Abstract
In this study, chitosan-grafted multiwalled carbon nanotubes were synthesized and characterized using Fourier transform infrared spectroscopy, transmission electron microscopy, X-ray diffraction, thermogravimetric analysis, and static and kinetic adsorption experiments. The results showed that the resulting material had good adsorption abilities and rapid dynamic properties for acrylamide. This material was used as a sorbent for development of matrix solid-phase dispersion extraction coupled with high-performance liquid chromatography for the determination of trace acrylamide in foods. Under the optimal conditions, the limit of detection, based on three times the signal to noise ratio of the baseline near the analyte peak, was 1.5 μg/L, and the relative standard deviation for five replicate extractions of 50 μg/L acrylamide was 4.3 %. Blank potato and flour samples spiked with acrylamide at different levels, namely, 2.25, 4.50, and 6.75 μg/kg, were extracted and determined by the developed method, with good recoveries ranging from 85.3 to 94.6 %. This method was used for quantitative analysis of acrylamide in different food samples, including twisted cruller, potato chip, and toast samples. Compared with other methods, this method for the extraction and determination of trace acrylamide in food samples is rapid and simple.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the Swedish National Food Administration and researchers at Stockholm University first announced, in April 2002, that high levels of acrylamide had been found in foods such as French fries and bread (Tareke et al. 2002), acrylamide has become a priority human health issue and attracted worldwide concern, because of its demonstrated neurotoxicity, genetoxicity, and reproductive development toxicity (Costa et al. 1991; IARC 1994). As a heat-induced food contaminant, it is probably carcinogenic to humans (Wenzl et al. 2007). Acrylamide is usually produced in starch-rich foodstuffs via the Maillard reaction at temperatures above 120 °C, especially at 140–180 °C (Fernandes and Soares 2007; Mottram et al. 2002). It is therefore necessary to control acrylamide production during food-heating processing to protect human health.
Many methods, including gas chromatography (GC) and liquid chromatography (LC), have been developed for determination of acrylamide in foods (Kim et al. 2011; Pedersen and Olsson 2003; Zhu et al. 2008; Zhang et al. 2007; Casella et al. 2006; Xu et al. 2013). GC method usually needs complex derivatization process. LC is the classic analytical technique, but the sensitivity of LC is low (Paleologos and Kontominas 2005); therefore, pretreatment is needed prior to LC. The traditional pretreatment is solid-phase extraction (SPE), but SPE procedures are usually labor intensive and time consuming. It is thus important to develop faster, more cost-effective, and environmentally friendly procedures. Matrix solid-phase dispersion extraction (MSPD) meets the above requirements (Smith 2003); this method not only can greatly reduce the analysis time but also requires less solvent. In recent years, a number of applications of MSPD in food analysis have been reported (Barker 2007; Bogialli and Di Corcia 2007; Kristenson et al. 2006; Soares and Fernandes 2009).
In the MSPD procedure, the most important factor is the adsorption performance of the sorbent. Traditional sorbents usually have poor adsorption abilities; so, the preparation of MSPD sorbents with good adsorption properties is crucial. Several reports have shown that multiwalled carbon nanotubes (MWCNTs) have better adsorption capacities than activated carbon in dioxin removal, because of their hollow and layered nanostructures and large specific surface areas (Long and Yang 2001; Li et al. 2002; Peng et al. 2003; Bacsa et al. 2000; Iijima 1991; Wijewardane 2009). They have been used as adsorbents in SPE for preconcentration and separation of metal ions and organic compounds in real samples (Wu 2007; Li et al. 2009; Liang et al. 2008; Gao et al. 2009; Sheng et al. 2010; Zhou et al. 2006; Min et al. 2008). However, MWCNTs also have drawbacks such as poor dispersion. Chitosan (CS), a natural polysaccharide with structural characteristics similar to those of cellulose, is usually obtained by deacetylation of chitin (Dai et al. 2012). CS has good film-forming, molecular separation, and adsorption abilities (Kumar 2000; Muzzarelli and Muzzarelli 2005; Chatterjee and Woo 2009) and has been used to improve the dispersion and application of MWCNTs (Wang et al. 2005).
