Abstract
A novel method for the separation/analysis of linuron in fruit and vegetable samples is described. The work is based on the utilization of ionic liquid ([C6min][PF6])-loaded β-cyclodextrin cross-linked polymer (IL-β-CDCP) for the effective adsorption of linuron coupled with ultraviolet spectrophotometry. The inclusion interaction of IL-β-CDCP with linuron is studied with FTIR and inclusion constants. Under optimum conditions, the preconcentration factor achieved for this method is approximately 10. Linear range, limit of detection (LOD), limit of quantification (LOQ), and relative standard deviation are 0.08–15.00 μg/mL, 5.8 ng/mL, 17.5 ng/mL and 0.96 % (n = 3, c = 4.0 μg/mL), respectively. This technique is successfully applied for determination of linuron in real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Linuron is one of the urea herbicides widely used in agriculture (Xu et al. 2006); it can exist in the environment for a long term, which would pollute soil and surface water, and further cause serious damage to groundwater and organisms. Toxicology studies indicate that these herbicides on humans have different degrees of toxicity, and even with carcinogenic effects (Zhang et al. 2012). Therefore, the accurate, sensitive, and at the same time quick and easy detection of linuron residues in fruits and vegetables is a method of great significance.
Various methods have been developed for the determination of linuron to date, including gas chromatography (Goncalvesa and Alpendurada 2003), HPLC (Mazzella et al. 2009), thin layer chromatography (Knorst et al. 1997), capillary electrophoresis (da Silva et al. 2003), etc. UV spectrometry has many advantages including simple operation, lower cost, and repeatable results, but it is often combined with separation and preconcentration techniques to improve the selectivity and sensitivity.
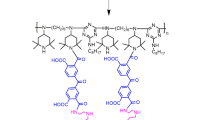
Solid-phase extraction (SPE) is of interest because of its simplicity, higher preconcentration factor, and rapid phase separation. Some novel functional materials such as activated carbon (Hennion 1999), zeolite (Liu et al. 2012), clay (Mafra and Blasco 2012), and cyclodextrin polymers (Yamasaki et al. 2006) have been used for SPE. Cyclodextrin polymers have attracted particular attention due to their unique physico-chemical characteristics, lower cost, availability, and presence of various reactive groups on its backbone chain (Crini 2005). The functionalization of cyclodextrin polymers can, in particular, alter their physical properties to make them suitable for a wide range of applications (Szeitjli 1998). Common methods for the functionalization of resin include load technology, bonding technology, and sol–gel technology.Load technology is proposed as an alternative green and economic process (Ren et al. 2011). Recent research on the application of functionalized cyclodextrin for separation/analysis include biological-based chitosan grafted β-cyclodextrin, benzoic acid (Chai and Ji 2012), β-cyclodextrin-ionic liquid polyurethanes, and organic pollutants/heavy metals (Mahlambi et al. 2010). Ionic liquid-loaded β-cyclodextrin cross-linked polymer as SPE material seems to be lacking.
In this work, ionic liquid was synthesized and loaded onto the β-cyclodextrin cross-linked polymer (IL-β-CDCP). FTIR and inclusion constants were used to study the inclusion interactions of IL-β-CDCP and linuron. IL-β-CDCP solid-phase extraction technique followed by UV was applied to separation/analysis of linuron in real samples.
Experimental
Materials and Reagents
Centrifuge (Anke Scientific Instrument Factory, Shanghai), timing multifunctional oscillator (Guohua Limited Company, China), digital water bath (Guohua Limited Company, China), and pH meter (Shanghai Jinjke Limited Company, China) were purchased from their respective companies.
FTIR spectra were measured with a Bruker Tensor27 spectrometer (Bruker Company, Germany). Samples were pressed in KBr pellets and recorded at frequencies from 4000 to 400 cm−1 with a resolution of 4 cm−1. A SEM Hitachi S-4800IIinstrument was used to obtain micrographs of the polymers. Absorbance monitoring was performed on a 2500 UV spectrophotometer (Shimadzu Corporation, Japan) with 1.0 nm spectrum bandwidth and slow speed scan.
