Abstract
A porous β-cyclodextrin polymer (P-CDP) was used as a new sorbent for simultaneous solid-phase extraction (SPE) and determination of bisphenol A (BPA), bisphenol F (BPF), and bisphenol AF (BPAF). The P-CDP was obtained via the cross-linking of β-cyclodextrin with tetrafluoroterephthalonitrile, and then was characterized by FT-IR spectroscopy, scanning electron microscopy, nitrogen adsorption-desorption isotherms, and thermo-gravimetric analysis. Adsorption tests showed that the P-CDP had good binding ability and fast uptake kinetics for three bisphenols. When used as a SPE sorbent for three bisphenols, P-CDP showed high extraction efficiencies, high enrichment factors, and good reusability. Based on the P-CDP sorbent, a new SPE-HPLC-UV method was developed and successfully applied to the detection of three bisphenols in real samples. The recoveries of BPF, BPA, and BPAF in water samples were 91.2–102.6% (RSD = 1.4–2.9%), 98.0–101.2% (RSD = 1.3–2.7%), and 96.3%–103.7% (RSD = 1.3–2.9%), respectively. Moreover, the recoveries of BPF, BPA, and BPAF in orange juice were 94.3–97.1% (RSD = 1.4–2.9%), 102.7–103.6% (RSD = 1.7–3.5%), and 94.5–101.0% (RSD = 1.3–2.8%), respectively. The limits of detection (S/N = 3) and the limits of quantification (S/N = 10) for all analytes were 0.3 and 1.0 ng/mL, respectively. Therefore, P-CDP can be used as a good SPE sorbent for the simultaneous determination of BPA, BPF, and BPAF in environmental water samples and beverages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is a high-yield industrial chemical mainly used in the production of polycarbonate plastics and epoxy resins (Caballero-Casero et al. 2016). These polymers are widely applied in food container, paper products, water pipes, toys, medical equipment, and electronics (Chen et al. 2016). Due to its leaching from these products to the surroundings, BPA has been found in packed food, beverages, environmental samples, and biological samples (Caballero-Casero et al. 2016). On the other hand, BPA is a well-known xenoestrogen (Jiménez-Díaz et al. 2015). Many studies have demonstrated the adverse effects of BPA on the neural, cardiovascular, metabolic, immune, reproduction, and development systems in human and animals (Chen et al. 2016; Jiménez-Díaz et al. 2015; Williams et al. 2016). The serious concerns on BPA led the industry to replace it with other bisphenols. Bisphenol F (BPF) and bisphenol AF (BPAF) are two structural analogues of BPA, which are used as the main substitutes of BPA in the manufacturing of polycarbonate plastics and epoxy resins (Chen et al. 2016). However, available studies have reported various toxic effects of these BPA analogues, including endocrine disruption, cytotoxicity, genotoxicity, reproductive toxicity, dioxin-like effects, and neurotoxicity (Chen et al. 2016). Therefore, robust analytical methodologies are urgently needed for monitoring BPA and its analogues.
Presently, HPLC and GC are the commonly used methods for determination of bisphenols (Caballero-Casero et al. 2016; Jiménez-Díaz et al. 2015); even some others approaches have also been reported including CE (Caballero-Casero et al. 2016; Jiménez-Díaz et al. 2015), enzyme-linked immunosorbent assay (Caballero-Casero et al. 2016; Jiménez-Díaz et al. 2015), electrochemical sensors (Yang et al. 2014a; Zhu et al. 2015), fluorescent biosensors (Long et al. 2014; Marks et al. 2014), and bioreporters (Rajasärkkä and Virta 2013). Due to the complexity of various real samples and the low concentration levels of bisphenols, an efficient sample pretreatment procedure is usually required before analysis to remove the interferences and concentrate the analytes. A variety of pretreatment methods have been reported for the extraction of bisphenols, such as solid-phase extraction (SPE), liquid-liquid extraction, and some other modern extraction techniques (e.g. solid-phase microextraction, liquid-phase microextraction, matrix solid-phase dispersion, ultrasound-assisted extraction, magnetic SPE, and dispersive SPE) (Caballero-Casero et al. 2016; Cerqueira et al. 2014; Jiménez-Díaz et al. 2015; Wu et al. 2017). Among these methods, SPE is the most frequently used one for analyzing bisphenols due to its high extraction efficiency, high enrichment factor, low cost, simple operation, and low consumption of organic solvent (Caballero-Casero et al. 2016; Jiménez-Díaz et al. 2015). The SPE sorbents currently used for bisphenols include C18 silica (Chen et al. 2012), Nexus® sorbent (Li et al. 2013b), Oasis® HLB (Yang et al. 2014b), Oasis® MAX (Yang et al. 2014b), and molecularly imprinted materials (Hu et al. 2016; Wu et al. 2016; Yang et al. 2015a; Yu et al. 2015). However, the new sorbents with higher adsorption capacities are still needed for SPE of bisphenols.
