Abstract
A novel unique liquid chromatographic method has been developed consisting of diode array and fluorescence detection, using phenyl-hexyl stationary phase of the column, for the determination of over thirty phenolic compounds (anthocyanins, flavonols, flavan-3-ols, hydroxybenzoic acids, hydroxycinammic acids, and stilbenes). The method was validated including the following parameters: linearity, limits of detection and quantification, precision, and reproducibility. Excellent selectivity and sensitivity have been achieved. Detection levels ranging from 1.79 × 10−5 mg/L to 0.26 mg/L were obtained. New fluorescence wavelengths for detection of flavan-3-ols (λ ex = 225 and λ em = 320 nm) were more sensitive than previously published. The new optimized method showed good repeatability and reproducibility, and values for relative standard deviations (RSD) were less than 3 %. Applicability of the method was demonstrated for five different matrices, white and red wine from Vitis vinifera cultivars, apple cider, blackberry wine, and grape skin extracts, showing that method is robust and can be applied for routine analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenolics from fruits and their derivate are structurally diverse, from simple molecules to oligomers and polymers usually designated as tannins (Terrier et al. 2009). They have an important impact on the organoleptic properties of fruits and wines as well the beneficial properties to health; thus, their analysis and quantification are of great importance. A large number of chromatography methods have been developed and applied for analysis of phenolic compounds in different fruits and wine over the past few decades (Merken and Beecher 2000). HPLC techniques are widely used for both separation and quantification of phenolic compounds (Monagas et al. 2005a; Lamuela-Raventos et al. 1994; Berente et al. 2001; Monagas et al. 2005b; Alonso et al. 1986; Negishi and Ozawa 1996; Vitrac at al. 2002).
HPLC systems are typically coupled with UV-vis, diode array (DAD), electrochemical (ED), mass spectrometry (MS), and fluorescence (FLD) detectors. The use of fluorescence detectors has allowed increasing selectivity and sensitivity for determination of flavan-3-ols and hydroxybenzoic acids (Sun et al. 2006a; Jeandet et al. 1997; Rodriguez Delgado et al. 2001; Gomez-Alonso et al. 2007).
Lately, there is often use of direct injection of wine samples without previous sample preparation apart from filtration in the HPLC system (Merken et al. 2000; Lamuela-Raventos et al. 1994). The great complexities of the chromatograms obtained from wine usually require sample preparation before analysis. Solid-phase extraction is the most commonly applied method for sample preparation (Sun et al. 2006b; Pinelo et al. 2006; Chilla et al. 1996; Dominguez et al. 2001; Matejiček et al. 2003; Baptiste et al. 2001; Del Almo et al. 2004; Zotou et al. 2008; Perez-Magarino et al. 2008).
C18 stationary phase is the most common column used for the separation of polyphenol compounds (Merken et al. 2000). These columns are generally packed with spherical particles of silica bonded with octadecyl chain (C18).
Numerous methods have been described dealing with specific matrices or specific classes of phenols. The aim of this paper was to develop method dealing with different matrices and wide range of different types of polyphenols. To the best of our knowledge, method dealing with phenyl-hexyl stationary phase for the simultaneous HPLC analysis of more than thirty phenolic compounds in fruit wine or grape extracts has not previously been published. Phenyl-hexyl stationary phase retains analytes via several different mechanisms, including π-π interactions between the overlap of the delocalized electrons on the analyte and the stationary phase phenyl group, and via partitioning between the mobile phase and the hydrophobic aryl-alkyl phase. A phenyl group is bonded to the silica surface via hexyl chain.
This article describes the development of a new reproducible analysis method of more than 30 phenolic compounds representative for the various different families (flavan-3-ols, flavonols, anthocyanins, stilbenes, hydroxycinammic, and hydroxybenzoic acids) by employing phenyl-hexyl stationary phase of columns. Two detectors, diode array detector, and fluorescence detector were utilized. This permits greater selectivity in the quantification of the compounds. Excitation and emission wavelengths of the fluorescence detection were improved in order to realize the first quantification of flavan-3-ols and hydroxybenzoic acids in different matrices by fluorimetry. In addition, a solid-phase extraction (SPE) method was used to provide a rapid technique for the isolation of monomeric polyphenol components.