β-Cyclodextrin, NaOH, HCl, carbinol, ethanol, and epoxy chloropropane were purchased from Shanghai Chemical Reagent Corporation (China). Linuron standard stock solution, with the standard 10 mg, was dissolved in 50.0 mL of ethanol and was stored in the dark at 4 °C. All chemicals and reagents were at least of analytical reagent grade, unless otherwise stated.
Synthesis of IL-β-CDCP
IL-β-CDCP was synthesized according to the literature (Ping et al. 2014). FTIR and SEM were used to certify that the solid particles were actually IL-β-CDCP.
The particle size of IL-β-CDCP was selected by using a standard screen before the experiment; the mesh size of the standard screen was 18, 24, 30, 45, and 80 mesh.
Real Sample Pretreatment
Water Sample
Lake water was collected from Slender West Lake in Yanzhou, China. Then, 50.0 mL of lake water sample was filtered through 0.45-μm membrane filters and stored at 4 °C.
Fruit and Vegetable Samples (Fan et al. 2007)
Apple and maize samples were supplied by our local market. Twenty grams of apple (or maize) slurry and 20 mL of anhydrous ethanol were placed in a 50-mL centrifuge, and the mixture was then shaken for 40 min. After centrifugation, the upper fluids in the tube were filtered and collected in a 50-mL volumetric flask and kept at 4 °C in the dark.
Static Adsorption and Elution of Linuron
At room temperature, the concentration of linuron 4.0 μg mL−1 and 0.1 g IL-β-CDCP was mixed at pH 7.0 and then fixed to 20.0 mL. The mixture was shaken on the multifunctional oscillator for 30.0 min and then centrifuged (2000 rpm, 10 min). Supernatant solution (solution after adsorption) was determined with UV at 246.0 nm.
At room temperature, the inclusion complex of linuron and IL-β-CDCP (IL-β-CDCP–linuron) was eluted with ethanol. The mixture (4.0 mL) was shaken on a multifunctional oscillator for 20.0 min and then centrifuged (2000 rpm, 10 min). Linuron in effluent solution was detected by UV at 246.0 nm.
Results and Discussion
Analytical Application
Factors affecting the adsorption process such as pH and particle size of IL-β-CDCP will be discussed.
Optimization of Adsorption Step
Effect of pH
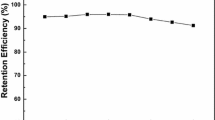
As shown in Fig. 1, (1) the adsorption behavior of linuron on IL-β-CDCP is similar to that on β-CDCP, but it cannot quantitatively absorb on β-CDCP (curve b). This results indicated that the adsorption ability of IL-β-CDCP for analyte is greatly improved due to the existence of ionic liquid which improved the hydrophobicity of polymer, (2) the retention efficiency of linuron on IL-β-CDCP remains above 85 % in the pH range of 3.0–9.0 and reaches a maximum at 7.0 (curve. a); the retention efficiency scarcely had any variation with pH. Thereafter, all experiments were conducted at pH 7.0.
Effect of IL-β-CDCP Size
It was pointed out that the smaller the particle size, the greater is the specific surface area and the bigger is the adsorption capacity (Lv and Tang 2008). The effect of IL-β-CDCP size (18–80 mesh) on the adsorption efficiency was investigated (Fig. 2). With the decrease of the IL-β-CDCP size (increase of mesh size), the adsorption efficiency of linuron on IL-β-CDCP increases. It quantitatively adsorbs linuron when the mesh size of IL-β-CDCP is larger than 45 mesh. In this study, the mesh size of IL-β-CDCP was 80 mesh.
