Abstract
A simple and rapid method based on magnetic solid-phase extraction (MSPE) combined with high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (HPLC–MS/MS) was used for the determination of 15 sulfonamides from milk samples. The extraction and cleanup used a graphene oxide-based magnetic nanocomposite (Fe3O4@GO) as an adsorbent. Various experimental parameters that could affect the extraction efficiencies, such as the amount of Fe3O4@GO, the extraction time, the ionic strength of sample solution, and the type of eluent, were investigated. Under optimized experimental conditions, good linearity was observed in the range of 2.0 to 100.0 μg L−1 for all of the analytes, with correlation coefficients (R2) ranging from 0.994 to 0.999. The limits of detection for the method ranged between 0.02 and 0.13 μg L−1. Mean values of the relative standard deviation of intraday and interday precision ranging from 1.0 to 7.3 % and from 1.7 to 8.1 % were obtained, respectively. The average recoveries were between 73.4 and 97.4 % at three different spiked levels. It was confirmed that the Fe3O4@GO nanocomposite was an effective MSPE material for use in sulfonamide analyses in milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfonamides (SAs) are a group of synthetic antimicrobials that are frequently employed for clinical and veterinary purposes to control bacterial infection (Roach et al. 2011). However, in animal husbandry, SAs are directly administered or added to animal feed to prevent and treat gastrointestinal and respiratory diseases. Uncontrolled use of SAs can lead to the accumulation of these drugs in animal-derived food (Pereira et al. 2012). The presence of these residues, despite their minimal amounts, can induce adverse effects in humans, such as allergic reactions in hypersensitive individuals, with long-term effects, including carcinogenicity, and prolonged exposure can result in the selection of resistant bacteria in the human body (Wang et al. 2012; Zhang et al. 2008). To ensure food safety, several international organizations, such as the European Union (EU) commission and Food and Drug Administration (FDA), have established a maximum reside limit (MRL) of 100 μg L−1 and 100 μg kg−1, respectively, for SAs in food of animal origin, such as milk (European Union 1999).
A variety of techniques have been employed for the determination of SAs contained in foodstuff, including fluorescence competitive immunoassay (Won et al. 2011), capillary electrophoresis (Soto-Chinchilla et al. 2006), gas chromatography mass spectrometry (GC-MS) (Reeves 1999), high-performance liquid chromatography (HPLC) (Hyun-Hee et al. 2009; Arroyo-Manzanares et al. 2014), and liquid chromatography tandem mass spectrometry (LC-MS/MS) (Gao et al. 2010; Chen et al. 2013). Among these techniques, LC–MS/MS has become a powerful tool for the analysis of SAs in complex samples. Not only does this method provide accurate quantification of multiple compounds but it also offers good sensitivity and short analysis times.
One of the key stages in the analysis of SAs is the extraction, cleanup, and preconcentration of the samples. Various sample preparation techniques have been employed for the extraction of SAs, such as liquid–liquid extraction (LLE), matrix solid-phase dispersion (MSPD), and solid phase extraction (SPE) (Garrido Frenich et al. 2010; Li et al. 2013; Tso et al. 2011). SPE is the most widely used method to perform a selective extraction of SA compounds from various matrices. C18 cartridges and Oasis HLB cartridges are common adsorbents for SPE (Heller et al. 2002; Meng et al. 2015). Although these methods can obtain satisfactory results, the SPE procedure requires a multistep, time-consuming operation and the commercial SPE cartridges are expensive.
Recently, a new magnetic solid-phase extraction (MSPE) method has been proposed for the preconcentration of a variety of inorganic and organic compounds from various matrices (Zheng et al. 2014; Synaridou et al. 2014; Huang et al. 2014; Ibarra et al. 2014; Luo et al. 2011). Graphene oxide (GO) is the oxidized derivative of graphene, which is usually produced through the strong oxidation of graphite. The two-dimensional single-atom thickness structure of GO possesses an ultrahigh-specific surface area (Sitko et al. 2013; Marcano et al. 2010; Wu et al. 2013). A magnetic graphene oxide nanocomposite (Fe3O4@GO) will not only have a high adsorption capacity but also the convenience of magnetically controllable separation (Han et al. 2012; Pan et al. 2014; Zeng et al. 2013; Sun et al. 2015). Recently, GO was used as an effective adsorbent for the removal of SAs from water (Chen et al. 2015; Hu et al. 2015). However, to the best of our knowledge, there has been no report on the enrichment of SAs in a milk matrix by using Fe3O4@GO as an adsorbent.