Materials and Methods
Materials
Delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, peonidin-3-O-glucoside, malvidin-3-O-glucoside, epigallocatechin, procyanidin B1, procyanidin B2, quercetin-3-O-rutinoside, quercetin-3-galactoside, and myricetin were purchased from Extrasynthese (Geney, France). Caffeic acid, caftaric acid, vanillic acid, p-coumaric acid, ferulic acid, gallic acid, (−)-epicatechin, (+)-catechin, epicatechin gallate, quercetin-3-O-glucoside, trans-reveratrol, quercetin, kaempferol, isorhamnetin, and syringic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
All chromatographic solvents were HPLC grade purchased from J.T.Baker (Deventer, Netherlands). Formic acid and 85 % -orthophosphoric acid were obtained from Fluka (Buchs, Switzerland). Tartaric acid was purchased from Riedel de Haen (Seelze, Germany). Ethanol and 1-M sodium hydroxide solution were provided from Kemika (Zagreb, Croatia).
A synthetic wine was prepared containing 12 % (v/v) ethanol and 3.5 g/L tartaric acid, and pH was adjusted to 3.5 with 1 M NaOH. Stock solution was prepared; each polyphenol standard was weighed and dissolved in methanol. Mixtures of standard solutions for calibration were prepared by diluting stock solutions in synthetic wine. Calibration curves were made in 5 points.
Samples
Commercial wines Merlot and Manzoni were obtained directly from Experiment station Jazbina, Faculty of Agriculture University of Zagreb, Croatia. Blackberry wine and apple cider samples were purchased in local stores in Zagreb, Croatia. Grape samples (cv. Cabernet Sauvignon) were obtained from a vineyard located at Experiment station Jazbina, Faculty of Agriculture University of Zagreb, Croatia. Grapes were harvest in state of full ripeness.
SPE Procedure
Prior HPLC analysis, wine samples, and apple cider were prepared by solid-phase extraction. Separation was achieved by solid-phase extraction on polymeric cartridge N-vinylpirolidon-divinylbenzen copolymer Strata X (33 μm, 500 mg, 3 mL), Phenomenex, Torrance, CA, USA. Originally this method was from Jefferey et al. (2008). The cartridge was conditioned with methanol, followed by water. One milliliter of red wine or 5 mL of white and cider was applied to cartridge. The cartridge was dried in vacuum for 5 min. All wanted components were eluted by 40 mL of 95 % acetonitrile/5 % 0.01 M HCl. The solvent was removed on a Hei-Vap Adventage G3 (Heidolph, Schwabach, Germany) rotary evaporator at 30 °C to dryness. The samples were dissolved in 1 mL of 12 % ethanol/0.1 % formic acid for HPLC analysis.
Analytical Procedure for SPE Recovery
The SPE recovery was determined using 17 commercially available and the most commonly contained standards in real samples. Standard mixture was prepared by adding 0.5 mg of standards and 1.2 mL of ethanol to a 10-mL volumetric flask and sonicating for 5 min. Then in the flask was added 5 mL of distilled water, and the solution was sonicated for further 5 min. Afterwards the flask was made up to the mark with water. The wines were spiked with 500 μL of prepared standard mixture in a 5-mL volumetric flask. For comparison, an additional sample of each wine was diluted to the same extent by adding 500 μL of 12 % v/v ethanol to the wine in a 5-mL volumetric flask.
Grape Extraction Procedure
The berry skins were manually removed from the pulp and air dried. Dry skins were grinded, and obtained powder (500 mg) was extracted by 10 mL of 70 % aqueous ethanol containing 1 % formic acid for one day in the dark. The extract was centrifuged in LC-321 centrifuge (Tehtnica, Železnik, Slovenia) for 20 min at 5000 rpm at room temperature. Supernatant was collected, concentrated under vacuum to remove ethanol (40 °C) on rotary evaporator and brought to final volume of 10 mL with mobile phase A. The extract was filtered with a Phenex-PTFE 0.20-μm syringe filter (Phenomenex, Torrance, USA) and analysed by HPLC.
