Abstract
This study deals with the determination of Cu, Al, Cd, Mo, Pb, V, Sr, Ni, Cr, Co, Zn, Mn, and Fe in tea, wheat grain, and wheat flour by inductively coupled plasma optical emission spectrometry (ICP OES) with axially viewed plasma. Ultrasonic nebulization (USN) or pneumatic nebulization with aerosol desolvation (PN/DES) is used to introduce the sample solution into the ICP. Solutions of nitric acid (HNO3) with different concentrations of the acid (4.6 to 9.1 mol L−1) and hydrogen peroxide (H2O2) were investigated for sample decomposition in closed vessel (made of TFM-polytetrafluoroethylene (PTFE)) under microwave irradiation. Certified reference materials of tea, wheat flour, or bush branches and leaves were analyzed (0.5 g of sample in the presence of 2 mL H2O2 + 3 mL HNO3 + 2 mL H2O). Element concentrations found were in accordance with those certified in most cases, excepting for Al and V. Plasma-related matrix effects were not observed as denoted by the signal of the Ar 420.069-nm emission line. The analyte recoveries in spiked chamomile tea and wheat flour samples ranged from 87 to 107 %. By using USN, the limits of quantification (LOQs) of Cu, Al, Cd, Mo, Pb, V, Sr, Ni, Cr, Co, Zn, Mn, and Fe were 0.033, 0.006, 0.023, 0.33, 2.0, 0.33, 0.001, 0.33, 0.07, 0.033, 0.07, 0.001, and 0.02 μg g−1, respectively. Quite similar LOQs were obtained for both nebulizers employed, with the exception of Pb; the LOQ of Pb was three times lower by using PN/DES. Commercialized tea, wheat grain, and wheat flour were analyzed, and the concentrations found were reported.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inductively coupled plasma optical emission spectrometry (ICP OES) and inductively coupled plasma mass spectrometry (ICP-MS) are usually employed for metal and metalloid determination in food and vegetable due to the wide linear response, low limits of detection (LODs), multielemental capability, fast analysis, good precision, and accuracy conferred by both techniques (Moreda-Piñeiro et al. 2005; Başgel and Erdemoğlu 2006; Gomez et al. 2007; Nardi et al. 2009; Altintig et al. 2014; Scheffler et al. 2014). Several toxic or nutrient elements are present in low concentration in food and vegetable. Thus, high efficiency nebulizers such as ultrasonic nebulization (USN) or pneumatic nebulization with desolvation system (PN/DES) can be employed as sample introduction systems in order to improve the LODs, mainly for ICP OES (Petry et al. 2005; Bentlin and Pozebon 2010; Scheffler and Pozebon 2013).
Determination of nutrient and toxic elements in tea has been conducted for checking element availability, quality control, or origin identification (Mierzwa et al. 1998; Fernández-Cáceres et al. 2001; Fernández et al. 2002; Nookabkaew et al. 2006; Gomez et al. 2007; Soylak et al. 2007; Ashraf and Mian 2008; Chen et al. 2009; Salahinejad and Aflaki 2010; Tokalioğlu 2012; Szymczycha-Madeja et al. 2012). As a rule, such determination has been carried out by means of ICP OES, ICP-MS, flame atomic absorption spectrometry (FAAS), and graphite furnace atomic absorption spectrometry (GFAAS), as reviewed by (Szymczycha-Madeja et al. 2012). These techniques have also been used for analysis of wheat flour in order to evaluate the concentration of nutrient and toxic elements and for wheat flour classification. Vrček and Vrček (2012) investigated toxic and essential elements in wheat flour from organic or conventionally cultivated wheat. Samples were submitted to microwave-assisted acid decomposition prior analyte detection by ICP-MS. They observed differences among the wheat flour samples and suggested that toxic elements would be higher in the wheat conventionally cultivated. Araujo et al. (2008) determined Ca, Cu, Fe, K, Mg, Mn, P, and Zn in wheat flour by ICP OES in order to classify the wheat flour consumed in Brazil. Multivariate analysis of the data provided useful information on the similarity among the samples, which could be divided in three groups.