β-Cyclodextrin (β-CD), a macrocyclic oligosaccharide consisting of seven glucose units, has a toroidal-shaped structure with a hydrophobic interior cavity and a hydrophilic exterior (Morin-Crini and Crini 2013; Xiao et al. 2017). Its hydrophobic cavity makes it easy for β-CD to selectively bind various organic compounds to form host-guest complexes, while its hydrophilicity causes the good water dispersibility of the β-CD-based materials. These properties make the β-CD-based materials the appropriate sorbents for organic molecules in water samples (Zhang et al. 2017). Meanwhile, most of the molecular β-CD derivatives are water-soluble; therefore, they are necessarily incorporated into polymers or grafted onto insoluble supports for the effective adsorption of the targets in water (Alzate-Sánchez et al. 2016). Several β-CD-based polymers have been obtained by the cross-linking of β-CD with epichlorohydrin (Morin-Crini and Crini 2013), monochlorotriazine (Cabrales et al. 2012), polyfunctional carboxylic acids (Agrawal and Warmoeskerken 2012; Machín et al. 2012), and acrylamide (Guo et al. 2016); however, they all exhibited low surface areas and poor uptake performance. Recently, Dichtel and co-workers reported a porous β-CD polymer (P-CDP) with high surface area, high adsorption capacity, and fast uptake kinetics, and applied it to the rapid removal of several different organic micropollutants from water (Alsbaiee et al. 2016). They also prepared a P-CDP-functionalized cotton fabric for capture of organic pollutants from contaminated air and water (Alzate-Sánchez et al. 2016). Very recently, a magnetic P-CDP was documented for the magnetic SPE of microcystins from water samples (Zhang et al. 2017).
In this work, a porous P-CDP was used as sorbent for the simultaneous SPE and determination of BPA, BPF, and BPAF. P-CDP was characterized by FT-IR spectroscopy (FT-IR), scanning electron microscopy (SEM), nitrogen adsorption-desorption isotherms, and thermo-gravimetric analysis (TGA). The static adsorption ability, binding kinetics, and SPE ability of P-CDP for three bisphenols were investigated. Finally, using P-CDP as the sorbent, a SPE-HPLC-UV method was developed and successfully applied to the determination of three bisphenols in water samples and orange juice.
Materials and Methods
Reagents and Materials
β-Dyclodextrin (β-CD) was bought from Sinopharm Chemical Reagent Co., Ltd., China. Tetrafluoroterephthalonitrile (TFP, 99%) was purchased from Shanghai Macklin Biochemical Co., Ltd., China. Bisphenol A (BPA, 96%), bisphenol F (BPF, 97%), bisphenol AF (BPAF, 99%), tetrahydrofuran (THF, superdry, 99.5%), and dimethylformamide (DMF, superdry, 99.8%) were all obtained from Beijing J&K Scientific Co., Ltd., China. Methanol was of HPLC grade supplied by Merck, Germany. The ultrapure water was obtained from the KL-III-40 purification system of AK, Taiwan. Tap water was taken from Wuhan city, and lake water from the Southern Lake in Wuhan city. Orange juice was bought from a supermarket in Wuhan city. All other reagents were of analytical grade.