HPLC Analysis
The analyses were performed on an HPLC Agilent 1100 (Agilent Technologies, USA) comprising a binary pump, an auto sampler, a diode array detector, and Agilent 1200 fluorescence detector. Separation was performed on a Luna Phenyl-Hexyl (Phenomenex, USA) column (250 mm × 4.6 mm i.d., 5 μm particle size) with a Phenyl guard column (4.0 × 3.0). The column was thermostated at 50 °C. The injection volume for all samples was 20 μL.
The solvent gradient described in Supplementary Table 1 was used for separation. Gradient consists of two phases: (A) water/phosphoric acid (99.5/0.5, v/v) and (B) acetonitrile/water/phosphoric acid (50/49.5/0.5, v/v/v).
For detection and quantification of compounds, the chromatograms were recorded at 280, 308, 320, 360, and 518 nm by diode array detector and at excitation wavelength 225 nm and emission wavelengths at 320, 365, and 392 nm by fluorescence detector. UV-vis spectra were recorded in range from 200 to 700 nm.
Quantification was obtained from calibration curves of external standards. Quantification of non-commercial available standards of anthocyanins was made according to calibration curves of malvidin-3-O-glucoside. In case of some flavonol glycosides and hydroxycinnamic acids, the calibration of structurally related compound was used. Samples were injected in triplicate.
HPLC-ESI-MS Analysis
For peak assignment, phenolic compounds were confirmed by HPLC-ESI-MS with Agilent 1200 Series system (Agilent, Germany) coupled on-line to an Agilent model 6410 mass spectrometer fitted with ESI source. The same column was used as previously described. The mobile phase was fixed to 0.5 mL/min. The solvents were as follows: (A) aqueous 0.1 % formic acid and (B) acetonitrile containing 0.1 % formic acid. Mass spectra were recorded from m/z = 100 to 1000 in a positive and negative ionization mode, alternately. The electrospray ionization (ESI) parameters were as follows: drying gas (N2) flow and temperature, 8 L/min and 300 °C, respectively; nebulizer pressure was 30 psi, capillary voltage was 4500 V for negative ion mode or −4500 V for positive ion mode. Fragmentation voltage was 135 V.
Results and Discussion
Optimization of the Chromatographic Conditions
Mixture of organic and aqueous solvent of phosphoric acid was chosen for mobile phases. Acetonitrile and methanol were tested as organic modifiers. The better fluorescence sensitivity and general performance was achieved using acetonitrile. According to Bonerz et al. (2008), this can be explained by air bubbles in the methanol eluent which is known to rapidly absorb air. In order to get the best separation of all phenolic compounds, we used mobile phases with very low pH. It is especially necessary for anthocyanins separation to get them in their most stable flavylium form.
To achieve excellent selectivity, it is necessary to choose proper detection wavelength. According to the literature, flavan-3-ols are widely quantified with UV-DAD at λ = 280 nm or using sensitive FLD at λ ex = 280 nm and λ em = 320 nm. In this study, the highest sensitivity was achieved at a wavelength of excitation λ ex = 225 nm and an emission wavelength λ em = 320 nm (Supplementary Figure 1). These observations can be explained by high excitation energy (5.51 eV), thus flavan-3-ols were quantified using FLD at specified wavelengths. Vanillic and syringic acids were quantified by UV-DAD at λ = 280 nm and for additional confirmation detected by FLD at the excitation wavelength λ ex = 225 nm and an emission wavelength λ em = 365 nm (Supplementary Figure 2).
All analysed compounds with their belonging detection wavelengths, absorption maximums, excitations, and emissions wavelengths are listed in Table 1.
Gradient slope (flow, time, and mobile phases composition) as well as gradient steps were optimized after injection of real samples to chromatographic system. Optimized gradient consist of four gradient steps. In the second gradient step elute, almost all target compounds except aglycons of flavonols which elute in the third and fourth gradient step, so it is necessary carefully optimize second gradient step. The greatest challenge was to achieve the good separation of flavonol glycosides. All flavonol glycosides have very similar structures and polarity so they elute in narrow range of mobile phases composition. For this reason, different gradient programs (time, flow, and temperature) were tested.