Hydrogen peroxide (H2O2), nitric (HNO3), hydrochloric (HCl), perchloric (HClO4), and hydrofluoric acid (HF) in open vessel and heating in hot plate/metallic block or in closed vessel in microwave oven have been used for tea and wheat flour decomposition. When the digestion has been conducted in closed vessel and heating in microwave oven, only HNO3 and H2O2 have been used in most cases. The main advantages of using HNO3 and H2O2 in comparison to HCl and HClO4 are the lower spectroscopic and/or matrix interference caused by both reagents in the determination of the analyte, mainly when ICP OES and ICP-MS are employed. Another advantage related with the use of HNO3 and H2O2 is the efficient sample decomposition in closed vessel under microwave irradiation, which reduces the risk of contamination and loss of volatile elements when compared to decomposition in open vessel. Usually, less than 10 mL of HNO3 is used for sample decomposition in closed vessel. However, even more acid may be necessary when the decomposition is conducted in open vessel and heating on hot plate or metallic block.
In order to reduce the amount of HNO3 used for sample decomposition in closed vessel under microwave irradiation, solutions of the acid instead of concentrated HNO3 have been investigated and proposed (Bizzi et al. 2011a, b, 2014). The oxidation efficiency of the HNO3 solution depends on the reactions that occur in the gas phase and in the presence of O2. These reactions involve nitrogen species generated during the oxidation of organic matter by O2, producing HNO3. Thus, regeneration of HNO3 occurs, and smaller quantities of the acid are then sufficient for the sample decomposition (Castro et al. 2009). Provided that there is sufficient amount of O2 in the sample decomposition flask, the amount of HNO3 needed for the decomposition is reduced, as observed by Bizzi et al. (2011a, b) when they introduced O2 in the sample decomposition flask. However, it has also been observed that the HNO3 oxidation efficiency in the presence of H2O2 was similar to that observed for O2 (Bizzi et al. 2014) when whole milk powder and bovine liver were analyzed. Therefore, the former procedure can be used, which is simpler and more easily conducted because it demands simpler instrumentation.
In the present work, diluted HNO3 in the presence H2O2 is investigated for tea, wheat, and wheat flour decomposition in closed flask in microwave oven. Ultrasonic nebulization and PN/DES are employed for introducing the sample solution into the ICP in order to improve the limits of quantification (LOQs). Matrix effect and the residual carbon content in the sample solution are also investigated. The main features of the method are (i) evaluation of diluted HNO3 for tea and wheat flour decomposition, (ii) comparison of the USN and PN/DES nebulizer performances with respect to sensitivity and accuracy for the investigated elements in tea and wheat flour, and (iii) evaluation of matrix effects.
Experimental
Instrumentation
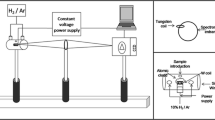
An Optima 2000DV ICP OES spectrometer (PerkinElmer, MA, USA) was used for elemental analysis. Operating conditions, accessories, and spectral lines monitored are summarized in Table 1. Argon (purity of 99.998 %, White Martins/Praxair, Brazil) was used as plasma gas, auxiliary, and nebulizer gas. Nitrogen (purity of 99.996 %, White Martins/Praxair, Brazil) was used as purging gas in the optical system of the spectrometer. A microwave oven (Berghof, Speedwave four, Germany) was employed to assist the sample decomposition. The microwave oven was equipped with 12 vessels (TFM-polytetrafluoroethylene (PTFE)) with a capacity of 60 mL, supporting 40 bars and 230 °C. The maximum power allowed is 1450 W.
Materials, Reagents, and Calibration Solutions
Water purified in a Milli-Q system (Millipore, USA), with conductivity of 18.2 ΩM cm−1 was used throughout the work. Nitric acid and H2O2 of suprapure grade were from Merck (Darmstadt, Germany). Calibration solutions (0.1 to 100 μg L−1) were prepared fresh daily by serial dilution of a 10 mg L−1 stock solution (SCP33MS, SCP Science, Quebec, Canada). The calibration solutions were prepared in 5 % (v/v) HNO3 or 15 % (v/v) HNO3. External calibration was used in the determination of all investigated elements.
Samples
Certified bush branches and leaves (NIM-GBW07602, NACIS, China), tea (NIM-GBW08505, NACIS, China), and wheat flour (SRM1567a, NIST, USA) were analyzed for method evaluation. The method was applied for analysis of commercialized tea (chamomile, boldo (Peumus boldus), spearmint (Mentha spicata), orange plus lotus flower green tea, white tea (Camellia sinensis), and black tea), wheat flour, wheat grain, and milled/sieved wheat grain. All samples were purchased in local markets. The wheat grain sample was ground in an agate mortar, sieved in a home-made nylon sieve (particles size <120 μm), and then stored in polypropylene vial. The tea was removed from its respective sachet and then analyzed. All samples were analyzed in triplicate accompanied by three replicates of the blank.