Preparation of P-CDP
The P-CDP sorbent was prepared according to a previous method (Alsbaiee et al. 2016). Briefly, β-CD and K2CO3 were predried at 120 °C under vacuum overnight. Then, dry β-CD (0.200 g, 0.176 mmol), TFP (0.100 g, 0.500 mmol), and dry K2CO3 (0.300 g, 2.17 mmol) were added into a flame-dried flask and were flushed with nitrogen for 5 min. After that, dry THF (7.2 mL) and dry DMF (0.8 mL) were added, followed by being bubbled with nitrogen for an additional 3 min. The mixture was placed in an oil bath at 80 °C and magnetically stirred for 48 h under nitrogen. After the mixture was cooled to room temperature, 1 mol/L HCl solution was added dropwise to remove residual K2CO3 until CO2 evolution stopped. The light yellow solid was isolated by filtration and then was washed by water, THF, and CH2Cl2 in sequence for three times. Finally, the solid was dried under vacuum at 77 K in a liquid nitrogen bath for 10 min and then at room temperature for 2 days. The finally obtained pale yellow powder was P-CDP.
For comparison, a non-porous β-CD polymer (NP-CDP) was similarly prepared using 6 mol/L NaOH instead of K2CO3 according to a previous method (Alsbaiee et al. 2016). The mixture containing β-CD, TFP, and NaOH solution was mixed vigorously until it was solidified. The solid was then washed with water, THF, and CH2Cl2 in sequence for three times, followed by drying under vacuum at room temperature for 2 days. The finally obtained solid was NP-PCDP.
Characterization of P-CDP
FT-IR spectra were collected using a Nicolet Avatar 360 FT-IR spectrometer (Thermo Fisher Scientific, USA). Thermo-gravimetric analysis (TGA) was performed on a TGA/DSC1 Thermal Analyzer (Mettler Toledo, Switzerland). Nitrogen adsorption was achieved on a TriStar II 3020 M automatic surface area and pore analyzer (Micromeritics, USA). The specific surface area and pore volume were calculated by the Brunauer-Emmett-Teller (BET) method, and the pore size distribution was obtained using the Barrett-Joyner-Halenda (BJH) theory. SEM images were observed on a Phenom Pro scanning electron microscope (Phenom-World BV, Netherlands).
Adsorption Experiment
For the static adsorption test, 20 mg of sorbent (P-CDP or NP-CDP) was added to 1 mL of the aqueous solution containing three bisphenols with different concentrations (10–800 μg/mL) and 15% methanol (v/v). The mixture was oscillated at 25 °C for 1 h. After centrifugation separation, the concentrations of bisphenols in the supernatant were measured by HPLC. The adsorption amounts (Q, mg/g) of bisphenols were calculated as Q = (c 0 − c)V/W, where c 0 and c are the initial and final concentrations of each bisphenol with unit of milligrams per milliliter, and V (mL) and W (g) are the volume of solution and the mass of sorbent, respectively. Finally, the adsorption isotherms of all bisphenols were plotted.
For binding kinetics test, 20 mg of sorbent (P-CDP or NP-CDP) was added to 1 mL of the aqueous solution containing three bisphenols with each concentration of 800 μg/mL and 15% methanol (v/v), and immediately the mixture was oscillated at 25 °C for different times (1–40 min). After fast filtration, the concentrations of the bisphenols in the filtrate were determined and subsequently the binding amount of each bisphenol was calculated according to the above method. The kinetic profiles of all bisphenols were plotted finally.
SPE Experiments
The SPE cartridge was prepared by packing 150 mg of P-CDP into a 3-mL empty polyethylene syringe between two sieve plates, and then it was conditioned with 20 mL methanol and 10 mL water sequentially. After that, 1 mL of bisphenol solution, containing BPF, BPA, and BPAF with different concentrations (0.2, 2, or 20 μg/mL) and 15% methanol (v/v), was loaded and passed through the cartridge. Subsequently, the cartridge was washed with 1 mL water, dried with nitrogen, and eluted with 2 mL methanol in sequence. After the solvent in the elution was evaporated completely by nitrogen, the residues were re-dissolved with 0.25 mL methanol and then analyzed by HPLC. The extraction efficiency (E, %) of three bisphenols was calculated as the ratio of the mass of analyte after SPE to that before SPE.
