Abstract
A fast and effective multiresidue method for the determination of 42 pesticides in golden berry was developed and validated. A modified QuEChERS method was established for sample preparation followed by ultra-high performance liquid chromatographic-tandem mass spectrometry (UHPLC-MS/MS) determination with electrospray ionization in a triple quadrupole system. Validation results were satisfactory, since the method presented recoveries between 70 and 114 % with relative standard deviations (RSD) <20 % for blank samples spiked from 5 to 25 μg kg−1. The method limit of detection and limit of quantification were 1.5 and 5 μg kg−1 , respectively. Matrix effect ranged from −32 to 218 % and was compensated using matrix-matched calibration. Method linearity was established from 2.5 to 100 μg kg−1 with r 2 ≥ 0.99. The proposed method combines the advantages of a simple and fast sample preparation step by a modified QuEChERS method with the high selectivity and sensitivity of the UHPLC-MS/MS system using selected reaction monitoring. The method was successfully applied to commercial samples, proving to be an efficient alternative for routine analysis. From the 16 analyzed samples, 13 presented residues of one or more pesticides (carbendazim, chlorpyrifos ethyl, dimethoate, propamocarb, and tebuconazole) in the concentration range of 2.0 to 55.6 μg kg−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Golden berry (Physalis peruviana L.) is an exotic fruit belonging to the family of Solanaceae and genus Physalis. This fruit is considered a functional food because of the physiological properties associated with a nutritional composition rich in proteins, carbohydrates, lipids, phytosterols, minerals, vitamins (A, B, C, E, and K1), and the steroidal lactones physalins and withanolides (Puente et al. 2011). Colombia is the largest producer and exporter of golden berry worldwide (Mazorra et al. 2006), and in 2012, the exportation totalized USD 29.2 million (Legiscomex 2013). In the last years, the production of golden berry in Brazil was small (2–3 t per year); however this fruit is well accepted by the local markets (Fischer et al. 2014). Thus, the introduction of this fruit is increasing and becoming a new alternative of fruit consumption as well as an income option for producers (Muniz et al. 2011). Pesticides are considered a useful tool to improve productivity; however, the indiscriminate use can leave residues in food in its original chemical form or as metabolites (Rekha and Prasad 2006). Consequently, monitoring of pesticide residues becomes an essential practical to ensure food safety and to prevent contamination through food chain (Melo et al. 2012).
In Colombia, the Resolution 2906, issued in 2007 by the Social Protection Ministry and the Agricultural and Rural Development Ministry, defines the maximum residue levels (MRLs) in food for human consumption, feed, or pasture (Colombia 2007). The European Union determined, through Regulation No. 600/2010, that golden berry would be classified in the “tomato” group, due to the similarity between these matrices (Restrepo et al. 2014). In Brazil, golden berry is considered a minor crop and there is still no MRLs established.
Due to the complexity of food matrices, the determination of pesticide residues becomes a challenge. Several sample preparation methods are on development in order to ensure isolation of analytes and to remove interfering compounds from complex matrices (Wilkowska and Biziuk 2011). Quick, easy, cheap, effective, rugged, and safe (QuEChERS) method proposed by Anastassiades and Lehotay (2003) is a suitable alternative for this purpose. QuEChERS is based in three steps: extraction, partition, and clean-up (Prestes et al. 2009). Chromatographic techniques such as ultra-high performance liquid chromatography (UHPLC) and gas chromatography (GC) coupled with tandem mass spectrometry (MS/MS) are the best choices for pesticide residues determination in food at low levels (Orso et al. 2016).
Restrepo et al. (2014) developed a multiclass multiresidue method using QuEChERS and GC-MS with single quadrupole for the determination of pesticide residues in tomato, tamarillo, and golden berry. The method was validated for the range from 0.02 to 0.20 mg kg−1 for 24 pesticides in tomatoes, 33 in tamarillos, and 28 in golden berries. From 46 target pesticides, only 28 compounds were successfully validated in golden berry. Interference by co-elution or lack of sensitivity was reported for several compounds.