Sample Preparation
About 0.5 of sample was exactly weighed and transferred to the TMF-PTFE flask accompanying the microwave oven employed in the present study. Subsequently, HNO3, H2O2, and water (see Table 3) were added to the sample, the flask closed and placed in the rotor that was then transferred to the microwave oven. Procedure 1 given in Table 3 was selected for sample analysis. All samples were decomposed according to the program given in Table 2, which is suggested by the microwave oven manufacturer. After cooling to room temperature, the flask was opened and the sample solution transferred to a polypropylene vial where the sample solution volume was elevated to 15 mL by water addition. An infusion of chamomile tea was also prepared; 30 mL of boiling water was added to 0.5 g of sample and left in contact for 20 min. Next, the mixture was filtered (in a Whatman paper-fast filtration), and 1.5 mL of HNO3 was added to the filtrate whose volume was completed to 30 mL by adding water.
Results and Discussion
Operating Conditions for USN
Ultrasonic nebulization improves sensitivity by an order of magnitude in comparison to conventional pneumatic nebulization. In the ultrasonic (US) nebulizer, the solution is pumped onto a piezoelectric transducer membrane surface, which is coupled to an oscillator that operates at the ultrasound frequency. The liquid breaks up into a very fine droplet aerosol on the oscillator, with a strict narrow droplet size distribution (the diameter of the particles is about 10 μm or lower). In the US, the aerosol is heated (about 140 °C) and subsequently condensed (about 3 °C). These processes increase the sample transport and give rise to a partially dried aerosol, which reduces plasma overloading and noise. It was observed that the sensitivity was little increased by increasing the temperature to 160 °C (the maximum allowed by the US nebulizer employed). However, matrix effects have also increased, as observed by the worse precision and accuracy. On the other hand, excessive cooling (temperature lower than 3 °C) impacted plasma robustness negatively, due to excessive water removal; water releases oxygen and hydrogen atomic species in the ICP where they are responsible for energy transfer. Thus, the heating and cooling temperatures (140 and 3 °C, respectively) recommended by the US nebulizer manufacturer were used in the present work.
Figure 1a shows the influence of the plasma power on the analyte signal (Mn(II)), plasma robustness (Mg(II)/Mg(I)), and plasma-related matrix effects (Ar(I) signal). It is observed in Fig. 1a that the ratio Mg(II)/Mg(I) remains almost constant from 1200 to 1500 W. Meanwhile, small signal variation is observed for Mn(II), Ar(I), Mg(I), and Mg(II) at plasma power higher than 1300 W. Thus, the plasma power was fixed in 1300 W for further measurements. Figure 1b illustrates the effects of the nebulizer gas flow rate. It can be seen that the plasma robustness (indicated by the ratio Mg(II)/Mg(I)) and the analyte Mn(II) signal decrease sharply when the nebulizer gas flow rate is greater than 0.5 L min−1. The ratio Mg(II)/Mg(I) indicates how efficient is the energy transfer in the ICP. If the ratio is ≥8.0, the ICP is considered robust, having more capability to support matrix effects. It is interesting to note the existence of a crossing point among the signal profiles of Mg(II) and Mg(I) at 0.8 L min−1, which means that ionization is predominant at low nebulizer gas flow rate. The intensity of Ar(I) decreases with the nebulizer gas flow rate increase, demonstrating that excitation conditions are worsened in the ICP. In order to obtain better sensitivity and plasma robustness, the nebulizer gas flow rate was fixed at 0.5 or 0.6 L min−1, depending on the emission signal observed for each element.