Effect of the Sample Volume
The ratio of sample volume to elution volume would lead to an improvement in preconcentration factor. The concentration of linuron was fixed at 4.0 μg mL−1, and the volume of the sample solution increased from 5.0 to 40.0 mL (Fig. 3). The retention efficiency was greater than 85.0 % in the sample volume of 5.0–30.0 mL and reached a maximum from 5.0 to 20.0 mL. In this work, the sample volume of 20.0 mL was adopted.
Effect of Retention Temperature and Time
In this experiment, the retention efficiency of linuron on IL-β-CDCP at different temperatures (5.0–50.0 °C) was studied. The retention efficiency decreases with the increased temperature and is above 90 % from 5.0C to 30.0 °C. Retention efficiency at different times (10.0–60.0 min) was also studied, and the adsorption process was completed within 30.0 min, with the retention efficiency kept constant until 60 min. So, room temperature for 30.0 min was chosen for next experiment.
Optimization of Desorption
Selection of Eluants
The IL-β-CDCP could be reproduced by eluants. Linuron could be adopted on IL-β-CDCP due to the hydrophobic grouping of linuron entering into IL-β-CDCP in an acidic solution, so some hydrophobic organic solvents and NaOH were selected as eluants. Different eluants as ethanol, carbinol, SDS (1 %), CTAB (1 %), and NaOH (0.1 mol/L) were investigated (Fig. 4). The order of elution efficiency was ethanol (95.0 %) > carbinol (89.0 %) > CTAB (58.7 %) > SDS (42.3 %) > NaOH (10.0 %). It indicates that linuron cannot be quantitatively eluted by NaOH, SDS, and CTAB but can be completely eluted by ethanol since the hydrophobicity of ethanol was stronger than the other eluants, and it was an environment-friendly eluant. So, ethanol was chosen as the eluant.
Then, elution efficiency of linuron with 2.0–10.0 mL of ethanol was studied. The elution efficiency of linuron is above 85 % from 3.0 to 10.0 mL and reaches 95 % in 4.0 mL. The preconcentration factor is 10 (the quotient of volume before absorption and after elution) (Song et al. 2013). Therefore, the optimum volume of ethanol solution chosen for this work is 4.0 mL.
Effect of Elution Time
The elution efficiency of linuron on IL-β-CDCP at different times (10.0–60.0 min) was studied. The elution process is completed within 20.0 min; the retention efficiency did not change in 60.0 min, with a stable elution efficiency of 95.0 %. The experiment was conducted at 20.0 min.
Adsorption Capacity
The adsorption capacity, defined as the maximum amount of linuron sorbed per gram of the polymer, is an important factor for the evaluation of the synthesized IL-β-CDCP. As shown in Fig. 5, when the concentration of linuron is 55.00 μg/mL, the retention reaches a maximum value. The retention capacity of the polymer for linuron is therefore calculated as 4.0 mg/g (for diuron, 3.6 mg/g).
Effect of Foreign Substances
Determination of linuron (4.0 μg/mL) in the presence of foreign substances was investigated. With a relative error of less than ±5 %, data on the tolerance limit for various foreign substances are presented in Table 1. Most of the evaluated inorganic ions do not produce serious changes in the measured absorbance of linuron. The higher interference in systems is produced by diuron, which produced an increase of the absorbance due to UV–visible absorption wavelengths near that of the linuron. In fact, the highest interferent concentration tested was around the corresponding solubility value (Piccirillia GN et al. 2008). The tolerance ratio of diuron was not usually exceeding 1 because the solubility of linuron was larger than that of diuron (Fischer et al. 2014; Lo et al. 2014). But in a real situation, diuron could be present but not linuron (i.e., a farmer has used diuron), and this could be a false positive result of diuron. In these cases, detection after SPE separation might be replaced by HPLC (Molins et al. 2000).