Amórtegui and Dallos (2015) proposed a method for the determination of 50 pesticide residues in exotic fruits (baby bananas, sweet granadilla, tamarillo, pitahaya, and golden berry) using QuEChERS and GC-MS with single quadrupole. Large volume injection up to 25 μL was used to improve sensitivity of the GC-MS system. A backflushing cleaning was required to deal with high injection volumes of sample extracts. Method limit of quantification was 0.01 mg kg−1 for most of the analytes; however, eight pesticides were only detected at this level and will need an alternative method for a reliable quantification of these compounds.
Despite the applicability of gas chromatography for pesticide residues analysis in food, many polar or ionic pesticides cannot be analyzed directly by this technique. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) is an effective alternative for separation, identification, and confirmation of substances with low volatility and/or thermal instability (Queiroz et al. 2012) and is frequently used for the determination of pesticides in multiresidues methods for food samples (Romero-González et al. 2008).
Considering the few studies described about the determination of pesticide residues in golden berry and that they are performed by GC-MS with single quadrupole, the aim of this work was to develop and validate a simple, rapid, and effective method for the determination of pesticide residues in golden berry using a modified QuEChERS method followed by UHPLC-MS/MS analysis using a triple quadrupole (QqQ) system.
Experimental
Chemicals and Apparatus
Analytical standards, all ≥93 % purity, and internal standard (IS) triphenylphosphate were purchased from Dr. Ehrenstorfer (Germany). Atrazine-d5, used as surrogate standard (SS), was acquired from CDN Isotopes (Canada). The analyzed pesticides were mainly selected based on compounds with MRL established for tomato and adopted for golden berry by different legislations (Colombia 2007; Codex Alimentarius 2013; European Union 2010). Purity was considered to calculate the concentration of each standard solution. Individual stock standard solutions (1000 mg L−1) were prepared in acetonitrile. From the stock solutions, a standard solution at 10.0 mg L−1 was prepared containing the mixture of the analytes listed in Table 1. These solutions were stored in amber glass vials at −10 °C.
Acetonitrile (MeCN), pesticide residue grade, was purchased from J.T. Baker (USA), and purified water was provided by a Direct QUV system (resistivity of 18.2 Ω) from Millipore (France). Anhydrous magnesium sulfate (MgSO4), sodium acetate (CH3COONa), and sodium chloride, all analytical grade, were acquired from J.T. Baker (USA). Sodium citrate tribasic dihydrate (C6H5Na3O7 .2H2O) and sodium hydrogencitrate sesquihydrate (C6H6Na2O7.1.5H2O), all analytical grade, were from Sigma Aldrich (USA). The sorbents Bondesil C18 (40 μm) and Bondesil primary secondary amine (PSA) (40 μm) were from Agilent Technologies (USA) and graphitized carbon black (GCB) Supelclean ENVI-Carb 120/400 mesh from Supelco (USA).
For the sample preparation procedure, different apparatus were used: analytical balances UX-420H from Shimadzu (Japan) and APX-200 from Denver Instruments (Brazil); refrigerated centrifuge NT 825 from Novatecnica (Brazil); TurboVap® LV Concentration Evaporator from Biotage (USA); vortex shaker (model QL-901) from Biomixer (Brazil); and food processor Walita (Brazil).
UHPLC-MS/MS Conditions
Chromatography analyses were performed using an UHPLC-MS/MS from Waters (USA) equipped with Acquity UPLC™ liquid chromatography, Xevo TQ™ MS/MS triple quadrupole detector, and the data acquisition software MassLynx V 4.1. Separations were carried out with an Acquity UPLC™ BEH C18 (50 × 2.1 mm, 1.7 μm) column from Waters (USA) maintained at 40 °C. Mass spectrometer was operated with electrospray ionization in positive mode (ESI+) for all compounds using selected reaction monitoring mode (SRM), with two transitions for each pesticide. The highest intensity was selected for quantification and the second highest intensity transitions for confirmation, as shown in Table 1.