Sample Decomposition
Typically, concentrated HNO3 and H2O2 are used for decomposition of samples with organic matrix. It has been demonstrated that even diluted HNO3 was effective for decomposition of olive leaves, apple leaves, peach leaves, and pine needles, bovine liver, and whole milk powder (Nóbrega et al. 2012 and references cited therein; Bizzi et al. 2014). In the present work, six solutions of HNO3 + H2O2 (see Table 3) were evaluated for tea and wheat flour decomposition. According to the analysis of variance (ANOVA) at confidence level of 95 %, for almost investigated elements, the respective concentration found in the samples treated with different amounts of the reagents was similar. The differences found can be seen in Fig. 2. According to Fig. 2a, the Mo, V, Cr, and Co concentrations found in tea are lower for procedure 4, whereas Al and Zn are for procedure 2. One can note that the concentrations of Mo and Co are also lower in wheat flour (Fig. 3b) treated according to procedure 4. When this sample was treated according to procedure 2, the concentration of Cr and Cd was lower. The concentration of Cd was also lower when the wheat flour was treated according to procedure 6. These results denote the influence of H2O2 and H2O. By comparing procedures 1 and 2, more water and less HNO3 are used in procedure 1 and the same amount of H2O2 is used in both procedures. However, the Zn and Al concentrations found in tea were lower for procedure 2. With respect to procedure 4, the amount HNO3 + H2O2 seems to be insufficient for total extraction/solubilization of Mo, V, Cr, and Co in tea and Cr, Cd, and Co in wheat flour. The same can be inferred for Cd in wheat flour treated according to procedure 6. By considering the results obtained and the lower amount of reagent employed, procedure 1 was selected and used in the subsequent determinations. Procedure 1 is compared in Table 4 to others reported, highlighting the lower amount of HNO3 used in the present work. The amount of HNO3 could be reduced even more by pressurizing with O2 the reaction medium (Bizzi et al. 2011a). However, a special flask is necessary in this case.
Element concentration in chamomile tea (a) and wheat flour (b) decomposed according to procedures 1 to 6 given in Table 3. V was not detected in the wheat flour sample. Ultrasonic nebulization was employed for introducing the sample solution in the ICP
Signal intensity (n = 3) of C and Ar in chamomile tea as a function of sample decomposition procedures (see Table 3). Ultrasonic nebulization was used to introduce the sample solution in the ICP
The carbon signal in the tea sample solutions did not differ markedly (Fig. 3a). Nevertheless, the lowest carbon emission signal was observed when more H2O2 was added (procedure 5)-H2O2 generates high-energy free radicals in situ, which are quite effective in oxidizing the organic matter. In Fig. 3a, one can also note that the carbon intensity was higher when the sample tea was decomposed following procedures 2 and 3, in which more HNO3 was used. However, less H2O2 was used in procedure 2, whereas water was not in procedure 3. These results emphasize that H2O2 is really necessary and that H2O also influences in the organic matter oxidation.
The signal of argon is a simple/reliable way to check shifts of the ICP equilibrium. To access possible plasma-related matrix effects, two emission lines of Ar were monitored (Fig. 3b). For all procedures, the residual sample matrix had the same influence on the ICP, since the Ar emission signal was similar for all solutions of the tea sample.
Accuracy and LOQ
Certified tea, wheat flour, and bush branch and leaves were analyzed in order to check the accuracy of the method. The results obtained are given in Table 5, together with the limits of quantification (LOQs). The LOQs were determined by the following equation: LD = m + 10s, where s is the standard deviation of ten consecutive measurements of the sample blank and m is the respective mean. The sample mass and dilutions involved were taken into account to calculate the LOQs.
In Table 5, it can be seen that the concentrations found are typically in agreement with those certified. However, lower Al concentration than that certified was found in all samples. In such matrices, it is possible that Al is bound to Si, whose compounds are not solubilized by HNO3/H2O2. Bizzi et al. (2011b) obtained accurate results for Al in botanical material (olive leaves, apple leaves, peach leaves, and pine needles) digested with diluted HNO3. The authors concluded that 3 mol L−1 HNO3 was suitable for an efficient digestion of 500 mg of botanical sample using 5 bars of O2 pressure in the microwave flask. By comparing the results obtained for Al in the present work with those reported for botanical material, it can be stated that purging with O2 is very important for complete solubilization of Al with diluted HNO3. By pressurizing with O2, Bizzi et al. (2011b) also obtained better results for Fe. In the present study, the concentration of Fe found in tea and in bush branch and leaves was lower than that certified. Concentration values lower than those certified were also obtained for V and Cr in bush branch and leaves and Zn in tea.