To evaluate the enrichment ability of the SPE cartridge, different volumes (1.0–12.0 mL) of the aqueous solutions containing three bisphenols with each concentration of 0.2 μg/mL and 15% methanol were tested. The SPE procedure was the same as the above method. The enrichment factor was calculated as the ratio of the volume of the bisphenol solution before SPE to that after SPE.
To investigate the reusability of the SPE cartridge, 8 mL of the aqueous solution, containing three bisphenols with each concentration of 50 ng/mL and 15% methanol, was tested. After each SPE test, the cartridge was washed with 20 mL methanol and 10 mL water sequentially and dried by nitrogen for the next SPE cycle.
For real sample analysis, lake water, tap water, and orange juice were filtered with a 0.22-μm membrane and then kept at 4 °C for further use. Finally, 8 mL of the as-pretreated sample was used for real sample analysis.
HPLC Analysis
All HPLC analyses were carried out on a Dionex UltiMate 3000 HPLC system (Thermo Fisher Scientific, USA), using a Dionex Acclaim™ 120 C18 column (250 mm × 4.6 mm, 5 μm) and a wavelength-variable UV detector. The column temperature was kept at 30 °C, detection wavelength was set at 276 nm, and injection volume was 20 μL. For all analyses, a gradient program was used at a flow rate of 1 mL/min, by combining solvent A (water) and solvent B (methanol) as follows: 35–80% (37 min).
Results and Discussion
Characterization of P-CDP
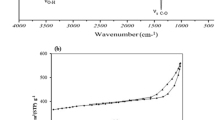
FT-IR spectra of β-CD, TFP, and P-CDP were collected first as shown in Fig. 1a. In the spectrum of TFP, the peaks at 2241 and 1271 cm−1 are attributed to the C≡N and C-F stretches, respectively, and those at 1681 and 1483 cm−1 belong to the C-C aromatic stretches. Meanwhile, these peaks also appear in the spectrum of P-CDP. Moreover, the peak of the C-O stretch at 1030 cm−1 in the spectrum of β-CD also occurs in that of P-CDP. These results verified well the successful synthesis of P-CDP.
To evaluate its stability, the TGA curve of P-CDP was measured at the temperature range of 50–1000 °C. As depicted in Fig. 1b, no obvious mass loss was observed below 270 °C, indicating the good thermal stability of P-CDP.
Figure 1c shows the nitrogen adsorption and desorption isotherms of P-CDP. At the same time, the nitrogen adsorption and desorption isotherms of NP-CDP were also measured. The BET area of P-CDP was 103.48 m2/g, which is much larger than that of NP-CDP (0.0838 m2/g). The average pore size of P-CDP was 2.45 nm, which is close to that in the previous report (Alsbaiee et al. 2016).
The SEM images of NP-CDP and P-CDP were taken for characterization of their morphologies. As shown in Fig. 2, NP-CDP and P-CDP all showed an irregular shape. However, P-CDP owned a coarser surface which might be attributed to its porous structure.
Adsorption Ability of P-CDP
The static adsorption ability of the sorbent for three bisphenols was investigated first. Figure 3a shows the adsorption isotherms of three bisphenols on P-CDP and NP-CDP. It can be seen that, within the concentration range of 10–800 μg/mL, the adsorption amounts of three bisphenols on P-CDP were much higher than those on NP-CDP. For example, at the concentration of 800 μg/mL, the adsorption amounts of BPAF, BPA, and BPF on P-CDP were 39.84, 37.72, and 37.53 mg/g, respectively, while those on NP-PCDP were 18.85, 24.19, and 24.33 mg/g. The binding amounts of bisphenols on P-CDP were much higher than those on other sorbents (Sun et al. 2014; Wu et al. 2016; Yang et al. 2015b; Yu et al. 2015). The high adsorption ability of P-CDP might be due to its porous structure.