In the first chosen conditions, gradient slope between the first two steps was 20–40 % B in 18 min, but these conditions did not give satisfied separation (Supplementary Figure 3a). Also, the described conditions with constant flow rate of 0.9 mL/min were enhanced by using column temperature program. The best resolution of anthocyanins especially for peonidin-3-O-glucoside and malvidin-3-O-glucoside was achieved by temperature 50 °C. According to the literature, optimal separation of flavonol glycosides is below 50 °C; thus, it was decided to apply temperature program of the column (Supplementary Table 2). Temperature program of column did not give desired results. Moreover, the best separation of all targeted compounds was achieved at 50 °C. It is possible that this is a consequence of the mechanism of separation on phenyl-hexyl stationary phase and π-π interactions. The van Deemter equation evaluates efficiency as a function of flow rate, so we try to decrease flow rate in that critical gradient step at 0.5 mL/min and 0.7 mL/min, respectively, but it gave only slight improvement (Supplementary Figure 3b). Based on these observations, gradient slope was decreased between first two gradient steps from 20–40 % B in 18 min to 20–40 % B in 28 min (Supplementary Table 1). These conditions showed great improvement in separation of flavonol glycosides as well as optimal separation for all other target phenolic compounds (Supplementary Figure 3c).
Improvement of sensitivity for the analysis of white wine and apple cider was enhanced by increasing injection volume from 20 to 50 μL, but it did not give satisfactory results. Thus, it was decided to use for solid-phase extraction.
Final chromatographic parameters of phenolic compounds for the proposed HPLC method are presented in Table 2. The variation coefficient for retention time obtained from 10 analyses was less than 1 %.
Linearity, Limits of Detection, and Quantification
Calibration curves obtained from standard solutions in 5 points showed good linearity, and correlation coefficients were greater than 0.999. Table 3 shows mass concentration range of standard solutions for calibration, regression equation, regression coefficients (R 2), limits of detection (LOD), and limits of quantification (LOQ). LOD and LOQ were defined as the amounts for which signal-to-noise ratios (S/N) were 3 and 10, respectively.
Repeatability and Reproducibility
Intraday repeatability and interday reproducibility was studied using standard solutions prepared in synthetic wine. One synthetic wine sample containing known amounts of added polyphenolic compounds was injected in chromatographic system five subsequent times (in 1 day) applying optimized HPLC method described above. The relative standard deviations (RSD) of the five replicates samples for individual compounds are presented in Table 4. Reproducibility was determined with replicate samples analysed in five different days (5 replicate × 3 injections × 5 days), and RSD for each polyphenolic compound was calculated (Table 4). The new optimized method showed good repeatability and reproducibility. Values for RSD were <3 %.
Method Application
The most important achievement for this method was that it could be used for polyphenol analysis of different types of matrices. In this article, the results of polyphenols in red (Merlot) and white (Manzoni) wines, grapes from cultivar Cabernet Sauvignon, blackberry wine, and apple cider are presented.
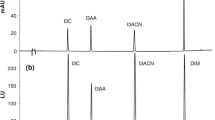
Wine samples and apple cider were submitted to sample preparation. Proposed SPE protocol, originally from Jefferey et al. (2008), used for rapid isolation of polymeric polyphenols proved to be excellent for pre-concentration of phenolic compounds in apple cider and white wines especially flavonols, also to avoid polymeric fractions in red wines, which occur as a big hump in the same interval as aglycones of flavonols elute at λ = 360 nm (Fig. 1a, b). Table 5 presents recoveries obtained in red and white wine. Recoveries (81–101 %) with RSD <10 % except for ferulic acid showed that the results were precise and reproducible.
Chromatograms of a Merlot wine at λ = 518 by direct injection, b Merlot wine at λ = 518 after SPE procedure on STRATA X cartridges, c hydroxycinammic acids profile of Manzoni wine recorded at λ = 320 nm, d grape skin extracts at λ = 518 nm, e grape skin extracts at λ = 360 nm, and f grape skin extracts at λ = 308 nm. For peak identification see Table 1
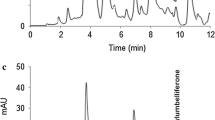
Table 6 shows the average values of triplicate analysis of the phenolic compounds in samples analysed using proposed HPLC method. In commercial white wine made from Manzoni grapes, the predominant phenolic acid is caftaric acid (Fig. 1c). Catechin was the most abundant flavonoid. In red wine made from Merlot, the most abundant flavonoid was malvidin-3-O-glucoside. Caftaric acid had the highest concentration of non-flavonoid compounds. In general, the concentration of individual compounds is in the range presented for Croatian wines from different geographical regions (Rastija et al. 2009; Katalinić et al. 2004; Plavša et al. 2012; Komes et al. 2007).