Although it was not investigated in the present work, the accuracy for Al could be improved by using HF in addition to HNO3 and H2O2. According to Table 4, several authors reported the use of HF and/or HClO4 and HCl. However, the latter two acids must be avoided because of spectral interference caused by Cl (in the case of using ICP-MS) and the risk of explosion associated with HClO4. Another alternative would be the use of quartz flask, rather than TFM-PTFE. A more efficient decomposition is possible in a quartz flask in view of the higher temperature of the reaction medium. As reported by Vrček and Vrček (2012), good accuracy was obtained for several elements (including Cr, V, Al) in wheat flour decomposed by HNO3 and microwave irradiation in closed quartz flask. In this case, efficient decomposition was achieved by using HNO3 only. In another work (Bizzi et al. 2014), accurate results were obtained for several elements determined in whole milk powder and bovine liver decomposed in quartz flask by means of very diluted HNO3 (1 mol L−1) and H2O2.
With respect to the results shown in Table 5, one can still observe that higher Cd concentration than that certified was found in tea and wheat flour. The main reasons for the incorrect Cd concentration determined could be contamination and/or spectral interference, which could not be identified. Additional studies were conducted to evaluate the accuracy of the method. The investigated elements were added to three replicates of chamomile tea and wheat flour before their decomposition, in order to obtain 5 μL−1 of Cu, Al, Cd, Mo, Pb, V, Sr, Ni, Cr, and Co or 50 μL−1 of Zn, Mn, and Fe in the final solution. The analyte recovery ranged from 87 to 107 %, which were considered satisfactory. Thus, it was concluded that the inaccurate concentration found in the certified materials was not due to matrix effects in sample transport and processes occurring in the ICP.
The performance of US was compared to that of PN/DES. According to the results presented in Table 5, it can be concluded that both nebulizers can be employed, but accuracy for Co, Cu, and Fe was better by employing PN/DES. The main advantage of PN/DES in comparison to the US is less sample consumption and waste generation (much longer washing time is required for the US nebulizer). In addition, the LOQs of PN/DES and USN are almost similar, with the exception of Pb (see Table 5).
Method application
Tea, wheat flour, and wheat grain samples were analyzed according to the proposed method. As shown in Table 6, higher concentrations of the investigated elements were found in spearmint tea, excepting Cd and Mn. Very toxic elements such as Cd and Pb were not detected in all samples of tea. The element concentrations found in the chamomile tea are quite similar of those reported by Başgel and Erdemoğlu (2006) for tea from Turkey. Conversely, higher element concentrations were reported by Chen et al. (2009) for tea from China. Szymczycha-Madeja et al. (2012) reviewed the elemental analysis of tea by spectrometric techniques. The concentration values of the investigated elements that are summarized in the review are in accordance with those found in the samples analyzed in the present work.
The chamomile tea was extracted with hot water in order to simulate the analyte concentration in the consumed infusion. The amounts of Cu, Al, Cd, Mo, V, Sr, Ni, Cr, Co, Zn, Mn, and Fe extracted were 18, 6, 49, 21, <1, 10, 38, 2, 22, 31, 10, and 1 %, respectively. Lead was not detected in the extract; the most extracted elements were Cd, Zn, and Ni, while Fe, Cr, and Al were the elements less extracted. These results are in accordance with those reported elsewhere (Nookabkaew et al. 2006; Szymczycha-Madeja et al. 2012, 2013). It is important to remark that Al is fortunately poorly extracted, which is a potentially toxic element and present in high concentration in tea.
With respect to wheat, in Table 6, one can see that the concentrations of nutrients elements such as Zn, Mn, and Fe are higher in the grain. The Cr concentration found in wheat flour is close to that reported by Tejera et al. (2013), whereas the Cu, Zn, Mn, and Fe concentrations agree with those reported by Araujo et al. (2008).
Conclusions
It was demonstrated that Cu, Mo, Pb, Sr, Ni, and Co can be accurately determined in tea, wheat flour, and wheat grain using diluted HNO3 (6.9 mol L−1) and H2O2 under microwave irradiation in closed vessel. Conversely, the Al and V concentrations found were lower than the certified concentration values. Lower concentrations than those certified were also found for Cr in wheat flour and bush branches and leaves as well as Zn and Fe in tea. It was concluded that additional studies about the use of diluted HNO3 are necessary, using quartz flask to decompose such samples, or addition of HF in the case of Al. Higher Cd concentration than that certified was found in tea and wheat flour, possibly due to contamination and/or spectral interference that could not be identified.
With respect to the HNO3 solutions investigated, the lowest carbon signal was observed when 1 mL H2O + 2 mL HNO3 + 4 mL H2O2 were used for the decomposition of chamomile tea. Meanwhile, the largest carbon signal was observed when 4 mL HNO3 + 3 ml H2O2 was used (without adding water). These results demonstrate the influence of H2O2 in the organic compound oxidation.
