Abstract
A new method for simultaneous determination of phoxim, chlorpyrifos, and pyridaben residues in edible mushrooms and the substrate at trace levels was developed using QuEChERS (quick, easy, cheap, efficient, rugged, and safe) analytical procedure and ultra-high-performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-MS/MS). The target compounds were determinated using UHPLC-MS/MS with an electrospray ionization source in positive mode (ESI+). The limit of detection (LOD) for the three compounds ranged from 1 to 10 μg kg−1, and the limit of quantification (LOQ) was 10 μg kg−1. Quantification was performed by calibration curves of standards from 0.01 to 1.0 mg kg−1 with correlation coefficients >0.994. Recovery studies were performed at three spiked levels of 0.01, 0.05, and 0.25 mg kg−1 in blank mushrooms and the substrate, and the overall average recoveries ranged from 75.5 to 98.4 %. The data demonstrated the good repeatability of the method with relative standard deviations (RSDs) lower than 11.4 % for all analytes. The results showed that the method is efficient and reliable for the simultaneous determination of phoxim, chlorpyrifos, and pyridaben residues in mushrooms and substrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edible mushrooms are not only high nutritive value, unique flavor, taste good but it is also recognized as healthy food with important positive health function and anti-tumor activity, more and more popular with consumers (Kalač 2013; Rop et al. 2009; Wisor et al. 2001). Among them in cultivation and consumption, Pleurotus ostreatus (oyster mushroom), Lentinus edodes (shiitake mushroom), Pleurotus eryngii (eryngii mushroom), and Agaricus bisporus (crimini mushroom) are the four of the most edible mushrooms. They can have a lot of beneficial nutrients to human body health, including amino acids, polysaccharide, fiber, chitin, and other substances; these ingredients are raising the usage value of these edible mushrooms (Gaglarirmak 2011; Nitschke et al. 2011). Edible mushroom production was for more than 20,200 thousand tons in China, and total output value was more than $20 billion. It is one of the world’s largest producers and exporters of edible fungi in 2009. Meanwhile, China, American, The Netherlands, France, and Spain are the most important producers of mushrooms in the world. Mushroom production in these countries accounted for 76 % of the world. But with the enlargement of the four kinds of edible mushroom cultivation, the incidence of pests and diseases has become more and more serious (Lewandowski et al. 2004; White 1981). Sciarid flies and Cecid, as dipteran insects, are widespread pests in mushroom all over the world (Binns 1981). Two pests were listed as the main research object in many mushroom production countries (van de Geijn 1983). In order to reduce the economic losses, a lot of fungicides and insecticides were used in mushrooms. It may lead to excessive pesticide residues and affect the export of mushrooms. Therefore, it is important to monitor residues in mushrooms to reduce the use of pesticides and improve the quality.
Phoxim is widely used in the production of highly efficient and low toxicity of organophosphorus pesticide (Li et al. 2010). It used to against a variety of Lepidoptera insect larvae and eggs and Dipteran insects (Wu et al. 1997). It can inhibit nerve in the body of the activity of acetylcholinesterase and destroys the normal nerve impulses conduction to play an insecticidal effect (Kwong 2002). Chlorpyrifos not only belongs to organophosphate pesticides, with triple function of stomach toxicity, contact poisoning, and fumigation, but also inhibits acetylcholinesterase activity (Han et al. 2013). Pyridaben is a kind of acaricide. It can be used to prevent and control miticidal and insecticidal activity (Hirata et al. 1995). It is mainly inhibition of muscle tissue, nerve tissue, and the synthesis of glutamate dehydrogenase on chromosome I in electron transfer system. In the process of mushroom cultivation, in order to prevent and treat pest of Sciarids and Cecid, these pesticides are often used on mushrooms to get more revenue. Therefore, in order to ensure food safety and the normal foreign trade, it has become more important to determine the residue of these insecticide and acaricide on mushrooms.