Instrumental parameters for MS/MS analysis were as follows: nitrogen (N2), employed as desolvation and cone gas, at a flow rate of 600 and 80 L h−1, respectively; capillary voltage, 2.0 kV; source temperature, 150 °C; desolvation temperature, 500 °C; and argon gas (Ar), used in the collision cell for ion fragmentation, at 0.15 mL min−1. In accordance with recent studies (Kemmerich et al. 2015; Rizzetti et al. 2016), mobile phases employing the additives formic acid and ammonium formate provided good results for the analysis of pesticide residues by UHPLC-MS/MS, improving the ionization efficiency. Thus, the mobile phase used in this work consisted of (A) water/methanol (98:2, v/v) and (B) methanol, both containing 5 mmol L−1 ammonium formate and 0.1 % formic acid. The gradient program started at 5 % of B (held 0.25 min), then increased linearly to reach 100 % B in 7.75 min (held 0.75 min), and decreases to 5 % of B at 8.51 min, holding until 10 min. The selected flow rate was 0.225 mL min−1 and the injection volume was 10 μL.
Sample Preparation
Blank samples of golden berry were cultivated in a local farm in Santa Maria (Brazil) without the use of pesticides. The whole fruits were processed and homogenized without the stems and the calyx, as described in Codex Alimentarius (2013). After processing, blank samples were maintained in polypropylene (PP) flasks and properly identified and stored in freezer at −10 °C until use.
Optimization of Sample Preparation
The three main variations of the QuEChERS method (original, acetate, and citrate) (Prestes et al. 2009) were tested in golden berry to evaluate which method would yield the best results in terms of recoveries and matrix interference. For all tests (T), 5 g of blank sample was transferred to 50 mL PP tube and spiked with the surrogate standard and pesticides at 20 μg kg−1. After that, the respective extraction solvent was added, the tube was shaken for 1 min, and then appropriate quantities of salts were added as follows:
-
T1 (QuEChERS original): 5 mL of acetonitrile; 2.0 g of MgSO4; and 0.5 g of NaCl
-
T2 (QuEChERS acetate): 5 mL of acetonitrile with 1 % (v/v) of acetic acid; 2.0 g of MgSO4; and 0.5 g of CH3COONa
-
T3 (QuEChERS citrate): 5 mL of acetonitrile; 2.0 g of MgSO4; 0.5 g of NaCl; 0.5 g of tri-sodium citrate dihydrate; and 0.25 g of di-sodium hydrogencitrate sesquihydrate
After salt addition, tubes were shaken during 1 min and centrifuged for 8 min at 2420×g. For the clean-up (C) step, different procedures were tested for 1 mL of extract:
-
C1 (for QuEChERS original and citrate): 25 mg PSA and 150 mg of MgSO4
-
C2 (for QuEChERS acetate): 50 mg PSA and 150 mg of MgSO4
-
C3 (for the three QuEChERS): 25 mg PSA, 25 mg of C18, 2.5 mg of GCB, and 150 mg of MgSO4
Gravimetric tests were performed (n = 6) in order to verify the influence of C18 in the clean-up step. The following tests were performed: without clean-up; without C18 in the clean-up; and clean-up with 25 mg of C18. For this evaluation, cleaned blank extracts (4 mL) were evaporated in TurboVap® LV under gentle nitrogen stream, and the mass of coextractives in the final extracted were gravimetrical determined. In order to assess the appropriate amount of C18, recovery tests (n = 3) with different amounts of sorbent added to 4 mL of blank extract spiked at 25 μg kg−1 were evaluated: (a) 150 mg; (b) 75 mg; and (c) 25 mg.