In our current work, Fe3O4@GO nanoparticles were synthesized and their application for the MSPE of SAs from milk samples was studied. The optimal conditions of MSPE and the instrument used were investigated to achieve higher sensitivity and higher specificity. The experimental results show that this method is a convenient and sensitive analytical method for monitoring SAs in milk samples.
Experimental
Chemicals and Materials
Graphite powder (99 %) was purchased from Alfa Aesar Co. Sodium nitrate (98 %), potassium permanganate (99 %), hydrogen peroxide (30 %), H2SO4 (98 %), iron (III) chloride hexahydrate (FeCl3·6H2O, 99 %), and iron (II) sulfate heptahydrate (FeSO4·7H2O, 99 %) were analytical grade and purchased from Beijing Chemical Factory (Beijing, China). Chromatographic grade acetonitrile, methanol, and acetone were purchased from Tedia Co. (Fairfield, OH, USA). Deionized water was obtained from a Milli-Q waters system (Millipore, Bedford, USA).
Sulfacetamide (SA), sulfamethizol (SMT), sulfisoxazole (SIZ), sulfachloropyridazine (SCP), sulfadiazine (SDZ), sulfamethoxazole (SMX), sulfathiazole (STZ), sulfamonomethoxine (SMM), sulfamerazine (SMR), sulfadoxin (SDX), sulfapyridine (SPD), sulfameter (SME), sulfamethazine (SMZ), sulfaphenazole (SP), and sulfadimethoxine (SDX) were supplied by Dr. Ehrenstorfer GmbH Co. (Augsburg, Germany, purity >98 %). A mixed stock solution of all standards was prepared in methanol at a concentration of 0.1 mg mL−1 and stored at 4 °C in darkness.
Synthesis of Fe3O4@GO
GO was synthesized win a slightly modified Hummers process (Wu et al. 2013). A 500-mL beaker equipped with a magnetic stirring bar was charged with 72 mL of H2SO4 and cooled at 0–5 °C. Graphite flakes (1.5 g) were added slowly with vigorous stirring, followed by the slow addition of 1.5 g of NaNO3 and 4.5 g of KMnO4, with the temperature of the reaction mixture maintained below 10 °C. The mixture was allowed to warm to room temperature and stirred for 1 h. The mixture was charged with deionized water (120 mL) and stirred for 30 min while the temperature was kept at 95 °C. The mixture was poured into deionized water (300 mL), followed by the slow addition of 10 mL of H2O2. The final reaction mixture was subjected to a wash with 5 % HCl and deionized water as well as repeated centrifugation. The resultant GO material was dried in a freeze-dryer overnight. GO (0.1 g) was exfoliated in 10 mL of DI water by sonication for 40 min. Iron(III) chloride hexahydrate (FeCl3·6H2O, 0.54 g) and iron(II) sulfate heptahydrate (FeSO4·7H2O, 0.28 g) were dissolved in deionized water (40 mL) and added slowly into the GO suspension. An ammonia (0.5 mL) solution was added to the solution and stirred for 1 h at 60 °C in a nitrogen atmosphere. The Fe3O4@GO product was collected by an external magnetic field and washed with deionized water three times and dried in a freeze-dryer overnight. The dry Fe3O4@GO was ground to powder using a mortar before use.
Characterization of Fe3O4@GO
The morphology and size of Fe3O4@GO were investigated using a JEOL JEM-2100 transmission electron microscope (TEM) at an accelerating voltage of 200 kV. Infrared analyses were conducted with a Bruker Vertex 80v Fourier transform infrared (FT-IR) spectrometer, where KBr was used to prepare the sample tablets. The magnetic properties of Fe3O4@GO were measured using a MPMS5 Quantum Design superconducting quantum interference device (SQUID) at 300 K over a range of applied fields from −10,000 to 10,000 Oe.