The Adsorption Mechanism of IL-β-CDCP and Linuron
The inclusion interactions of IL-β-CDCP and linuron are the primary mechanism for the adsorption of linuron on IL-β-CDCP (Yang et al. 2011). In this paper, it is evidenced by FTIR and inclusion constants.
FTIR Analysis
Figure 6 shows the FTIR spectra of IL-β-CDCP, IL-β-CDCP–linuron, and linuron. The intensity of σ C=C peaks 1625 cm−1 in imidazole ring of IL-β-CDCP–linuron appears (curve b) and is weaker than that of in linuron (curve c), which is not in IL-β-CDCP (curve a). It is probably due to the fact that the ionic liquid bond interactions play a crucial role in the inclusion of IL-β-CDCP–linuron. The σ O–H peak (3430 cm−1) in IL-β-CDCP–linuron increases, which demonstrated that the hydroxy part in linuron is enclosed in the hydrophobic cavity of IL-β-CDCP–linuron (curves b and c).
Inclusion Interactions of IL-β-CDCP/β-CD and Linuron
The inclusion constant K is a significant parameter which illustrates inclusion interactions of host–guest molecules. The inclusion complex can be easily formed at a higher K. The inclusion constants of the monomers of two kinds of polymers (β-CD and IL + β-CD) and linuron were measured. The form of inclusion and the inclusion constant can be calculated by UV–visible absorption spectroscopy and Hildebrrand–Benesi equation (Bao et al. 2004; Zarzycki and Lamparczyk 1998).
The double reciprocal plots of the β-CD–linuron inclusion complex (curve a) and (IL-β-CD)–linuron inclusion complex (curve b) are shown in Fig. 7. It can be concluded that both β-CD and IL-β-CD form the inclusion complexes with linuron at a ratio of 1:1. The two double reciprocal plots show good linearity with correlation coefficients of 0.9921 for β-CD and 0.9958 for IL-β-CD. The inclusion constant of β-CD–linuron inclusion complex and for (IL-β-CD)–linuron inclusion complex K is 1.60 × 103 and 2.98 × 103 L/mol, respectively, which are acquired by the slope and intercept of the double reciprocal plots. Inclusion constant reflects the inclusion ability of a host molecule to a guest molecule. All of the above illustrate that the inclusion ability of IL-β-CD towards linuron is stronger than of β-CD. That is one reason why IL-β-CDCP has a higher adsorption efficiency than β-CDCP.
Analytical Parameters
Under optimum conditions described above, IL-β-CDCP shows a linear calibration curve within the concentration range of 0.08 to 15.00 μg/mL. The least square equation is:
The limit of detection, defined as C DL =3S b/m, where S b is the standard deviation of 11 replicate blank signals and m is the slope of the linear section of the calibration curve after preconcentration, is found to be 5.8 ng/mL for a sample volume of 20.0 mL, and the limit of quantification is determined as 3 × LODs 17.5 ng/mL (Nardelli et al 2010). The relative standard deviation for three separate batch experiments with 0.1 g of sorbent for determination of 4.0 μg mL−1 linuron in 20.0 mL of water is 0.96 %. The preconcentration factor was calculated to be 10. The recoveries for the spiked samples ranged from 92.0 to 104.0 % (Table 2).
Sample Analysis
The proposed method was applied to determine linuron in water, fruits, and vegetable samples. The results along with the recovery of linuron in the spiked samples are given in Table 2. The results indicated that the present method was feasible for the analysis of linuron in complicated matrices.
Comparison with Other Methods
The figures of merit of the present method of extraction and determination of linuron were compared with other reported methods. Table 3 presents data on comparison of the RSD and DL for the analysis of linuron in real samples with those reported in literatures. It is obvious that the present work was mainly focused on good precision and high sensitivity and could be operated easily.
Conclusion
IL-β-CDCP used as solid-phase extraction material to pre-concentrate/separate linuron coupled with UV for the analysis of linuron is established. The proposed method has some advantages, such as increase of contact area between IL and water, reduction of amount of ionic liquid, little loss of ionic liquid, and easy, safe, and inexpensive methodology for the separation and determination of linuron in fruit and vegetable samples.