Optimized Procedure
The optimized sample preparation procedure presented in Fig. 1 consisted of weight 5 g of homogenized sample in a 50-mL PP tube, spike with the surrogate standard (atrazine-d5) and with standard solution of mixed pesticides in order to give a concentration level of 20 μg kg−1. The sample was extracted with 5 mL of acetonitrile shaking in vortex for 1 min. After this step, 2 g of MgSO4, 0.5 g of NaCl, 0.5 g of C6H5Na3O7 .2H2O, and 0.25 g of C6H6Na2O7.1.5H2O were added. Then, the mixture was manually shaken for 1 min and centrifuged at 2420×g for 8 min. After that, 1 mL of the supernatant was transferred to a 15-mL PP tube containing 150 mg of MgSO4, 25 mg PSA, 20 mg C18, and 2.5 mg of GCB for clean-up step by dispersive solid-phase extraction (d-SPE). Tubes were centrifuged for 8 min at 2420×g: the supernatant was filtered in nylon membrane filter (0.2 μm) and diluted with ultrapure water in a ratio of 1:4 (v/v). Before analysis in the UHPLC-MS/MS system, the internal standard triphenyl phosphate was added at a final concentration of 20 μg L−1.
Validation Conditions
The optimized method was validated according to the SANTE/11945/2015 guideline (SANTE 2015). The developed UHPLC-MS/MS method includes selectivity, matrix effect, linearity and working range, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision (repeatability and intermediate precision).
The linearity was determined using curves in triplicate prepared in solvent (acetonitrile) and in blank matrix extract at 0.5, 1.0, 2.0, 5.0, 10.0, and 20.0 μg L−1. Matrix effect was calculated comparing the slopes of the analytical curves in solvent and in matrix extracts (Ferrer et al. 2011). Method limit of quantification (LOQm) were established experimentally as the lowest concentration of the spiked blank matrix that provided recoveries between 70 and 120 % with relative standard deviations (RSD)% ≤20 % in accordance with SANTE (2015). The LODm was calculated by dividing the LOQm by 3.3. Instrumental limits of detection (LODi) and quantification (LOQi) were obtained considering the final dilution of 1:4 (v/v) introduced at the end of the sample preparation step.
Results and Discussion
UHPLC-MS/MS Analysis
UHPLC-MSMS analysis allowed the determination of the selected pesticides in golden berry, with good selectivity and sensitivity. Mobile phase, gradient program, and the other parameters were selected in accordance with previous studies of Kemmerich et al. (2015) and Rizzetti et al. (2016). Figure 2 shows the chromatogram obtained by UHPLC-MS/MS in SRM mode from an analytical solution prepared in matrix extract at 20 μg L−1.
Sample Preparation Optimization
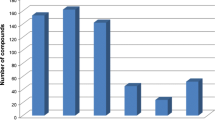
To choose the best clean-up, three versions of QuEChERS method were investigated: T1 (QuEChERS original), T2 (QuEChERS acetate), and T3 (QuEChERS citrate) with different amounts of the sorbent PSA (25 mg for T1 and T3, 50 mg for T2) and 150 mg of MgSO4 per mL of extract for all tests. The obtained extracts were not considered clean enough for injection in the UHPLC-MS/MS system since there were too many pigments present. To improve the clean-up, 2.5 mg of GCB per mL was added to the three tests (T1–T3). Moreover, C18 (25 mg per mL) was used to promote an adequate clean-up for comparison of recoveries. Satisfactory recovery results were similar between the tests (Fig. 3); however, T3 was chosen for the following tests. The best results for T3 can be explained by the acidity of goldenberry matrix, since pH is around 3.5; nevertheless, the higher extraction of coextractives at acid pH was observed as described by Sousa et al. (2013) for pesticides in potato, apple, and soil matrices. Thus, it is essential to promote a buffering effect increasing the pH. At the test T3, the mixture of sodium citrate tribasic dihydrate and sodium hydrogencitrate sesquihydrate increased the pH to 5.0–5.5 (Prestes et al. 2011). The test T2 showed recoveries from 70 to 120 % for 95 % of the analyzed compounds; however, this test was not considered a good extraction option due to the presence of turbidity even after the clean-up with GCB. Better recoveries were obtained with test T3, providing adequate recovery for 98 % of the compounds. The only exception was propamocarb that is as unstable compound (Burrows et al. 2002) and presented mean recovery below 56 % for all three tests.