Sample Preparation
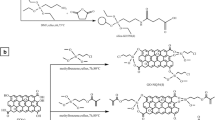
Milk samples were purchased from local retail markets and stored at 4 °C. A schematic representation of the proposed extraction process is shown in Fig. 1. The procedure included the following steps: a 1-mL milk sample was placed in a 5-mL centrifuge tube, and a 100-μL 10 % perchloric acid solution was added with subsequent vortex-mixing for 1 min. Samples were diluted to 4 mL with phosphate buffer (20 mM, pH 5.0) and again vortex-mixed for 2 min, followed by centrifugation at 10,000 rpm for 5 min; the supernatant was used for MSPE. An 80-mg sample of the MGO was added to the above extract, and the mixture was shaken for 5 min on a slow-moving platform shaker; the SAs-adsorbed Fe3O4@GO samples were magnetically separated with the assistance of a magnet, and then the SAs were desorbed with 1.0 mL of acetonitrile containing a 5 % ammonium hydroxide solution (v/v) with a 1-min vortex time. This desorption step was repeated two times. The eluents were combined and evaporated to dryness under a gentle N2 stream at 45 °C. The residue was dissolved in 500 μL of initial mobile phase, and 20 μL of the sample solution was injected into a LC–MS/MS system for analysis.
LC–MS/MS Apparatus and Conditions
The samples were analyzed using a Shimadzu liquid chromatographic system consisting of LC20-AD binary pumps, a DGU-20A degasser, a CTO-20AC column oven, and a SIL-20AC autosampler (Shimadzu, Tokyo, Japan) connected to a 4000 QTRAP Mass spectrometer (Foster City, CA, USA).
Separation was carried out on a Shimadzu reverse phase SHIM-PACK XR-ODS column (I.D. 3.0 mm × 75 mm, 2.2 μm). The flow rate of the mobile phase was 0.3 mL/min. The column oven was set to 30 °C. Eluent A was water, and eluent B was acetonitrile, both of which contained 0.1 % formic acid. Gradient elution was performed by changing the mobile phase composition as follows: 0–4 min, 15 % B; 5–12 min, 15–50 % B; 12–12.1 min, 50–15 % B; and 12.1–15 min, 15 % B.
For LC–MS/MS analyses, the ESI interface was used in positive ion mode with the following settings: temperature (TEM) 350 °C; curtain gas (CUR), nitrogen, 10 psi; nebulizer gas (GS1), air, 50 psi; heater gas (GS2), air, 50 psi; and ion spray voltage 5500 V. The mass spectrometer operated in multiple reaction monitoring (MRM) mode by monitoring two transitions (one quantifier, one qualifier) for each compound. The optimized declustering potential (DP), entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP) are listed in Table 1.
Results and Discussion
Characterization of Fe3O4@GO
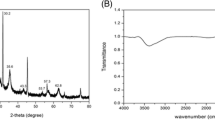
The morphologies of the GO and Fe3O4@GO were characterized by TEM. Figure 2a reveals that the GO sheet exhibited an irregular shape and contained some wrinkles, which maintained a large surface area. Figure 2b demonstrates the distribution of the magnetic nanoparticles on a GO sheet. The SQUID was used to investigate the magnetic properties of Fe3O4@GO. Figure 2c demonstrates that Fe3O4@GO exhibited good super paramagnetic properties with a saturation magnetization value (Ms) ∼ 32 emu g−1 at 300 K and a very low coercivity (Hc) ∼7 Oe. The Fe3O4@GO was aggregated (within 1.0 min) onto the vial wall by an external magnetic field. The FT-IR spectrum of Fe3O4@GO shows O–H stretching of approximately 3350 cm−1, as well as signals for surface carboxylic groups C=O–O (1720 cm−1), aromatic C=C (1620 cm−1), carboxyl O=C–O (1360 cm−1), and alkoxy C–O (1050 cm−1). The FT-IR spectrum of MGO reveals that aromatic alkene C=C (1570 cm−1), carboxyl O=C–O (1360 cm−1), and C–O (1200 cm−1) are the remaining functional groups, demonstrating that a number of oxygen functionalities have successfully been reduced. The Fe–O bond (575 cm−1) and amine N–H broad band (3000–3300 cm−1 centered at 3200 cm−1) confirm the magnetic functionalities of Fe3O4@GO (Fig. 2d).