To our knowledge, some reports use liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) to analysis phoxim of residue in tea and grain (Greulich and Alder 2008; Huang et al. 2009). Residues of chlorpyrifos and pyridaben also have been reported in vegetables and fruits by liquid chromatography coupled to tandem mass spectrometry (Kmellár et al. 2008; Lehotay et al. 2005; Ortelli et al. 2005; Sannino et al. 2004; Tran et al. 2012). And there are several papers that applied QuEChERS for the extraction of pesticides from mushrooms, and determination of residue used GC, GC-MS/MS (Cengiz et al. 2014; Peruga et al. 2013; Tran et al. 2012), or LC-MS/MS (Du et al. 2013). But a multi-residue analysis method for simultaneous determination of phoxim, chlorpyrifos, and pyridaben in the mushrooms by UHPLC-MS/MS has not yet been reported. In recent years, liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) is growing in popularity in pesticide residue analysis because of its high sensitivity and selectivity (Pan et al. 2008; Takatori et al. 2008; Lv et al. 2009). It has become the main tool for pesticide residue analysis in different complicated matrices. Moreover, it makes sample pretreatment steps become easier, and quantification and confirmation become more accurately at the low concentration level. In addition, the QuEChERS (quick, easy, cheap, efficient, rugged, and safe) method was introduced by Anastassiades et al. (Anastassiades et al. 2003); it has been developed as an attractive method for pesticide multi-residue analysis in the process of sample preparation. And the QuEChERS approach takes advantage of the wide analytical scope and high degree of selectivity and sensitivity provided by the mass spectrometry for detection (Hu et al. 2015).
Therefore, the objective of the present study is to develop a simple, economical, sensitive, and accurate analytical method for simultaneous determination of phoxim, chlorpyrifos, and pyridaben residues in oyster mushroom, shiitake, eryngii, crimini mushroom, and the substrate of mushroom by UHPLC-MS/MS. The reliability of analytical method developed was confirmed by its application to the analysis of authentic samples.
Materials and Methods
Chemicals and Materials
The analytical standards of phoxim (purity, 98.4 %) and pyridaben (purity, 98.5 %) were bought from Qinchengyixin Technology Development Co., Ltd. (Beijing, China). The analytical standard chlorpyrifos (98.0 % purity) was obtained from the National Institute of Metrology (Beijing, China). Chromatography grade formic acid, acetonitrile, and methanol were bought from Honeywell International Inc. (New Jersey, USA). Acetonitrile, sodium chloride (NaCl), and anhydrous magnesium sulfate (anhydrous MgSO4) for pesticide residue analysis were analytical grade and purchased from Beijing Chemical and Reagent Company (Beijing, China). Primary-secondary amine (PSA), octadecylsilane (C18), and Florisil were obtained from Agela Technologies Inc. (Newark, DE, USA).
Standard stock solutions (100 mg l−1) of phoxim, chlorpyrifos, and pyridaben were prepared in chromatography grade acetonitrile. The solvent solutions and matrix-matched standard solutions were prepared (0.01, 0.05, 0.1, 0.5, and 1.0 mg l−1) from the standard stock solution by serial dilution with acetonitrile. All the solutions were protected against light with aluminum foil and stored in a refrigerator in the dark at 4 °C until use.
Instrumentation and Chromatographic Conditions
Chromatographic separation employed a Waters ACQUITY UHPLC BEH C18 column (100 × 2.1 mm, 1.7-μm particle size) on a Waters ACQUITY ultra-performance liquid chromatography system with the mobile phase consisting of (A) 0.2 % formic acid in ultrapure water and (B) acetonitrile (chromatography grade). The gradient elution program was as follows: 0–1.5 min, 90–10 % A; 1.5–3 min, retain 10 % A; 3–3.1 min, 10–90 % A; 3.1–5 min, retain 90 % A; equilibration of the column. The mobile phase was pumped at a flow rate of 0.3 ml min−1. The separation and stabilization were finished in 5.0 min. The column oven temperature was maintained at 45 °C in order to decrease viscosity, and the temperature in the sample manager was set at 5 °C. The sample volume injected was at 5 μl.