The binding kinetics of bisphenols on sorbents was also studied. As shown in Fig. 3b, P-CDP showed a fast binding kinetics for three bisphenols with the adsorption equilibrium time less than 1 min, which might be also attributed to its porous structure. Moreover, the results further verified that the adsorption ability of P-CDP for bisphenols was much higher than that of NP-CDP.
SPE Ability of P-CDP
Three bisphenol mixtures with different concentrations (0.2, 2, or 20 μg/mL) were used to investigate the SPE ability of the P-CDP cartridge. As shown in Fig. 4a, the extraction efficiencies of BPA, BPF, and BPAF were 96.2–103.1, 94.1–100.2, and 97.1–103.8%, respectively. These results showed that the P-CDP cartridge had high extraction ability and therefore it could be used as a good SPE sorbent for bisphenols.
In order to evaluate the validation of the new SPE method, the enrichment factor of bisphenol mixtures on the P-CDP cartridge was studied. Different volumes (1.0–12 mL) of bisphenol aqueous solutions with the concentration of 0.2 μg/mL for each bisphenol were tested. Results are shown in Fig. 4b. When 1.0–10 mL of bisphenol solutions was used with the enrichment factors of 4–40 folds, the extraction efficiencies of BPF, BPA, and BPAF were 95.1–104.8, 97.0–105.8, and 96.2–104.9%, respectively. However, when 12 mL of bisphenol solution was used, the extraction efficiencies of BPF, BPA, and BPAF decreased to 86.9, 89.8, and 85.2%, respectively. To obtain satisfactory extraction efficiency, 8 mL of sample solution was used with the enrichment factor of 32 folds for the following tests.
Reusability of the P-CDP Cartridge
The reusability of the P-CDP cartridge was also studied. Eight milliliters of the aqueous solution containing 50 ng/mL BPA, BPF, and BPAF was used for test. After each SPE test, the cartridge was washed with methanol and water in sequence for the next extraction cycle. The results are shown in Fig. 5. It can be seen that, even after eight extraction cycles, the P-CDP cartridge still presented a good SPE ability for BPF, BPA, and BPAF with the extraction efficiencies of 94.0, 103.0, and 94.0%, respectively, indicating its good reusability.
Analysis of Real Samples
The calibration curves for determination of bisphenols were measured by directly analyzing six standard solutions on HPLC with concentrations of 0.1–120.0 μg/mL for each bisphenol. The obtained linear regression equations were A = 0.0246 + 0.3363c (r 2 = 0.9999) for BPF, A = 0.0191 + 0.2982c (r 2 = 0.9999) for BPA, and A = 0.0099 + 0.1392c (r 2 = 0.9999) for BPAF, where A and c (μg/mL) are the peak area and concentration of each analyte, respectively.
A new SPE-HPLC-UV method was developed by using P-CDP as sorbent for determination of three bisphenols in real samples (tap water, lake water, and orange juice). Since the found contents of three bisphenols in these real samples were all lower than the limits of detection (LODs), different amounts of bisphenol were spiked with three concentration levels (10.0, 20.0, or 50 ng/mL for each analyte) for test. As listed in Table 1, the recoveries and precisions for determination of three bisphenols were all satisfactory. For water samples, the obtained recoveries of BPF, BPA, and BPAF were 91.2–102.6% (RSD = 1.4–2.9%), 98.0–101.2% (RSD = 1.3–2.7%), and 96.3–103.7% (RSD = 1.3–2.9%), respectively. For orange juice, the recoveries of BPF, BPA, and BPAF were 94.3–97.1% (RSD = 1.4–2.9%), 102.7–103.6% (RSD = 1.7–3.5%), and 94.5–101.0% (RSD = 1.3–2.8%), respectively.