Optimization of Adsorption and Desorption Conditions
Effect of the Amount of Fe3O4@GO
The effect of the amount of Fe3O4@GO on the SAs extraction was investigated and the range as modulated from 20 to 120 mg. As shown in Fig. 3, the peak area of the SAs rapidly increased as the amount of adsorbent increased, and the maximum plateau of adsorption was achieved when the amount of Fe3O4@GO increased to 80 mg. Afterward, the extracted amount was almost constant, indicating that 80 mg of sorbent was sufficient to extract Fe3O4@GO.
Effect of the Extraction Time
The effect of extraction time was investigated in the range of 1 to 15 min. As shown in Fig. 4, the peak area reached a maximum for SAs at 5 min. It can be concluded that extraction equilibrium between the aqueous phases and the sorbents was nearly reached after 5 min. To shorten the analysis time, all further extractions of SAs were carried out at the optimized extraction time of 5 min.
Effect of the Solution Ionic Strength
In the MSPE process, the ionic strength of the sample solution is an important parameter that influences the extraction efficiency. In this study, the effect of the ionic strength was investigated. NaCl was added to adjust the ionic strength of the solution. As shown in Fig. 5, the peak area of the SAs decreased rapidly with the NaCl concentration (0∼5.0 %). Therefore, there is no need to adjust the ionic strength of sample solution.
Desorption Conditions
Desorption of the SA analytes from Fe3O4@GO was studied by using different organic solvents (methanol, acetonitrile, acetone, all containing 5 % ammonium hydroxide solution). The results showed that acetonitrile containing a 5 % ammonium hydroxide solution had the strongest desorption power among these eluents (Fig. 6).
Reusability of Fe3O4@GO
After the sorption of the SAs from the magnetic adsorbent, the Fe3O4@GO sorbent was reused for the MSPE of the SAs. The results showed that the Fe3O4@GO could be reused at least six times without a significant decrease in the sorption capacity. It is evident that Fe3O4@GO nanocomposites have the advantage of reusability, which can significantly reduce the cost of sample preparation.
Analytical Performance and Applications
For HPLC separation, different organic modifiers (acetonitrile and methanol with or without formic acid) were compared. The results showed that both acetonitrile and water containing 0.1 % formic acid (v/v) was the optimum additive, with highest sensitivity and resolution. The gradient was optimized to render a rough separation between the target compounds in a short run time. The 15 SA compounds in milk samples were identified and confirmed by comparing the retention time and MS spectrum of the reference standards.
For HPLC/MS/MS detection, the matrix effect is a factor affecting accurate quantitative analysis, and this paper examines the milk samples for matrix effects in 15 types of SA. We found that SDZ, SPD, and SMM have a strong matrix enhancement effect and that SCP, SIZ, and SP demonstrate a substrate inhibition effect, with the results shown in Fig. 7. Therefore, matrix-matched calibration curves were selected to compensate for potential matrix effects occurring during sample preparation and analysis. The sample solutions were spiked with stock solution to obtain final SA concentrations of 2.0, 5.0, 10.0, 25.0, 50.0, and 100 μg L−1. As shown in Table 2, satisfactory correlation coefficients ranging from 0.994 to 0.999 were obtained. The determination of the analytical limits was conducted by spiking samples with a low concentration of analytes, followed by MSPE–HPLC–MS/MS analysis, and the limits of detection (LOD) and limits of quantitation (LOQ) were calculated using signal-to-noise ratios of 3 and 10, respectively. Intraday precision was examined by analyzing the standard solution within 1 day, and interday precision was determined for six independent days. These results are shown in Table 3. The recovery was determined by spiking milk sample with three mass concentrations (20, 50, and 100 μg L−1) for each compound. The concentration levels and the detailed results are summarized in Table 4. The results showed the average recoveries were in the range of 73.4 to 97.4 %. As we know, C18 cartridges are common adsorbents, it was reported recoveries of which were 51–101 % for extraction of SA compounds (Heller et al. 2002). While with Oasis HLB cartridges as adsorbents, recoveries were 61–115 % (Meng et al. 2015). The MSPE method used in this study differs from traditional SPE method in that the Fe3O4@GO adsorbents are well dispersed in sample solution and can rapidly contact the analytes by vortexing. The weak-polar SAs are associated with Fe3O4@GO surface via π–π and cation–π interactions. The hydrophilic and more polar components remain in the sample solution. The SAs-adsorbed Fe3O4@GO adsorbents can be easily isolated from the sample solution using an external magnet. Figure 8 shows LC-MS/MS chromatograms of a milk sample containing 50 μg L−1 of each sulfonamide monitored at their specific MRM transitions.