The use of USN or PN/DES enhanced sensitivity, allowing the determination of low concentrations of the investigated elements using ICP OES. Both US and PN/DES led to quite similar LODs, but with the use of PN/DES, the sample consumption is lower as well as waste generation. At robust plasma conditions, no matrix effects were observed even using diluted HNO3 for sample decomposition.
References
Altintig E, Altundag H, Tuzen M (2014) Bull Chem Soc Ethiop 28:9
Araujo RGO, Macedo SM, Korn MGA, Pimentel MF, Bruns RE, Ferreira SLC (2008) J Braz Chem Soc 19:935
Ashraf W, Mian AA (2008) Bull Environ Contam Toxicol 81:101
Başgel S, Erdemoğlu SB (2006) Sci Total Environ 359:82
Bentlin FRS, Pozebon D (2010) J Braz Chem Soc 21(4):627
Bizzi CA, Barin JS, Garcia EE, Nóbrega JA, Dressler VL, Flores EMM (2011a) Spectrochim Acta B 66(5):394
Bizzi CA, Barin SJ, Muller EI, Schmidt L, Nóbrega JA, Flores EMM (2011b) Talanta 83:1324
Bizzi CA, Flores ELM, Nóbrega JA, Oliveira SSO, Oliveira JSS, Schmidt L, Mortari S (2014) J Anal At Spectrom 29:332
Castro JT, Santos EC, Santos WPC, Costa LM, Korn M, Nóbrega JA, Korn MGA (2009) Talanta 78:1378
Chen Y, Yu M, Xu J, Chen X, Shi J (2009) J Sci Food Agric 89:2350
Fernández PL, Pablos F, Martín MJ, González AG (2002) Food Chem 76:483
Fernández-Cáceres PL, Martín MJ, Pablos F, González G (2001) J Agric Food Chem 49:4775
Gomez MR, Cerutti S, Sombra LL, Silva MF, Martínez LD (2007) Food Chem Toxicol 45:1060
Mierzwa J, Sun YC, Chung YT, Yang MH (1998) Talanta 47:1263
Moreda-Piñeiro A, Fisher A, Hill SJ (2005) J Food Comp Anal 16:195
Nardi EP, Evangelista FS, Tormen L, SantPierre TD, Curtius AJ, Souza SS, Barbosa F Jr (2009) Food Chem 112:727
Nóbrega JA, Pirola C, Fialho LL, Rota G, de Campos JCEKMA, Pollo F (2012) Talanta 98:272
Nookabkaew S, Rangkadilok N, Satayavivad J (2006) J Agric Food Chem 54:6939
Petry CF, Pozebon D, Bentlin FRS (2005) At Spectrosc 26:19
Salahinejad M, Aflaki F (2010) Biol Trace Elem Res 134:109
Scheffler GL, Pozebon D (2013) Anal Chim Acta 789:33
Scheffler GL, Dressler VL, Pozebon D (2014) Food Anal Methods 7:1415
Soylak M, Tuzen M, Souza AS, Korn MGA, Ferreira SLC (2007) J Hazard Mater 149:264
Szymczycha-Madeja A, Welna M, Pohl P (2012) Trac-Trend Anal Chem 35:165
Szymczycha-Madeja A, Welna M, Zyrnicki W (2013) J Braz Chem Soc 24:777
Tejera RL, Luis G, González-Weller D, Caballero JM, Gutiérrez AJ, Rubio C, Hardisson A (2013) Nutr Hosp 28:506
Tokalioğlu S (2012) Food Chem 134:2504
Vrček V, Vrček IV (2012) Int J Food Sci Technol 47:1777
Acknowledgments
The authors acknowledge FAPERGS for financial support and CNPq and CAPES for scholarships.
Conflict of Interest
C.A. Martins declares that she has no conflict of interest. C. Cerveira declares that she has no conflict of interest. G.L. Scheffler declares that he has no conflict of interest. D. Pozebon declares that she has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, C.A., Cerveira, C., Scheffler, G.L. et al. Metal Determination in Tea, Wheat, and Wheat Flour Using Diluted Nitric Acid, High-Efficiency Nebulizer, and Axially Viewed ICP OES. Food Anal. Methods 8, 1652–1660 (2015). https://doi.org/10.1007/s12161-014-0044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0044-z