues of the detected pesticides were 0.010–0.149 mg/Kg. Only one sample was found contaminated with deltamethrin at a level above the respective EU-MRLs (European commission 2005). The detected pesticides were chlorpyrifos, cypermethrin, deltamethrin, and fluopicolide. Chlorpyrifos was the most frequently found pesticide followed by deltamethrin. Eighteen samples (14 % of the total no. of samples) were found contaminated with chlorpyrifos and 15 (11 % of the total no. of samples) with deltamethrin.
Traces of few pesticides such as propamocarb hydrochloride, chlorpyrifos, and deltamethrin were also detected, whereas other selected pesticides such as indoxacarb and pirimicarb were not detected in any of the analyzed samples. Of the analyzed samples, 11 (8 % of the total no. of samples) had residues at levels below the LOQ but above the LOD. Six samples were found contaminated with propamocarb hydrochloride, four with chlorpyrifos and one with deltamethrin.
Variability Factors Obtained from the Marketed Samples
Variability factors were calculated using the SPSS statistical software and according to the reference procedure defined as the 97.5th percentile of the residue levels in the samples divided by the average residue of that lot (FAO/WHO 2005). When units did not contain detectable concentrations, these were assumed to contain half of the quantification limit. This approach was supported by Earl et al. 2000; Caldas et al. 2006; FAO/WHO 2005). To estimate the unit to unit residue variability, a total of 132 samples of cabbage were analyzed. The estimated variability factors for cabbage are presented in Table 6. The estimated variability factors ranged from 1.00 to 6.75 for cabbage. A wide range of VF was found in this study as the collected samples from the market originated from different fields and thus different variability factors were found.
The results of the present study are supported by the findings of several researchers. Yu-feng et al. (2011) estimated a VF value of 7 for 42 types of vegetables including eggplant, and cauliflower, when contaminated with chlorpyrifos, cypermethrin, and deltamethrin, and VF values ranging from 3 to 6 were reported for celery (Pesticides Safety Directorates 1998). Hill and Reynolds (2002) estimated VF values ranging from 1.4 to 4.8 for celery with chlorpyrifos, VF values ranging from 2.4 to 8.7 for several pesticide residues found in palm including chlorpyrifos, pirimicarb and dimethoate and VF values ranging from 2.9 to 6.8 for peach and nectarine with dimethoate, and also VF values ranging from 1.9 to 7.8 were found for chlorpyrifos and dimethoate along with few other pesticides in oranges.
EFSA (2005) reported a VF value of 8.0 for tomato with acephate and a VF ranging from 3.1 to 4.7 for apple with chlorpyrifos. They also estimated VF values ranging from 1 to 10.5 for different pesticides including chlorpyrifos, pirimicarb, and dimethoate in palms. A VF value ranging from 2.9 to 5.7 for orange with chlorpyrifos and values ranging from 5.1 to 5.5 for orange with dimethoate was also stated by them.
VF values ranging from 3 to 34 were reported for several pesticides, including chlorpyrifos, pirimicarb, and dimethoate in palms (Pesticides Safety Directorate 1998). VF values ranging from 5 to 9 were reported for chlorpyrifos in apple (ACP 1997). Harris (1998) estimated a VF value of 6 for chlorpyrifos, 6 to 16 for pirimicarb, and 23 for dimethoate in palms.
Although the estimated variability factors, derived from the residue data from marketed samples of cabbage, are in a good agreement with relevant data already reported by many researchers, nevertheless, they are higher than the default variability factor of 3, which is currently used in estimating acute dietary intake at the international level recommended by the JMPR (FAO/WHO 2005).
Conclusions
A LC-MS/MS multi-residue method for the analysis of five insecticides and two fungicides in cabbage using QuEChERS extraction technique was developed and validated by evaluating the accuracy, precision, linearity, limit of detection (LOD), and limit of quantification (LOQ). A very good accuracy (average recoveries ranged from 80 to 110 %) and precision (RSDr ≤16 % and RSDR ≤12 %) was found for all the analytes. Moreover, the amount of QuEChERS cleanup materials were optimized in this method. Six hundred milligrams of anhydrous MgSO4 and 75 mg of PSA per 3 mL extract performed better than the other combinations used in this method. Matrix effects of cabbage for the selected pesticides were estimated in this study. The effect of the matrix was prominent for cabbage with cypermethrin and deltamethrin and it was +49 and +97 %, respectively. To overcome this effect, use of the matrix-matched standards is the best way for quantification. Finally, the analytical method was applied successfully for pesticide residue analysis of 132 cabbage samples collected from different market places in Thessaloniki, Greece. Among the analyzed samples, 41 (31 % of the total no. of samples) had pesticide residues, of which two had multiple pesticide residues and 39 had a single pesticide residue. One sample was found contaminated with deltamethrin at a level above the respective EU-MRLs (European commission 2005). Thus, the proposed method can be used successfully to monitor multiple pesticide residues in cabbage and this type of vegetables.