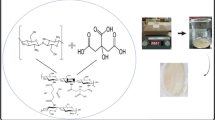
In this paper, we describe the synthesis of a CS/MWCNT-OX composite by grafting CS onto purified MWCNT (MWCNT-OX) surfaces. This material was used as the sorbent to develop a simple and sensitive MSPD coupled with HPLC, for the preconcentration, separation, and determination of trace acrylamide. The factors affecting the MSPD and the detection sensitivity of the method are discussed in detail. The accuracy and applicability of the method are also evaluated. So far, there have been no other reports on the use of CS-modified MWCNT-OX in MSPD, coupled with HPLC, for the separation and determination of trace acrylamide.
Materials and Methods
Materials
Potato, flour, twisted cruller, potato chip, and toast samples were purchased from the supermarket of Tai’an (Shandong, China) in April 2014.
Chemicals
Acrylamide (99 %) was purchased from the Meryer Chemical Technology Co., Ltd. (Shanghai, China). CS (degree of deacetylation more than 90 %) was obtained from the Shanghai Yuanye Biotechnology Co., Ltd. (Taizhou, China). MWCNTs were provided by the Beijing NaChen Science Co., Ltd. (Beijing, China). The lengths of the MWCNTs were in the range 10–20 μm, and their outer diameters were in the range 60–100 nm. Glutaraldehyde (50 %) and sodium dodecyl sulfate (SDS, 59 %) were purchased from the Tianjin Chemical Reagent Co., Ltd. (Tianjin, China). n-Hexane was purchased from the Yongda Chemical Reagents Center (Tianjin, China). HPLC-grade methanol was purchased from the Shandong Yuwang Industrial Co., Ltd. (Dezhou, China). Doubly deionized water (DDW), obtained from a Water Pro Water System (Labconco Corp., Kansas City, MO, USA), was used throughout the experiments. All other chemicals used were of analytical grade, and all solutions were prepared using DDW.
Instrumentation
Fourier transform infrared (FT-IR) spectroscopy (4,000–400 cm−1; KBr) was performed using a Vector 22 spectrometer (Bruker, Ettlingen, Germany). A JEM-100CX II transmission electron microscopy (TEM; JEOL, Tokyo, Japan) was used to observe the CS/MWCNT-OX structure. X-ray diffraction (XRD) patterns were obtained using a D8-advance diffractometer (Bruker). A 721 ultraviolet (UV) spectrometer (Shimadzu, Kyoto, Japan) and a DTG-60AH thermogravimetric analyzer (Shimadzu) were also used. A UCT SPE column (30 mL) was purchased from Pribo Lab Pte., Ltd. (Singapore).
Methods
MWCNT Purification
For removal of catalyst and addition of hydroxyl groups, MWCNTs (2.0 g) were refluxed in 100 mL of sulfuric acid (98 %)/nitric acid (65 %) (1:3, v/v) for 3.0 h. After filtration, the resulting MWCNT-OX was washed with DDW until neutral.
CS/MWCNT-OX Synthesis
Using MWCNT-OX as support material, the CS/MWCNT-OX was produced by grafting CS onto the MWCNT-OX surface as follows. First, MWCNT-OX (100 mg) was dispersed in 20 mL of CS solution (0.1 g of CS dissolved in 100 mL of 2 % acetic acid solution). To improve the MWCNT-OX dispersion (Vaisman et al. 2006; Shvartzman-Cohen et al. 2004), SDS (100 mg) was added. The mixture was sonicated for 30 min, and then, 1 % glutaraldehyde (1.0 mL) was added. The mixed suspensions were homogenized in an ultrasonic bath for 120 min, and then, the mixed solution was centrifuged at 10,000 rpm for 30 min; the product was collected and Soxhlet extracted with 150 mL of DDW/methanol (50:50, v/v) for 8 h. Finally, the resulting CS/MWCNT-OX was dried in a vacuum oven at 60 °C for 24 h.