Blackberry wines were analysed by direct injection because of high content of phenolic compounds and lack of polymeric forms. The most abundant anthocyanin was cyanidin-3-O-glucoside. The predominant flavonol was quercetin-3-O-rutinoside.
In apple cider, the most abundant compound was chlorogenic acid. Among other families of phenolic compounds, flavan-3-ols are present in the highest concentration. Predominant flavan-3-ol was epigallocatechin.
The chosen conditions for extraction of polyphenols from grape skins were selective enough for extraction of simple polyphenol compounds; thus, SPE procedure is not required. Most abundant compounds are anthocyanins, especially malvidin-3-O-glucoside. Flavonols were presented only in glycoside form. Among them, quercetin-3-O-glucoside was the most abundant. Resveratrol was present only in glucosidic form. It was also observed a high content of caftaric acid. Chromatogram with specific compounds contained in grape extracts is presented in Fig. 1d–e.
Conclusion
A phenyl-hexyl stationary phase was used for the chromatographic determination of the great number (more than 30 compounds) of different phenolic compounds in grape, wine, apple cider, and blackberry wine using two detectors (DAD and fluorescence) in tandem for increased specificity and sensitivity. New excitation and emission wavelengths were established for determination of flavan-3-ols. Column was very stable under chosen conditions. Low pH (1.8) and high temperature of column (50 °C) did not harm to its performance even after 1500 injections of different types of wine samples and grape extracts.
References
Alonso E, Estrilla MI, Revilla E (1986) HPLC separation of flavonol glycosides in wines. Chromatographia 22:7–12
Baptiste JAB, da Tavares JFP, Carvalho RCB (2001) Comparison of polyphenols and aroma in red wines from Portuguese mainland Azores Islands. Food Res Int 34:345–355
Berente B, Reichenbächer M, Danzer K (2001) Improvement of the HPLC analysis of anthocyanins in red wines by use of recently developed columns. Fres J Anal Chem 371:68–72
Bonerz DPM, Pour Nikafardjam MS, Creasy GL (2008) A new RP-HPLC method for analysis of polyphenols and indole-3-acetic acid in wine. Am J Enol Vitic 59:106–109
Chilla C, Guillen DA, Barroso CG, Perez-Bustamante JA (1996) Automated on line solid phase extraction-high performance liquid chromatography-diode array detection of phenolic compounds in sherry wine. J Chromatogr A 750:209–214
Del Almo M, Casado L, Hernandez V, Jimenez JJ (2004) Determination of free molecular phenolics and catechins in wine by solid phase extraction on polymeric cartridges and liquid chromatography with diode array detection. J Chromatogr A 1049:97–105
Dominguez C, Guillen DA, Barroso CG (2001) Automated solid-phase extraction for sample preparation followed by high-performance liquid chromatography with diode array and mass spectrometric detection for the analysis of resveratrol derivates in wine. J Chromatogr A 918:303–310
Gomez-Alonso S, Garcia-Romero E, Hermosin-Gutierrez I (2007) HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J Food Comp Anal 20:618–626
Jeandet P, Brenil AC, Adrian M, Weston LA, Debord S, Meunier P, Maume G, Bessis R (1997) HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorometry. Anal Chem 69:5172–5177
Jeffery DW, Mercurio MD, Herderich MJ, Hayasaka Y, Smith PA (2008) Rapid isolation of red wine polymeric polyphenols by solid phase extraction. J Agric Food Chem 56:2571–2580
Katalinić V, Miloš M, Modun D, Boban M (2004) Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem 86:593–600
Komes D, Urlich D, Kovačević Ganić K, Lovrić T (2007) Study of phenols and volatile composition of white wine during fermentation and a short time of storage. Vitis 46:77–84
Lamuela-Raventos RM, Waterhouse AL (1994) A direct HPLC separation of wine phenolics. Am J Enol Vitic 45:1–5