References
Bao ZJ, Dai L, Miao ZT (2004) Chin J Yunnan Univ (Nat Sci Ed) 26:66
Chai KG, Ji HB (2012) Chem Eng J 203:309
Crini G (2005) Prog Polym Sci 30:38
Da Silva CL, de Lima EC, Tavares MFM (2003) J Chromatogr A 1014:109
Fan YM, Gu XJ, Yu XJ et al (2007) Anal Sci (Chinese) 23:583
Fischer LG, Baum EW, Crouse GD et al (2014) US Patent No. 20140274688A1 20140918
Goncalvesa C, Alpendurada MF (2003) J Chromatogr A 19:173
Hennion MC (1999) J Chromatogr A 856:3
Knorst MT, Neubert R, Wohlrab W (1997) J Pharm Biomed 15:1627
Li H, Shi LM, Zhou JK (2013) Chin J Food Ferment Technol 49:78
Liu Y, Guo YP, Gao W et al (2012) J Clean Prod 32:204
Lo WC, Hunter JE, Watson GB et al (2014) US Patent No. 20140171314 A1 20140619
Lv M, Tang L (2008) Chin J Metall Anal 28:75
Mafra L, Blasco T (2012) Annu Rep NMR Spectrosc 77:259
Mahlambi MM, Malefetse TJ, Mamba BB (2010) J Polym Res 17:589
Mazzella N, Delmas F, Delest B et al (2009) J Environ Monit 11:108
Molins C, Hogendoorn EA, Dijkman E et al (2000) J Chromatogr A 869:487
Nardelli V, dell’Oro D, Palermo C, Centonze D (2010) J Chromatogr A 1217:4996
Piccirillia GN, Escandar GM, Ca˜nada F et al (2008) Talanta 77:852
Ping WH, Zhu XS, Wang B (2014) Anal Lett 3:504
Ren RB, Wang Y, Zhang R et al (2011) Talanta 83:1392
Song XY, Shi YP, Chen J (2013) Food Chem 139:246
Szeitjli J (1998) Chem Rev 98:1743
Xiao L, Wang YC, Chen MR (2010) Chin J Tea Sci 1:52
Xu Y, Zhao Y, Zhu J et al (2006) Agrochemicals 2:19
Yamasaki H, Makihata Y, Fukunaga K (2006) J Chem Tech Biotech 81:1271
Yang ZJ, Chai KG, Ji HB (2011) Sep Purif Technol 80:209
Zarzycki PK, Lamparczyk H (1998) J Pharm Biomed 18:165
Zhang Y, Zhang GW, Zhang YP (2012) Chin J Anal Lab 31:36
Zhao JY, Xin JH, Guo YN et al (2004) Chin J Anal Chem 32:939
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (21375117, 21155001) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Laboratory Open Fund of Environmental Engineering and environmental Materials Jiangsu Province.
Conflict of Interest
The authors have a financial relationship with the organizations that sponsored the research as follows: Prof. Xiashi Zhu has received research grants from the National Natural Foundation of China (21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions; Gang Feng declares that he has no conflict of interest; and Wenhui Ping, Xingxiu Qin, and Jie Liu are currently master’s degree students in Yangzhou University. They declare that they have no conflict of interest. The research proposed in this article does not contain any studies with human or animals subjects. There are no ethical issues with human or animal subjects in our studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, G., Ping, W., Qin, X.X. et al. Ionic-Liquid-Loaded β-Cyclodextrin-Cross-Linked Polymer Solid-Phase Extraction for the Separation/Analysis of Linuron in Fruit and Vegetable Samples. Food Anal. Methods 8, 2315–2320 (2015). https://doi.org/10.1007/s12161-015-0118-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0118-6