The presence of coextractives can affect the response of the chromatographic system; thus, the cleaning of the extracts is essential to promote robustness and reliability of the results. Increasing the efficiency of the clean-up step decreases the matrix effect (Pinho et al. 2009). The use of PSA in the clean-up step is important since golden berry composition has 19.6 % of carbohydrate (Restrepo et al. 2014). PSA can remove fatty acids, sugars, some pigments, and other coextractives that form hydrogen bonds (Maštovská and Lehotay 2004). Golden berry composition also contains 1.6 % of carotenoids. Thus, the use of GCB was necessary to improve the removal of pigments and other interferences (Lehotay et al. 2010).
From the gravimetric tests, applied in order to verify the influence of C18 in the clean-up step, it was observed that the use of C18 is essential. The results of the gravimetric tests proved that there is a difference in terms of weight of coextractives when C18 is used as sorbent. This sorbent is known for high removal of nonpolar compounds; however, the amount of C18 can influence the recovery of target compounds. From the test to evaluate the appropriate amount of C18 sorbent, it can be seen that 84 % of the evaluated compounds at test A had recoveries between 70 and 120 %, 95 % at test B, and 91 % at test C. An intermediate amount of C18 was considered suitable, leading to an efficient removal of coextractives without interfering on the recovery of analytes. Therefore, the addition of 20 mg of C18/mL of extract was selected for the validation step.
Restrepo et al. (2014) used QuEChERS citrate method for the determination of pesticides in golden berry by GC-MS. With the optimized clean-up step (150 mg of MgSO4, 25 mg of C18, and 25 mg of PSA/mL of extract), only 28 of the 46 target pesticides presented adequate recovery. The clean-up step selected in our work was similar except by the use of 2.5 mg of GCB and a lower amount of C18 (20 mg).
Method Validation
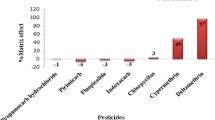
Matrix effects caused by influence of coextractives ranged from −32 % for deltamethrin to 218 % for dimethoate and are shown in Fig. 4. All target compounds, with the exception of benalaxyl, presented matrix effect. For 30 compounds, matrix effect ranged between −20 and +20 %, but for other compounds, the matrix effect was higher. The coefficients of determination (r 2) of curves prepared in blank matrix (matrix-matched calibration) are presented in Table 1. Considering the coefficients of determination and matrix effect from the curves in solvent and matrix-matched, a higher number of compounds can be analyzed using matrix-matched calibration because it can compensate signal suppression or enhancement. With this calibration mode, standards and sample extracts have similar amount of coextractives and the ionization efficiency of the analytes in the mass spectrometer source will be comparable (Zanella et al. 2015). Regarding these facts, matrix-matched calibration was selected for this work.
Andrade et al. (2015) evaluated 57 pesticide residues in tomato using QuEChERS acetate followed by LC-MS/MS analyses. Considering the common compounds to our work, azoxystrobin, benalaxyl, carbaryl, carbofuran, chlorpyrifos ethyl, fenamiphos, indoxacarb, malathion, oxamyl, tebuconazole, and trifloxystrobin presented less pronounced matrix effect in golden berry. Five compounds (cymoxanil, dimethoate, fenpropathrin, imidacloprid, and triadimenol) presented higher values of matrix effect in golden berry, and pirimicarb had the same value in both matrices.
Except for propamocarb that presented mean recovery of 56 %, the validated method provided satisfactory recovery results (70–120 %) and precision (RSD ≤20 %) for all compounds for all spike levels (5, 10, and 20 μg kg−1) as presented in Table 1. For repeatability and intermediate precision assays, recoveries were from 70 to 114 %, with RSD from 1 to 20 % and from 2 to 20 %, respectively, as shown in Table 1. In both assays, propamocarb presented recoveries between 50 and 57 % at all levels. This behavior can be explained by the tendency of this compound to undergo degradation (Burrows et al. 2002).