Matejiček D, Klejdus B, Mikeš O, Šterbova D, Kuban V (2003) Application of solid phase extraction for determination of phenolic compounds in barrique wines. Anal Bioanal Chem 377:340–345
Merken HM, Beecher GR (2000) Measurement of food flavonoids by high performance liquid chromatography: a review. J Agric Food Chem 48:577–599
Monagas M, Bartolome B, Gomez-Cordoves C (2005a) Updated knowledge about the presence of phenolic compounds in wine. Crit Rev Food Sci Nutr 45:85–118
Monagas M, Suarez R, Gomez-Cordoves C, Bartolome B (2005b) Simultaneous determination of non-anthocyanin phenolic compound in red wines by HPLC-DAD/ESI-MS. Am J Enol Vitic 56:139–147
Negishi O, Ozawa T (1996) Determination of hydroxycinammic acids, hydroxybenzoic acids, hydroxylbenzaldehydes, hydroxybenzyl alcohols and their glucosides by high performance liquid chromatography. J Chromatogr A 756:129–136
Perez-Magarino S, Ortega-Heras M, Cano-Mozo E (2008) Optimization of a solid-phase extraction method using copolymer sorbents for isolation of phenolic compounds in red wines and quantification by HPLC. J Agric Food Chem 56:11560–11570
Pinelo M, Laurie VF, Waterhouse AL (2006) A simple method to separate red wine nonpolymeric and polymeric phenols by solid phase extraction. J Agric Food Chem 54:2839–2844
Plavša T, Jurinjak N, Antunović D, Peršurić Đ, Ganić Kovačević K (2012) The influence of skin maceration time on the phenolic composition and antioxidant activity of red wine Teran (Vitis vinifera L.). Food Technol Biotechnol 50:152–158
Rastija V, Srečnik G, Medić-Šarić M (2009) Polyphenolic composition of Croatian wines with different geographical origins. Food Chem 115:54–60
Rodriguez Delgado MA, Malovana S, Perez JP, Borges T, Garcia Montelongo FJ (2001) Separation of phenolic compounds by high performance liquid chromatography with absorbance and fluorimetric detection. J Chromatogr A 912:249–257
Sun B, Ribes AM, Leandro M, Belchior AP, Spranger MI (2006a) Stilbenes: quantitative extraction from grape skins, contribution of grape solids to wine and variation during wine maturation. Anal Chim Acta 563:382–390
Sun B, Leandro MC, De Freitas V, Spranger MI (2006b) Fractionation of red wine polyphenols by solid phase extraction and liquid chromatography. J Chromatogr A 1128:27–38
Terrier N, Poncet-Legrand C, Cheynier V (2009) Flavanols, flavonols and dihydroflavonols. In: Polo MC, Moreno-Arribas MV (eds) Wine chemistry and biochemistry. Springer, New York, pp 463–507
Vitrac X, Monti JP, Vercauteren J, Defieux G, Merillon JM (2002) Direct liquid chromatographic analysis of resveratrol derivates and flavanonols in wines with absorbance and fluorescence detection. Anal Chim Acta 458:103–110
Zotou A, Frangi E (2008) Development and validation of an SPE-LC method for the simultaneous determination of trans-resveratrol and selected flavonoids in wine. Chromatographia 67:789–793
Conflict of Interest
Ivana Tomaz declares that she has no conflict of interest. Luna Maslov declares that she has no conflict of interest.
Compliance with Ethics Requirements
This is an original research article that has neither been published previously nor considered presently for publication elsewhere. All authors named in a manuscript are entitled to the authorship and have approved the final version of the submitted manuscript. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ivana Tomaz and Luna Maslov contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 200 kb)
Rights and permissions
About this article
Cite this article
Tomaz, I., Maslov, L. Simultaneous Determination of Phenolic Compounds in Different Matrices using Phenyl-Hexyl Stationary Phase. Food Anal. Methods 9, 401–410 (2016). https://doi.org/10.1007/s12161-015-0206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0206-7