Analyses of phoxim, chlorpyrifos, and pyridaben were carried out on a triple-quadrupole mass spectrometer (TQD, Waters Corp., USA) equipped with an electrospray ionization (ESI). The capillary voltage was set at 3.0 kV. The source temperature and desolvation temperature were kept at 120 and 350 °C, respectively. The desolvation and cone gas were set at flow of 600 and 50 l h−1, respectively. All other settings were as determined during the regular tuning and calibration of the instrument. Both positive and negative ionization modes had been conducted to optimize the mass parameters by infusion of each compound. All other ESI and MS parameters were optimized individually for each target compound and listed in Table 1. Data were collected using the Masslynx NT v.4.1 (Waters, USA) software. The approximate retention time of phoxim, chlorpyrifos, and pyridaben were 2.73, 3.01, and 3.26 min under the described conditions, respectively.
Sample Preparation
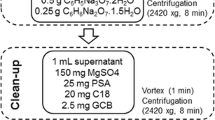
The mushroom samples were prepared using modified QuEChERS method; 10 g of samples were weighed into a 50-ml Teflon centrifuge tube. Appropriate volumes of mixed working standard solution were added to blank samples to validate recovery studies. Then, the tubes containing spiked samples were vortexed for 30 s and allowed to stand for 30 min at room temperature. Water (5 ml) was added for the substrate of mushroom, and then acetonitrile (10 ml) were added to extract the pesticides. Further, the mixtures were vigorously shaken for 10 min on the vortex at the maximum setting. Subsequently, 4 g anhydrous MgSO4 and 1 g sodium chloride (NaCl) were added. The tubes were shaken vigorously for 5 min, immediately. The tubes were then centrifuged at RCF 2811×g for 5 min to provide a well-defined phase separation. For clean-up, a volume of 1.5 ml of extraction solvent was transferred into a single-use centrifuge tube containing 50 mg PSA and 150 mg anhydrous MgSO4. The samples were capped and vortexed for 1 min and then centrifuged for 5 min at RCF 2077×g. The resulting supernate was immediately filtered by a 0.22-μm nylon syringe filter and then transferred to an auto-sampler vial. Then, the extract was analyzed for UHPLC-MS/MS injection.
Method Validation
In order to verify the absence of interfering substances around the retention time of analytes, the blank samples were analyzed. The linearity of the method was evaluated by linear regression analysis of the standard solutions and the different matrix-matched standard solutions at five concentrations from 0.01 to 1 mg kg−1. The LOQ was established as the lowest concentration level validated with satisfactory values of recovery (70–120 %) and RSD ≤ 20 %. The LOD is taken as the lowest concentration of the three compounds in the five matrices that can be detected under the stated conditions of the test. To investigate the accuracy and precision of the method, the recovery assays were carried out that five replicates of spiked samples at three different levels (0.01, 0.05, and 0.25 mg kg−1) were prepared on three different days. The recoveries were determined by compared the extracted spiked samples with the matrix-matched standards at the same concentration.
Results and Discussion
Optimization of MS/MS Parameters
The MRM transitions and associated acquisition parameters were optimized for the maximum abundance of fragmented ions under ESI mode conditions. The MS/MS parameters of the three target compounds was tested in both positive and negative ion modes, and the ESI in positive mode was selected for the analysis because it can obtain higher precursor ion signal intensities and better fragmentation patterns than the negative mode. The more abundant ion transition and the less abundant ion transition of chlorpyrifos and pyridaben were used for quantitation and identification, respectively. While the phoxim is different from them, the more abundant ion transition was used for identification, and the less abundant ion transition was used for quantitation. For all the analytes, the molecular weights, precursor ions, daughter ions, cone voltages, corresponding collision voltages, etc. are provided in Table 1.
Optimization of Chromatography
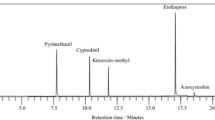
UHPLC was optimized to ensure the three kinds of target compounds’ complete separation, and obtain a suitable uptime and optimal peak shape. The chromatography condition was also optimized by means of different mobile phases. Thus, methanol, acetonitrile and water, or water (containing 0.2 % formic acid) was tested as mobile phases. The results demonstrated that the most efficient chromatographic conditions were obtained when the system consisting of acetonitrile and water (containing 0.2 % formic acid) was used. It may be that the formic acid in water improves the protonation of target compounds. The chromatograms of the three compounds were obtained from the analysis of blank eryngii sample and eryngii sample spiked at 10 μg kg−1 (Fig. 1a, b). As shown in Fig. 1, no interference peaks of blank samples were detected at the retention time of the fortified samples, and the analysis time of phoxim, chlorpyrifos, and pyridaben was shorter than 3.3 min.