With the exception of propamocarb that presented low recovery, method LOQ, established as the lowest spiked level that provided adequate results in the validation, was 5 μg kg−1. Method LOD corresponds to 1.5 μg kg−1. This LOQm level can be considered adequate because it is below the maximum residue levels established for golden berry or to the tomato group by different legislations. Considering the final dilution of 1:4 (v/v) adopted before analysis by UHPLC-MS/MS, the instrumental limits of detection (LODi) and quantification (LOQi) correspond to 0.3 and 1.0 μg L−1, respectively.
Restrepo et al. (2014) obtained similar results for the determination of pesticide residues in tomato, golden berry, and tamarillo by GC-MS. However, the authors obtained lower recovery for carbaryl (53.6 %) if compared with our study (87 to 97 %).
In a recent study, Golge and Kabak (2015) used a modified QuEChERS for the determination of 109 multiclass pesticides by liquid chromatography-triple-quadrupole mass spectrometry in tomatoes achieving recoveries between 77.1 and 113.2 %. A clean-up step with 50 mg of PSA and 150 mg of MgSO4 per mL of extract was used. Arias et al. (2014) also used the QuEChERS acetate method with clean-up using 25 mg of PSA and 150 mg of MgSO4 per mL of extract for the determination of 24 pesticides in fresh tomatoes.
The MRL values established or adopted (from the tomato group) for golden berry ranged from 0.01 to 2 mg kg−1 according to Codex Alimentarius (2013), from 0.01 to 5 mg kg−1 by the Resolution 2906/07 (Colombia 2007), and from 0.002 to 10.0 mg kg−1 by the European Union (2010). Thus, considering that the experimental method limit of quantification (0.005 mg kg−1) obtained by the proposed method is below the MRLs, with exception of the MRL established by the European Union for carbofuran (0.002 mg kg−1), the proposed method is suitable for monitoring purpose. It is noteworthy that the method LOD (0.0015 mg kg−1) is lower than this MRL. Since propamocarb presented recoveries between 50 and 57 %, it has not reached validation criteria. However, as its MRL is 1 mg kg−1 or higher, further studies are recommendable in this concentration range.
Application to Commercial Samples
From the 16 samples of golden berry evaluated in the application of the proposed method, two samples (S3 and S4) were from the Rio Grande do Sul state (Brazil) and the other 14 samples were from Colombia. Table 2 shows the samples that presented quantified pesticide residues.
From the analyzed samples, 81.3 % presented residues of one or more pesticides in the concentration range from 2.0 to 55.6 μg kg−1. Carbendazim, chlorpyrifos ethyl, dimethoate, propamocarb, and tebuconazole presented residues below the MRL values established or adopted for golden berry. The fungicide carbendazim is present in almost all samples in concentration between 4.8 and 55.6 μg kg−1. Propamocarb and tebuconazole, both fungicides, were found in S1 at 18.8 and 3.8 μg kg−1, respectively, emphasizing that the result for propamocarb is an estimation since the same was not validated. The inseticide chlorpyrifos ethyl was detected only in S13, at 2.7 μg kg−1. Dimethoate is an acaricide and insecticide with MRLs established at 20 μg kg−1 by the European Union for the tomato group, but no MRLs is available from Codex Alimentarius or by the Colombian and Brazilian legislations. However, the occurrence of this pesticide from 2.0 to 6.8 μg kg−1 in the samples evaluated suggest the necessity of regulation for this pesticide.
Conclusions
The optimization of the QuEChERS method followed by UHPLC-MS/MS analysis proved to be effective for the determination of 42 pesticide residues in golden berry. The moderate use of the solvent acetonitrile in the extraction step (5 mL) is advantageous and environmentally friendly. Moreover, the clean-up choice proved to be essential to improve the recoveries and decrease matrix effect caused by the presence of coextractives for most of the compounds, reducing the need of maintenance of the analysis system. The developed method presented suitable recoveries (70–114 %) and RSD (<20 %) for all the target compounds, except for propamocarb. From the studied pesticides, only carbendazim, chlorpyrifos ethyl, dimethoate, propamocarb, and tebuconazole were present in the commercial samples, but all these pesticides presented residues below the MRL values established or adopted for golden berry.