In this study, variability of pesticide residues originated from the samples of cabbage purchased from different market places of Thessaloniki in Greece were estimated. The estimated average VF for cabbage with the detected pesticides was 5.00 and it was higher than the default variability factors of 3, which is currently used in estimating acute dietary intake at international levels recommended by the JMPR (FAO/WHO 2005). However, the findings from this study are in a good agreement with the previously proposed default variability factor of 5 for large-sized commodities (FAO/WHO 1999), which is also used currently in the EU (Board for the authorisation of plant protection products and biocides 2014). It is recommended that a VF value should be reconsidered when more data are available, especially data concerning large-sized crops.
References
Advisory Committee on Pesticides (ACP) (1997) Unit to unit variation of pesticide residues in fruit and vegetables (York, UK: Advisory Committee on Pesticides, PSD)
Ambrus A (2006) Variability of pesticide residues in crop units. Pest Manag Sci 62:693–714
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Andersson A (2000) Comparison of pesticide residues in composite samples and in individual units: the Swedish approach to sampling. Food Addit Contam 17(7):547–550
Board for the authorisation of plant protection products and biocides (2014) Evaluation Manual for the authorisation of plant protection products and biocides according to Regulation (EC) No 1107/2009. EU part; Plant protection products. Chapter 5 Residues; risk to consumers, version 2.0
Caldas E, Jardim A, Ambrus A, Souza LC (2006) Variability of organophosphorus insecticide residues in large size crops grown in commercial farms in Brazil. Food Addit Contam 23:148–158
Earl M, Kaethner M, Uihlein M (2000) Unit to unit variation of pesticide residues—options for acute dietary risk assessment. Food Addit Contam 17:83–89
EURACHEM (1998) The fitness for purpose of analytical methods.www.eurachem.com
European commission (2005) EU pesticide residue MRLs. regulation no. 396/2005. http://ec.europa.eu/sanco_pesticides/public/index.cfm
European commission (2013) Method validation and quality control procedures for pesticides residues analysis in food & feed. Document no. SANCO/12571/2013
FAO/WHO (2005) Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO core assessment group on pesticide residues. FAO Plant Production and protection paper 183
FAO/WHO (1999) Pesticide residues in Food 1999, report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO core assessment group on pesticide residues. FAO Plant Production and protection paper, 153
European Food Safety Authority (EFSA) (2005) Opinion of the scientific panel on plant health, plant protection products and their residues on a request from the commission related to the appropriate variability factor (s) to be used for acute dietary exposure assessment of pesticide residues in fruit and vegetables. EFSA J 177:1–61
Fernández Moreno JL, Garrido Frenich A, Plaza Bolaños P, Martínez Vidal JL (2008) Multiresidue method for the analysis of more than 140 pesticide residues in fruits and vegetables by gas chromatography coupled to triple quadrupole mass spectrometry. J Mass Spectrom 43(9):1235–54. doi:10.1002/jms.1400
Fujita M, Yajima T, Iijima K, Satoh K (2012a) Effect of sampling size on the determination of accurate pesticide residue levels in Japanese agricultural commodities. J Agric Food Chem 60:4457–4464
Fujita M, Yajima T, Iijima K, Satoh K (2012b) Comparison of the variability in the levels of pesticide residue observed in Japanese cabbage and grape units. J Agric Food Chem 60:1516–1521
Gilbert-Lopez B, Juan Garcia-Reyes F, Lozano A, Fernandez-Alba AR, Molina-Diaz A (2010) Large-scale pesticide testing in olives by liquid chromatography–electrospray tandem mass spectrometry using two sample preparation methods based on matrix solid-phase dispersion and QuEChERS. J Chromatogr A 1217:6022–6035
Hajslova J, Holadova K, Kocourek V, Poustka J, Godula M, Cuhra P, Kempny M (1998) Matrix-induced effects: a critical point in the gas chromatographic analysis of pesticide residues. J Chromatogr A 800:283–295
Hajslova J, Zrostlikova J (2003) Matrix effects in (ultra) trace analysis of pesticide residues in food and biotic matrices. J Chromatogr A 1000(1–2):181–197
Hans GJM, Ruud van Dam CJ, Odile MS (2003) Determination of polar organophosphorus pesticides in vegetables and fruits using liquid chromatography with tandem mass spectrometry: selection of extraction solvent. J Chromatogr A 1015:119–127
Harris C (1998) Unit to unit variability of pesticide residues in celery, plums and kiwi fruit, Report by the Pesticides Safety Directorate, York