Optimization of Purification Procedure
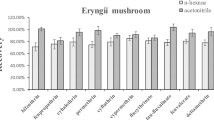
In order to choose an appropriate cleanup agent, four types of sorbent, PSA, C18, Florisil, and a combination of C18 and PSA, were tested to compare purification effect and recovery rate of the compounds extracts in matrices. Comparison of the four sorbents of purification effect was performed by spiking three replicate samples (oyster mushroom, shiitake, eryngii, crimini mushroom, and the substrate of mushroom) at the same concentration level (0.05 mg kg−1). PSA (50 mg), C18 (50 mg), Florisil (50 mg), and a combination of C18 (30 mg) and PSA (20 mg) were used in the experiments, respectively. PSA is mainly used to remove all kinds of polar organic acids, polar pigments, and some sugars from the non-polar samples. In addition, Florisil is also used to remove polar compounds from the non-polar samples. On the contrary, C18 applies to extract non-polar and medium-polar compounds from the polar samples (Chen et al. 2014). As the results showed in Fig. 2, the recovery and RSD were both satisfied when PSA and C18 were used in shiitake, eryngii, and the substrate of mushroom, but poor result when Florisil used in all the matrix. It may be due to the some target compounds were selectively retained by Florisil in that specific matrix conditions. Furthermore, better recoveries were obtained when the PSA was used except for the substrate of mushroom. Finally, considering these results, PSA used as the absorbent for mushroom clean-up was better than other cleaning agent in purification effect and recovery rate, while for the substrate of mushroom requires used C18 purification, and the recovery rate is the highest.
Matrix Effects
It is well known that ionization of the target compounds can be affected by the presence of matrix components which have been co-extracted from the sample when ESI is used (Li et al. 2011). Therefore, in the current study, the matrix effect on the MS/MS (MRM mode) detector using the proposed method was investigated in these matrices by comparing the standards in the solvent with the matrix-matched standards. The slopes in the calibration were obtained for the phoxim, chlorpyrifos, and pyridaben (0.01–1 mg kg−1) using the matrix-matched standards compared with those obtained using the solvent standards. Table 2 shows the slope ratio of the matrix to the solvent for each pesticide of phoxim, chlorpyrifos, and pyridaben. As a result, the co-extracted substances from the sample matrix were shown to cause an obvious suppression of the three pesticide peak responses in the matrix compared to that of the same concentration of the three pesticides in the solvent. Therefore, a calibration was carried out for these three pesticides using the external matrix-matched standards to eliminate the matrix effect and to obtain more realistic results in all samples in this study.
Linearity, LOD, and LOQ
The calibration curves obtained for the phoxim, chlorpyrifos, and pyridaben (from 0.01 to 1 mg kg−1) in different matrices were shown in Table 2. The regression equations and coefficients (R 2) of the linearity for all the pesticides were also shown in Table 2. Satisfactory linearity was obtained with correlation coefficients (R 2) higher than 0.994. The LODs for the three pesticides in all the matrices were estimated to be 1–10 μg kg−1, and the LOQs for the three target compounds were 10 μg kg−1. The LOQ for phoxim was equal to the maximum residue limit (MRL) required by the EU (0.01 mg kg−1 in mushrooms). However, the LOQ for the other two compounds was much below the maximum residue limit (MRL) required by the EU (chlorpyrifos, 0.05 mg kg−1 in mushrooms; pyridaben, 0.05 mg kg−1 in mushrooms).