Figure 6a, b shows the chromatograms of 8 mL tap water spiked with 1 ng/mL bisphenols after and before SPE. No signal of bisphenols could be detected in the chromatogram of the spiked tap water before SPE, while obvious peaks of three analytes were observed after SPE. The LODs (S/N = 3) and limits of quantification (LOQs, S/N = 10) for the three bisphenols in real samples were 0.3 and 1.0 ng/mL, respectively. Therefore, the new SPE-HPLC-UV method can be applied to the simultaneous determination of BPA, BPF, and BPAF in environmental water samples or beverages. In addition, the performance of the present method was compared with other SPE-HPLC-UV methods in previous reports as listed in Table 2. It can be seen that the LOD of the present method is lower than those of most of the previous methods (Chu et al. 2015; Filippou et al. 2017; Grumetto et al. 2008; Li et al. 2013; Reyes-Gallardo et al. 2016; Yang et al. 2015a, b), close to those of four previous methods (Hu et al. 2016; Li et al. 2017; Wu et al. 2017; Yu et al. 2015), while higher than those of two previous methods (Sun et al. 2014; Wu et al. 2016). Therefore, the present method is sensitive enough for detection of trace bisphenols in environmental samples and juices. Moreover, due to the effective combination of SPE with the HPLC method, the selectivity of the present method is high enough for measurement of bisphenols in these samples.
Conclusions
A new SPE-HPLC-UV method was developed using P-CDP as sorbent for the simultaneous determination of BPA, BPF, and BPAF. The P-CDP sorbent showed good adsorption ability and fast binding kinetics for three bisphenols. When used as SPE sorbent, P-CDP presented high extraction efficiencies, high enrichment factor, and good reusability for three analytes. Based on the P-CDP sorbent, a new SPE-HPLC-UV method was developed and applied successfully to the detection of BPA, BPF, and BPAF in two water samples and orange juice. The new method showed high recoveries, good precisions, and low LODs for all analytes. Therefore, P-CDP can be used as a good SPE sorbent for the simultaneous determination of BPA, BPE, and BPAF in environmental water samples and beverage.
References
Agrawal PB, Warmoeskerken MMCG (2012) Permanent fixation of β-cyclodextrin on cotton surface-an assessment between innovative and established approaches. J Appl Polym Sci 124(5):4090–4097. https://doi.org/10.1002/app.35291
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 529(7585):190–194. https://doi.org/10.1038/nature16185
Alzate-Sánchez DM, Smith BJ, Alsbaiee A, Hinestroza JP, Dichtel WR (2016) Cotton fabric functionalized with a β-cyclodextrin polymer captures organic pollutants from contaminated air and water. Chem Mater 28(22):8340–8346. https://doi.org/10.1021/acs.chemmater.6b03624
Caballero-Casero N, Lunar L, Rubio S (2016) Analytical methods for the determination of mixtures of bisphenols and derivatives in human and environmental exposure sources and biological fluids: a review. Anal Chim Acta 908:22–53. https://doi.org/10.1016/j.aca.2015.12.034
Cabrales L, Abidi N, Hammond A, Hamood A (2012) Cotton fabric functionalization with cyclodextrins. J Mater Environ Sci 3:561–574
Cerqueira MBR, Caldas SS, Primel EG (2014) New sorbents in dispersive solid phase extraction step of quick, easy, cheap, effective, rugged, and safe for the extraction of organic contaminants in drinking water treatment sludge. J Chromatogr A 1336:10–22. https://doi.org/10.1016/j.chroma.2014.02.002
Chen D, Kannan K, Tan H, Zheng Z, Feng Y, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity: a review. Environ Sci Technol 50(11):5438–5453. https://doi.org/10.1021/acs.est.5b05387
Chen M, Tao L, Collins EM, Austin C, Lu C (2012) Simultaneous determination of multiple phthalate metabolites and bisphenol a in human urine by liquid chromatography-tandem mass spectrometry. J Chromatogr B 904:73–80. https://doi.org/10.1016/j.jchromb.2012.07.022