Sample Analysis
The proposed methodology was applied in the determination of SAs in 10 commercial milk samples from different brands. Low concentrations of SDZ (0.88 μg L−1) and SDX (1.14 μg L−1) were found in two different milk samples, and no residues were detected in the other eight samples. The concentration values in the positive samples did not exceed the legal limit, and their contamination levels do not seem to pose a threat to public health.
Conclusions
In the current study, a magnetic graphene oxide nanocomposite (Fe3O4@GO) was synthesized and, for the first time, was used as an effective adsorbent for the extraction and purification of SAs in milk samples prior to HPLC–MS/MS. The use of Fe3O4@GO as an MSPE adsorbent endowed the method with an easy separation of the adsorbent from the sample solution and a high extraction capacity, while avoiding the time-consuming SPE cartridge handling process that is required in conventional SPE methods. The Fe3O4@GO nanocomposite synthesis procedure is mild, fast, and can be repeatedly used as an MSPE sorbent. Good repeatabilities and recoveries were obtained. The results indicated the developed method is suitable for the extraction and determination of trace SAs contained in milk samples.
References
Arroyo-Manzanares N, Gámiz-Gracia L, García-Campaña AM (2014) Alternative sample treatments for the determination of sulfonamides in milk by HPLC with fluorescence detection. Food Chem 143:459–464
Chen L, Zhou T, Zhang Y, Lu Y (2013) Rapid determination of trace sulfonamides in fish by graphene-based SPE coupled with UPLC/MS/MS. Anal Methods 5:4363–4370
Chen H, Gao B, Li H (2015) Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J Hazard Mater 282:201–207
European Union (1999) Commission Regulation 281/96 of 14 February 1996 amending Annexes I and III to Council Regulation (EEC) No. 2377/90 laying down a Community procedure for establishment of maximum residue limits of veterinary medical products in foodstuff of animal origin. Off J L37:9
Gao Q, Luo D, Ding J, Feng Y (2010) Rapid magnetic solid-phase extraction based on magnetite/silica/poly (methacrylic acid-co- ethylene glycol dimethacrylate) composite microspheres for the determination of sulfonamide in milk samples. J Chromatogr A 1217:5602–5609
Garrido Frenich A, Aguilera-Luiz Mdel M, Martínez Vidal JL, Romero-González R (2010) Comparison of several extraction techniques for multiclass analysis of veterinary drugs in eggs using ultra-high pressure liquid chromatography–tandem mass spectrometry. Anal Chim Acta 661:150–160
Han Q, Wang Z, Xia J, Chen S, Zhang X, Ding M (2012) Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 101:388–395
Heller DN, Ngoh MA, Donoghue D, Podhorniak L, Righter H, Thomas MH (2002) Identification of incurred sulfonamide residues in eggs: methods for confirmation by liquid chromatography–tandem mass spectrometry and quantitation by liquid chromatography with ultraviolet detection. J Chromatogr B 774:39–52
Hu S, Cao W, Da J, Dai H, Cao J, Ye L, Li X, Chu C (2015) Dispersive micro solid-phase extraction with graphene oxide for the determination of phenolic compounds in dietary supplements by ultra high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Food Anal Methods 8:833–840
Huang D, Deng C, Zhang X (2014) Functionalized magnetic nanomaterials as solid-phase extraction adsorbents for organic pollutants in environmental analysis. Anal Methods 6:7130–7141
Hyun-Hee C, Jung-Bin L, Yun-Hee C, Kwang-Geun L (2009) Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using microbial assays and high performance liquid chromatography. Food Chem 113:297–301
Ibarra IS, Miranda JM, Rodriguez JA, Nebot C, Cepeda A (2014) Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milk samples. Food Chem 157:511–517
Li H, Sun H, Zhang J, Pang K (2013) Highly sensitive and simultaneous determination of sixteen sulphonamide antibiotics, four acetyled metabolites and trimethoprim in meat by rapid resolution liquid chromatography-tandem mass spectrometry. Food Control 31:359–365
Luo Y, Shi Z, Gao Q, Feng Y (2011) Magnetic retrieval of graphene: extraction of sulfonamide antibiotics from environmental water samples. J Chromatogr B 1218:1353–1358