Precision and Accuracy
Evaluation of the recoveries and RSDs of these three pesticides were applied to validate the UHPLC-MS/MS method by spiking the blank samples at different levels (0.01, 0.05, and 0.25 mg kg−1). The precision of the method was determined by the repeatability and reproducibility studies, and expressed as the RSD. As shown in Table 3, the mean recoveries of the method ranged from 75.5 to 98.4 %, with 1.0–11.2 % intra-day RSD and 1.8–11.4 % inter-day RSD as summarized in Table 3. The mean recoveries for phoxim ranged from 75.5 to 96.3 % with RSDs of 1.0–11.1 %, whereas the mean recoveries ranged from 78.9 to 98.4 % with RSDs of 2.6–11.4 % for chlorpyrifos, and 81.9 to 96.2 % with RSDs of 1.4–8.2 % for pyridaben. The results of the recovery studies demonstrated that the UHPLC-MS/MS method can achieve a satisfactory accuracy, precision, and sensitivity for residue analysis of these three pesticides in oyster mushroom, shiitake, eryngii, crimini mushroom, and the substrate of mushroom. Figure 1 showed the typical chromatograms of these three pesticides.
Application to Real Samples
In order to evaluate the effectiveness and applicability of this method in measuring trace levels of phoxim, chlorpyrifos, and pyridaben, it was monitored by analyzing oyster mushroom, shiitake, eryngii, and crimini mushroom. Fifty samples were determined which were purchased from the local market (Beijing, China). Pyridaben was detected in crimini mushroom, and the concentration ranged from 0.008 to 0.015 mg kg−1. However, all of these three pesticides were not found in other tested samples.
Conclusions
In this work, a UHPLC-MS/MS method for the trace analysis of the three pesticide in mushrooms (oyster mushroom, shiitake, eryngii, and crimini mushroom) and the substrate of mushroom was successfully developed and validated. The developed UHPLC-MS/MS analysis method combined with modified QuEChERS method followed by purification provided sufficient selectivity and sensitivity for the simultaneous determination of the three compounds. And this developed method not only showed satisfactory validation parameters in terms of linearity, lower limits, accuracy, and precision but the time, labor, and volume of reagent consumption were also minimized. The chromatographic separation was performed on a BEH C18 column using binary gradient elution containing acetonitrile and 0.2 % formic acid in ultrapure water. This method provided baseline separation of the target compounds within 3.3 min with good specificity. The LOQ were below 10 μg kg−1, and the mean recoveries for the three pesticides ranged from 75.5 to 98.4 % in the different matrices. In a word, the proposed method was suitable for routine monitoring of phoxim, chlorpyrifos, and pyridaben residues in mushrooms and the substrate of mushroom samples to ensure food safety.
References
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Binns E (1981) Sciarids as migrants. Mushroom J 108:415–423
Cengiz MF, Catal M, Erler F, Bilgin AK (2014) Rapid and sensitive determination of the prochloraz residues in the cultivated mushroom Agaricus bisporus (Lange). Imbach Anal Methods 6:1970–1976
Chen X, Xu J, Liu X, Tao Y, Pan X, Zheng Y, Dong F (2014) Simultaneous determination of trifloxystrobin and trifloxystrobin acid residue in rice and soil by a modified quick, easy, cheap, effective, rugged, and safe method using ultra high performance liquid chromatography with tandem mass spectrometry. J Sep Sci 37:1640–1647
Du P et al (2013) Rapid residue analysis of pyriproxyfen, avermectins and diflubenzuron in mushrooms by ultra-performance liquid chromatography coupled with tandem mass spectrometry. Anal Methods 5:6741–6747
Gaglarirmak N (2011) Chemical composition and nutrition value of dried cultivated culinary-medicinal mushrooms from Turkey International Journal of Medicinal Mushrooms 13
Greulich K, Alder L (2008) Fast multiresidue screening of 300 pesticides in water for human consumption by LC-MS/MS. Anal BioanalChem 391:183–197
Han Y et al (2013) The behavior of chlorpyrifos and its metabolite 3, 5, 6-trichloro-2-pyridinol in tomatoes during home canning. Food Control 31:560–565
Hirata K, Kawamura Y, Kudo M, Igarashi H (1995) Development of a new acaricide, pyridaben. Nippon Noyaku Gakkaishi 20:177–179