Chu GH, Cai WS, Shao XG (2015) Preparation of 4-butylaniline-bonded attapulgite for pre-concentration of bisphenol A in trace quantity. Talanta 136:29–34. https://doi.org/10.1016/j.talanta.2014.12.051
Filippou O, Deliyanni EA, Samanidou VF (2017) Fabrication and evaluation of magnetic activated carbon as adsorbent for ultrasonic assisted magnetic solid phase dispersive extraction of bisphenol A from milk prior to high performance liquid chromatographic analysis with ultraviolet detection. J Chromatogr A 1479:20–31. https://doi.org/10.1016/j.chroma.2016.12.002
Grumetto L, Montesano D, Seccia S, Albrizio S, Barbato F (2008) Determination of bisphenol A and bisphenol B residues in canned peeled tomatoes by reversed-phase liquid chromatography. J Agric Food Chem 56(22):10633–10637. https://doi.org/10.1021/jf802297z
Guo H, Xiong J, Ma W, Wu M, Yan L, Li K, Liu Y (2016) Synthesis of molecularly imprinted polymers using acrylamide-beta-cyclodextrin as a cofunctional monomer for the specific capture of tea saponins from the defatted cake extract of Camellia oleifera. J Sep Sci 39(22):4439–4448. https://doi.org/10.1002/jssc.201600834
Hu X, Wu X, Yang F, Wang Q, He C, Liu S (2016) Novel surface dummy molecularly imprinted silica as sorbent for solid-phase extraction of bisphenol A from water samples. Talanta 148:29–36. https://doi.org/10.1016/j.talanta.2015.10.057
Jiménez-Díaz I, Vela-Soria F, Rodríguez-Gómez R, Zafra-Gómez A, Ballesteros O, Navalón A (2015) Analytical methods for the assessment of endocrine disrupting chemical exposure during human fetal and lactation stages: a review. Anal Chim Acta 892:27–48. https://doi.org/10.1016/j.aca.2015.08.008
Li J, Zhang XB, Liu YX, Tong HW, Xu YP, Liu SM (2013a) Preparation of a hollow porous molecularly imprinted polymer using tetrabromobisphenol A as a dummy template and its application as SPE sorbent for determination of bisphenol A in tap water. Talanta 117:281–287. https://doi.org/10.1016/j.talanta.2013.09.022
Li L, Chen L, Meng X, Chen B, Chen S, Zhao Y, Zhao L, Liang Y, Zhang Y (2013b) Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One 8(e62526):1–9
Li Y, Cheng J, Lu P, Guo W, Wang Q, He C (2017) Quartz-wool-supported surface dummy molecularly imprinted silica as a novel solid-phase extraction sorbent for determination of bisphenol A in water samples and orange juice. Food Anal Methods 10(6):1922–1930. https://doi.org/10.1007/s12161-016-0765-2
Long F, Zhu A, Shi H, Wang H (2014) Hapten-grafted graphene as a transducer for homogeneous competitive immunoassay of small molecules. Anal Chem 86(6):2862–2866. https://doi.org/10.1021/ac500347n
Machín R, Isasi JR, Vélaz I (2012) β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr Polym 87(3):2024–2030. https://doi.org/10.1016/j.carbpol.2011.10.024
Marks HL, Michael VP, Jackson GW, Coté GL (2014) Rational design of a bisphenol A aptamer selective surface-enhanced Raman scattering nanoprobe. Anal Chem 86(23):11614–11619. https://doi.org/10.1021/ac502541v
Morin-Crini N, Crini G (2013) Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog Polym Sci 38(2):344–368. https://doi.org/10.1016/j.progpolymsci.2012.06.005
Rajasärkkä J, Virta M (2013) Characterization of a bisphenol A specific yeast bioreporter utilizing the bisphenol A-targeted receptor. Anal Chem 85(21):10067–10074. https://doi.org/10.1021/ac401614c
Reyes-Gallardo EM, Lucena R, Cardenas S, Valcarcel M (2016) Dispersive micro-solid phase extraction of bisphenol A from milk using magnetic nylon 6 composite and its final determination by HPLC-UV. Microchem J 124:751–756. https://doi.org/10.1016/j.microc.2015.10.025
Sun X, Wang J, Li Y, Jin J, Zhang B, Shah SM, Wang X, Chen J (2014) Highly selective dummy molecularly imprinted polymers as a solid-phase extraction sorbent for five bisphenols in tap and river water. J Chromatogr A 1343:33–41. https://doi.org/10.1016/j.chroma.2014.03.063