Harris CA, Davis TJ (1998) Unit to unit variation of pesticide residues in celery, plums and kiwi fruit. Pesticide Safety Directorate, 1–62
Harris CA, Mashall JR, Warren SFP, Crossley SJ (2000) Summary report of the international conference on pesticide residues variability and acute dietary risk assessment. Food Addit Contam 17(7):481–485
Heimstra M, Kok AD (2007) Comprehensive multi-residue method for the target analysis of pesticides in crops using liquid chromatography–tandem mass spectrometry. J Chromatogr A 1154:3–25
Hill ARC, Reynolds SL (2002) Unit-to-unit variability of pesticide residues in fruit and vegetables. Food Addit Contam 19(8):733–747
Jansson C, Pihlström T, Österdahl BG, Markides KE (2004) A new multi-residue method for analysis of pesticide residues in fruit and vegetables using liquid chromatography with tandem mass spectrometric detection. J Chromatogr A 1023:93–104
Kaethner M (2002) Determination of residue variability in table and wine grapes after a tank-mix application of anilinopyrimidine, triazole, pyrethroid, organophosphate, and di-carboximide crop protection products – France/Germany 2000 to 2001, Summary Report by the ECPA Residues Expert Group, 2002, BASF DocID 2002/1007077
Lehotay SJ, Kyung AS, Kwon H, Koesukwiwat U, Fu W, Mastovska K, Hoh E, Leepipatpiboon N (2010) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A 1217:2548–2560
Lehotay SJ, De Kok A, Hiemstra M, Van BP (2005) Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int 88(2):595–614
Ministry of Rural Development and Food of Hellenic Republic (2012). Available on: http://wwww.minagric.gr/syspest/SYSPEST_CROPS_eng.aspx.
Organic Facts (2015) Health benefits of cabbage. https://www.organicfacts.net/…benefits/…/health-benefits-of-cabbage.htm…
Pang GF, Fan CL, Liu YM, Cao YZ, Zhang JJ, Li XM, Li ZY, Wu YP, Guo TT (2006) Determination of residues of 446 pesticides in fruits and vegetables by three-cartridge solid-phase extraction-gas chromatography–mass spectrometry and liquid chromatography-tandem mass spectrometry. J AOAC Int 89(3):740–71
PSD (Pesticides Safety Directorate) (1998) Unit to unit variability of pesticide residues in celery, plums and kiwi fruit (York, UK: Pesticides Safety Directorate)
Prodhan MDH, Papadakis EN, Papadopoulou-Mourkidou E (2015) Analysis of pesticide residues in melon using QuEChERS extraction and liquid chromatography triple quadrupole mass spectrometry. Int J Environ Anal Chem 95(13):1219–1229. doi:10.1080/03067319.2015.1025227
Romero-GonzAlez R, Garrido Frenich A, Martínez Vidal JL (2008) Multiresidue method for fast determination of pesticides in fruit juices by ultra performance liquid chromatography coupled to tandem mass spectrometry. Talanta 76(1):211–25. doi:10.1016/j.talanta.2008.02.041
Takatori S, Yamamoto H, Fukui N, Yamaguchi S, Kitagawa Y, Kakimoto Y, Osakada M, Okihashi M, Kajimura K, Obana H (2013) Validation study on a rapid multi-residue method for determination of pesticide residues in vegetables and fruits by LC-MS/MS. J Food Hyg Safety sci 54(3):237–249
Singh B, Kar A, Mandal K, Kumar R, Sahoo SK (2012) Development and validation of QuEChERS method for estimation of chlorantraniliprole residue in vegetables. J Food Sci 77(12):T208–15. doi:10.1111/j.1750-3841.2012.02801.x
Yu-feng S, Xiao LU, Feng-shan REN (2011) Variability of pesticide residues in vegetables from the marketplaces in Jinan city. Agric Sci China 10(10):1646–1652
Acknowledgments
The principal author is grateful to the State Scholarship Foundation (IKY), Greece, for their financial support to pursue his PhD. The authors are thankful to Kotopoulou Athina, kintzikoglou Katherina, and Tsampoula Aggeliki in the Pesticide Science Laboratory of AUTH for their cooperation during the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable
Conflict of Interest
M. D. H. Prodhan declares that he has no conflict of interest. Emmanouil-N. Papadakis declares that he has no conflict of interest. Euphemia Papadopoulou-Mourkidou declares that she has no conflict of interest.
Ethical Approval
The manuscript has not been published previously and has not been submitted to another journal for simultaneous consideration. This article does not contain any studies with human or animal subjects.
Informed Consent
Not applicable
Rights and permissions
About this article
Cite this article
Prodhan, M.D.H., Papadakis, EN. & Papadopoulou-Mourkidou, E. Analysis of Pesticide Residues and Their Variability in Cabbage Using QuEChERS Extraction in Combination with LC-MS/MS. Food Anal. Methods 9, 3470–3478 (2016). https://doi.org/10.1007/s12161-016-0537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0537-z