Hu M, Liu X, Dong F, Xu J, Li S, Xu H, Zheng Y (2015) Determination of ametoctradin residue in fruits and vegetables by modified quick, easy, cheap, effective, rugged, and safe method using ultra-performance liquid chromatography/tandem mass spectrometry. Food Chem 175:395–400
Huang Z et al (2009) Simultaneous determination of 103 pesticide residues in tea samples by LC‐MS/MS. J Sep Sci 32:1294–1301
Kalač P (2013) A review of chemical composition and nutritional value of wild‐growing and cultivated mushrooms. J Sci Food Agric 93:209–218
Kmellár B, Fodor P, Pareja L, Ferrer C, Martínez-Uroz M, Valverde A, Fernandez-Alba A (2008) Validation and uncertainty study of a comprehensive list of 160 pesticide residues in multi-class vegetables by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1215:37–50
Kwong TC (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24:144–149
Lehotay SJ, Maštovská K, Lightfield AR (2005) Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J AOAC Int 88:615–629
Lewandowski M, Sznyk A, Bednarek A (2004) Biology and morphometry of Lycoriella ingenua (Diptera: Sciaridae). Biol Lett 41:41–50
Li B, Wang Y, Liu H, Xu Y, Wei Z, Chen Y, Shen W (2010) Resistance comparison of domesticated silkworm (Bombyx mori L.) and wild silkworm (M.) to phoxim insecticide. Afr J Biotechnol 9:1771–1775
Li Y et al (2011) Simultaneous enantioselective determination of fenbuconazole and its main metabolites in soil and water by chiral liquid chromatography/tandem mass spectrometry. J Chromatogr A 1218:6667–6674
Lv Z, Gao L, Gao H, Hou Z, Zhang B (2009) Improved determination of phoxim residue in stored wheat by HPLC with DAD. J Food Sci 74:T37–T41
Nitschke J, Altenbach H-J, Malolepszy T, Mölleken H (2011) A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr Res 346:1307–1310
Ortelli D, Edder P, Corvi C (2005) Pesticide residues survey in citrus fruits. Food Addit Contam 22:423–428
Pan J, Xia X-X, Liang J (2008) Analysis of pesticide multi-residues in leafy vegetables by ultrasonic solvent extraction and liquid chromatography-tandem mass spectrometry. Ultrason Sonochem 15:25–32
Peruga A, Barreda M, Beltrán J, Hernández F (2013) A robust GC-MS/MS method for the determination of chlorothalonil in fruits and vegetables. Food Addit Contam: Part A 30:298–307
Rop O, Mlcek J, Jurikova T (2009) Beta‐glucans in higher fungi and their health effects. Nutr Rev 67:624–631
Sannino A, Bolzoni L, Bandini M (2004) Application of liquid chromatography with electrospray tandem mass spectrometry to the determination of a new generation of pesticides in processed fruits and vegetables. J Chromatogr A 1036:161–169
Takatori S et al (2008) A rapid and easy multiresidue method for the determination of pesticide residues in vegetables, fruits, and cereals using liquid chromatography/tandem mass spectrometry. J AOAC Int 91:871–883
Tran K, Eide D, Nickols SM, Cromer MR, Sabaa-Srur A, Smith RE (2012) Finding of pesticides in fashionable fruit juices by LC–MS/MS and GC–MS/MS. Food Chem 134:2398–2405
van de Geijn J (1983) Prevention and control of pests on Dutch mushroom farms. Mushroom J 28:390–396
White P (1981) Spread of the mushroom disease Verticillium fungicola by Megaselia halterata (Diptera: Phoridae) Protection Ecology (Netherlands)
Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM (2001) Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21:1787–1794
Wu K, Liang G, Guo Y (1997) Phoxim resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in China. J Econ Entomol 90:868–872
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31371970 and 31272070).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This is an original research article that no part of this paper has been published or submitted elsewhere.
Informed Consent
All authors named in a manuscript are entitled to the authorship and have approved the final version of the submitted manuscript. This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tian, F., Liu, X., Xu, J. et al. Simultaneous Determination of Phoxim, Chlorpyrifos, and Pyridaben Residues in Edible Mushrooms by High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Anal. Methods 9, 2917–2924 (2016). https://doi.org/10.1007/s12161-016-0490-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0490-x