Williams KE, Lemieux GA, Hassis ME, Olshen AB, Fisher SJ, Werb Z (2016) Quantitative proteomic analyses of mammary organoids reveals distinct signatures after exposure to environmental chemicals. Proc Natl Acad Sci U S A 113(10):E1343–E1351. https://doi.org/10.1073/pnas.1600645113
Wu X, Li Y, Zhu X, He C, Wang Q, Liu S (2017) Dummy molecularly imprinted magnetic nanoparticles for dispersive solid-phase extraction and determination of bisphenol A in water samples and orange juice. Talanta 162:57–64. https://doi.org/10.1016/j.talanta.2016.10.007
Wu X, Wang X, Lu W, Wang X, Li J, You H, Xiong H, Chen L (2016) Water-compatible temperature and magnetic dual-responsive molecularly imprinted polymers for recognition and extraction of bisphenol A. J Chromatogr A 1435:30–38. https://doi.org/10.1016/j.chroma.2016.01.040
Xiao L, Ling Y, Alsbaiee A, Li C, Helbling DE, Dichtel WR (2017) β-Cyclodextrin polymer network sequesters perfluorooctanoic acid at environmentally relevant concentrations. J Am Chem Soc 139(23):7689–7692. https://doi.org/10.1021/jacs.7b02381
Yang J, Li Y, Wang J, Sun X, Shah S, Cao R, Chen J (2015a) Novel sponge-like molecularly imprinted mesoporous silica material for selective isolation of bisphenol A and its analogues from sediment extracts. Anal Chim Acta 853:311–319. https://doi.org/10.1016/j.aca.2014.09.051
Yang J, Li Y, Wang J, Sun X, Cao R, Sun H, Huang C, Chen J (2015b) Molecularly imprinted polymer microspheres prepared by Pickering emulsion polymerization for selective solid-phase extraction of eight bisphenols from human urine samples. Anal Chim Acta 872:35–45. https://doi.org/10.1016/j.aca.2015.02.058
Yang J, Wang X, Zhang D, Wang L, Li Q, Zhang L (2014a) Simultaneous determination of endocrine disrupting compounds bisphenol F and bisphenol AF using carboxyl functionalized multi-wall carbon nanotubes modified electrode. Talanta 130:207–212. https://doi.org/10.1016/j.talanta.2014.06.056
Yang Y, Lu L, Zhang J, Yang Y, Wu Y, Shao B (2014b) Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 1328:26–34. https://doi.org/10.1016/j.chroma.2013.12.074
Yu D, Hu X, Wei S, Wang Q, He C, Liu S (2015) Dummy molecularly imprinted mesoporous silica prepared by hybrid imprinting method for solid-phase extraction of bisphenol A. J Chromatogr A 1396:17–24. https://doi.org/10.1016/j.chroma.2015.04.006
Zhang W, Lin M, Wang M, Tong P, Lu Q, Zhang L (2017) Magnetic porous β-cyclodextrin polymer for magnetic solid-phase extraction of microcystins from environmental water samples. J Chromatogr A 1503:1–11. https://doi.org/10.1016/j.chroma.2017.04.063
Zhu Y, Zhou C, Yan X, Yan Y, Wang Q (2015) Aptamer-functionalized nanoporous gold film for high-performance direct electrochemical detection of bisphenol A in human serum. Anal Chim Acta 883:81–89. https://doi.org/10.1016/j.aca.2015.05.002
Acknowledgements
This work was supported by the National Nature Science Foundation of China (21277106) and the Science and Technology Program of Wuhan (2015060101010034).
Funding
Dr. Qiang Wang has received research grant from the National Natural Science Foundation of China. Dr. Chiyang He has research grant from the Science and Technology Program of Wuhan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yarong Li declares that she has no conflict of interest. Pengpeng Lu declares that he has no conflict of interest. Jincheng Cheng declares that she has no conflict of interest. Qiang Wang declares that he has no conflict of interest. Chiyang He declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Li, Y., Lu, P., Cheng, J. et al. Simultaneous Solid-Phase Extraction and Determination of Three Bisphenols in Water Samples and Orange Juice by a Porous β-Cyclodextrin Polymer. Food Anal. Methods 11, 1476–1484 (2018). https://doi.org/10.1007/s12161-017-1131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1131-8