Considering the few studies available for the determination of pesticide residues in golden berry, and especially that this is the first method described for this fruit using liquid chromatography, the developed method is a good alternative for multiresidue analysis in sample routine since the procedure is easy, fast, and effective.
References
Amórtegui JCE, Dallos JAG (2015) Practical aspects in gas chromatography-mass spectrometry for the analysis of pesticide residues in exotic fruits. Food Chem 182:14–22
Anastassiades M, Lehotay SJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Andrade GCRM, Monteiro SH, Francisco JG, Figueiredo LA, Botelho RG, Tornisielo VL (2015) Liquid chromatography-electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chem 175:57–65
Arias LA, Bojacá CR, Ahumada DA, Schrevens E (2014) Monitoring of pesticide residues in tomato marketed in Bogota, Colombia. Food Control 35:213–217
Burrows HD, Canle LM, Santaballa JA, Steenken S (2002) Reaction pathways and mechanisms of photodegradation of pesticides. J Photochem Photobiol B 67:71–108
Codex Alimentarius: International Food Standards (2013) IOP Publishing Codex Alimentarius. http://www.fao.org/fao-who-codexalimentarius/standards/pestres/commodities-detail/en/?c_id=320. Accessed 03 Feb 2016
Colombia, Ministerio De La Protección Social (2007) Por la cual se establecen los Límites Máximos de Residuos de Plaguicidas – LMR em alimentos para consumo humano y em piensos o forrajes. Resolución 2906 (p. 50). Bogotá, D.C. Issued 22.08.07
European Commission (2010) Regulation EU 600/2010. IOP Publishing European Commission.http://ec.europa.eu/food/plant/pesticides/legislation/max_residue_levels_en.htm. Accessed 06 Feb 2016
Ferrer C, Lozano A, Agüera A, Girón AJ, Fernández-Alba AR (2011) Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A 1218:7634–7639
Fischer G, Almanza-Merchán PJ, Miranda D (2014) Importancia y cultivo de la uchuva (Physalis peruviana L.). Rev Bras Frutic 36:1–15
Golge O, Kabak B (2015) Evaluation of QuEChERS sample preparation and liquid chromatography-triple-quadrupole mass spectrometry method for the determination of 109 pesticde residues in tomatoes. Food Chem 176:319–332
Kemmerich M, Rizzetti T, Martins ML, Prestes OD, Adaime MB, Zanella R (2015) Optimization by central composite design os a modified QuEChERS method for extraction of pesticide multiresidue in sweet pepper and analysis by ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Anal Methods 8:728–739
Legiscomex (2013) Inteligencia de mercados - Exportación de frutas exóticas colombianas. IOP Publishing Legiscomex. http://legiscomex.com/BancoMedios/Documentos%20PDF/exportaciones-estudio-frutas-exoticas.pdf. Accessed 03 Feb 2016
Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fud W, Mastovska K, Hoh E, Leepipatpiboon N (2010) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A 1217:2548–2560
Maštovská K, Lehotay SJ (2004) Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues. J Chromatogr A 1040:259–272
Mazorra MF, Quintana AP, Miranda D, Fischer G, Valencia MC (2006) Aspectos anatómicos de la formación y crecimiento del fruto de uchuva Physalis peruviana (Solanaceae). Acta Biol Colomb 11:69–81
Melo A, Cunha SC, Mansilha C, Aguiar A, Pinho O, Ferreira IMPLVO (2012) Monitoring pesticide residues in greenhouse tomato by combining acetonitrile-based extraction with dispersive liquid-liquid microextraction followed by gas-chromatography-mass spectrometry. Food Chem 135:1071–1077
Muniz J, Kretzschmar AA, Rufato L, Pelizza TR, Marchi T, Duarte AE, Lima APF, Garanhani F (2011) Sistemas de condução para o cultivo de Physalis no planalto catarinense. Rev Bras Frutic 33:830–838