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun ZZ, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Meng Z, Shi Z, Liang S, Dong X, Li H, Sun H (2015) Residues investigation of fluoroquinolones and sulphonamides and their metabolites in bovine milk by quantification and confirmation using ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem 174:597–605
Pan S, Zhou L, Zhao Y, Chen X, Shen H, Cai M, Jin M (2014) Amine-functional magnetic polymer modified graphene oxide as magnetic solid-phase extraction materials combined with liquid chromatography-tandem mass spectrometry for chlorophenols analysis in environmental water. J Chromatogr A 1362:34–42
Pereira R, Eustáquia de Freitas E, Fabiano de Alkimim J, Azevedo E, Vasconcellos D, Augusti R (2012) Development and validation of a method for the determination of sulfonamides in animal feed by modified QuEChERS and LC-MS/MS analysis. Food Control 28:192–198
Reeves VB (1999) Confirmation of multiple sulfonamide residues in bovine milk by gas chromatography-positive chemical ionization mass spectrometry. J Chromatogr B 723:127–137
Roach JA, DiBussolo JM, Krynitsky A, Noonan GO (2011) Evaluation and single laboratory validation of an on-line turbulent flow extraction tandem mass spectrometry method for melamine in infant formula. J Chromatogr A 218:4284–4290
Sitko R, Zawisza B, Malicka E (2013) Graphene as a new sorbent in analytical chemistry. Trends Anal Chem 51:33–43
Soto-Chinchilla JJ, García-Campaña AM, Gámiz-Gracia L, Ruces-Blanco CC (2006) Application of capillary zone electrophoresis with large-volume sample stacking to the sensitive determination of sulfonamides in meat and ground water. Electrophoresis 27:4060–4068
Sun J, Liang Q, Han Q, Zhang X, Ding M (2015) One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 132:557–563
Synaridou ME, Sakkas VA, Stalikas CD, Albanis TA (2014) Evaluation of magnetic nanoparticles to serve as solid-phase extraction sorbents for the determination of endocrine disruptors in milk samples by gas chromatography mass spectrometry. J Chromatogr A 1348:71–79
Tso J, Dutta S, Inamdar S, Aga DS (2011) Simultaneous analysis of free and conjugated estrogens, sulfonamides, and tetracyclines in runoff water and soils using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Agric Food Chem 59:2213–2222
Wang L, Wu J, Wang Q, He C, Zhou L, Wang J, Pu Q (2012) Rapid and sensitive determination of sulfonamide residues in milk and chicken muscle by microfluidic chip electrophoresis. J Agric Food Chem 60:1613–1618
Won SY, Lee CH, Chang HS, Kim SO, Lee SH, Kim DS (2011) Monitoring of 14 sulfonamide antibiotic residues in marine products using HPLC-PDA and LC-MS/MS. Food Control 22:1101–1107
Wu MC, Deokar AR, Liao JH, Shi PY, Ling YC (2013) Graphene-based photothermal agent for rapid and effctive killing of bacteria. ACS Nano 7:1281–1290
Zeng S, Gan N, Weideman-Mera R, Cao Y, Li T, Sang W (2013) Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem Eng J 218:108–115
Zhang H, Zhang Y, Wang S (2008) Development of flow-through and dip-stick immunoassays for screening of sulfonamide residues. J Immunol Methods 337:1–6
Zheng H, Mo J, Zhang Y, Gao Q, Ding J, Yu Q, Fen Y (2014) Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples. J Chromatogr A 1329:17–23
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Basic Science Research Foundation (grant number: 2452015203) of Northwest A&F University.
Conflict of Interest
Yutang Wang declares that he has no conflict of interest.
Laping Liu declares that he has no conflict of interest.
Chunxia Xiao declares that she has no conflict of interest.
Lin Chen declares that she has no conflict of interest.
Peng Yang declares that he has no conflict of interest.
Qian Liu declares that she has no conflict of interest.
Jianlong Wang declares that he has no conflict of interest.
Xuebo Liu declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Yutang Wang and Laping Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, L., Xiao, C. et al. Rapid Determination of Trace Sulfonamides in Milk by Graphene Oxide-Based Magnetic Solid Phase Extraction Coupled with HPLC–MS/MS. Food Anal. Methods 9, 2521–2530 (2016). https://doi.org/10.1007/s12161-016-0433-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0433-6