Orso D, Floriano L, Ribeiro LC, Bandeira NMG, Prestes OD, Zanella R (2016) Simultaneous determination of multiclass pesticides and antibiotics in honey samples based on ultra-high performance liquid chromatography-tandem mass spectrometry. Food Anal Methods 9:1638–1653
Pinho GP, Neves AA, Queiroz MELR, Silvério FO (2009) Matrix effect in pesticide quantification by gas chromatography. Quim Nov. 32:987–995
Prestes OD, Adaime MB, Zanella R (2011) QuEChERS: possibilities and trends in sample preparation for multiresidue determination of pesticides in food. Scientia Chromatogr 3:51–64
Prestes OD, Friggi CA, Adaime MB, Zanella R (2009) QuEChERS—a modern sample preparation method for pesticide multiresidue determination in food by chromatographic methods coupled to mass spectrometry. Quim Nov. 32:1620–1634
Puente LA, Pinto-Muñoz CA, Castro ES, Cortés M (2011) Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res Int 44:1733–1740
Queiroz SCN, Ferracini VL, Rosa MA (2012) Validação de método multirresíduo para determinação de pesticidas em alimentos empregando QuEChERS e UPLC-MS/MS. Quim Nov. 35:185–192
Rekha NSN, Prasad R (2006) Pesticide residue in organic and conventional food-risk analysis. J Chem Health Saf 13:12–19
Restrepo AR, Ortiz AFG, Ossa DEH, Mesa GAP (2014) QuEChERS GC-MS validation and monitoring of pesticide residues in different foods in the tomato classification group. Food Chem 158:153–161
Rizzetti T, Kemmerich M, Martins ML, Prestes OD, Adaime MB, Zanella R (2016) Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in the orange juice by UHPLC-MS/MS. Food Chem 196:25–33
Romero-González R, Garrido Frenich A, Martínez Vidal JL (2008) Multiresidue method for fast determination of pesticides in fruit juices by ultra performance liquid chromatography coupled to tandem mass spectrometry. Talanta 76:211–225
SANTE, EUROPEAN COMMISSION 2015 Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. Document n° SANTE/11945/2015
Sousa FA, Costa AIG, Queiroz MELR, Teófilo RF, Pinho GP, Neves AA (2013) Influence of pH and matrix components in the chromatographic response of pesticides. Chromatographia 76:67–73
Wilkowska A, Biziuk M (2011) Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem 125:803–812
Zanella R, Prestes OD, Martins ML, Adaime MB (2015) Quantitative analysis and method validation. In: Tuzimski T, Sherma J (eds) High performance liquid chromatography in pesticide residue analysis. CRC Press, New York, pp. 303–323
Acknowledgments
The authors are grateful to the financial support and fellowship grants from the Brazilian agencies CNPq and CAPES. Natalia Cadavid Muñoz thanks the “Programa de Alianzas para la Educación y la Capacitación” OEA-GCUB 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Council of Scientific and Technological Development (CNPq), Brazil.
Conflict of Interest
Natalia C. Muñoz declares that she has no conflict of interest. Luana Floriano declares that she has no conflict of interest. Maiara P. de Souza declares that she has no conflict of interest. Nelson Bandeira declares that he has no conflict of interest. Osmar D. Prestes declares that he has no conflict of interest. Renato Zanella declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Muñoz, N.C., Floriano, L., de Souza, M.P. et al. Determination of Pesticide Residues in Golden Berry (Physalis peruviana L.) by Modified QuEChERS Method and Ultra-High Performance Liquid Chromatography-Tandem Quadrupole Mass Spectrometry. Food Anal. Methods 10, 320–329 (2017). https://doi.org/10.1007/s12161-016-0582-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0582-7