Characterization of Adsorption Performance
For investigation of the adsorption performance of CS/MWCNT-OX material, 20 mg of CS/MWCNT-OX or MWCNTs was equilibrated with 10 mL of standard solutions containing acrylamide at concentrations of 20–160 mg/L. The mixtures were mechanically shaken for 4 h at room temperature, and then separated centrifugally (10,000 rpm) for 10 min. Unextracted acrylamide in the supernatant was detected using UV spectrometry at 198 nm, and the adsorption capacity (Q) was calculated, respectively.
The uptake kinetics of the prepared CS/MWCNT-OX toward acrylamide was also evaluated. CS/MWCNT-OX (20 mg) was added to 10 mL of 40-mg/L acrylamide solution, and the mixture was mechanically shaken for 5, 15, 30, 60, 90, 120, or 140 min at room temperature. The adsorption capacities were determined.
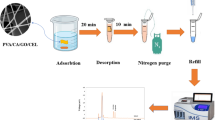
MSPD-HPLC Procedure
Acrylamide analysis was performed using an HPLC system (Shimadzu) equipped with two LC-20AT pumps and a Shimadzu SPD-M20A UV detector. All separations were achieved on an analytical reversed phase Thermo C18 column (4.6 × 250 mm; Agela Technology, Newark, DE, USA), at a mobile phase flow rate of 0.8 mL/min. The oven temperature was held at 40 °C. Class-vp software was used to acquire and process spectral and chromatographic data. The mobile phase was methanol/DDW (20:80, v/v). The sample volume injected was 50 μL, and detection was performed at 210 nm.
To investigate the applicability of CS/MWCNT-OX as a sorbent for extraction and separation of trace acrylamide in foods, CS/MWCNT-OX (2.0 g) and a grinded food sample (1.0 g) were packed into a glass mortar. The mixture was blended using a glass pestle for 5 min, and then packed into an empty UCT SPE column. The cartridge was first thoroughly conditioned with 2.0 mL of n-hexane and 2.0 mL of methanol/DDW (40:60, v/v) to remove impurities. The target analytes adsorbed on the CS/MWCNT-OX were eluted with 4.0 mL of methanol/DDW/acetic acid (90:10:2, v/v/v). The effluents were collected and condensed to dryness under a gentle flow of nitrogen, and then accurately redissolved in 0.3 mL of DDW. After filtration through a 0.22-μm filter membrane, 50 μL of filtrate was injected into the HPLC for detection.
For comparison, the same procedure was performed using MWCNT cartridges for acrylamide extraction.
Sample Preparation
To check the accuracy of the developed MSPD-HPLC using the CS/MWNT-OX sorbent, fortified potato and flour samples were prepared, which were confirmed to be free of acrylamide by HPLC before spiking. Briefly, blank potato or flour samples (1.0 g) were weighed into 100-mL conical flasks, spiked with 1.0 mL of standard acrylamide solution (2.25, 4.50, and 6.75 μg/L) containing 2.25, 4.50, and 6.75 ng of acrylamide. After incubation for 4.0 h, the spiked samples were extracted and analyzed according to the MSPD-HPLC procedure. The HPLC signals were recorded.
The acrylamide levels in twisted cruller, potato chip, and toast samples were determined by blending the sample (1.0 g) with the sorbent (2.0 g), followed by extraction, separation, and determination according to the MSPD-HPLC procedure.
Results and Discussion
CS/MWCNT-OX Characterization
The FT-IR spectra of MWCNT-OX (a), CS/MWCNTs-OX (b), and MWCNTs (c) are compared in Fig. 1. The band at 1,380 cm−1 was ascribed to –CH3 functional groups. Unlike the MWCNTs, the MWCNT-OX had strong absorptions at 3,440 and 1,640 cm−1, which were attributed to hydroxyl groups and nitro groups, respectively. The results show that carboxyl groups had been grafted onto the MWCNT surface by concentrated acid treatment. The peak at 2,925 cm−1 was the stretching vibration of –NH (Sugimoto et al. 1998), indicating that the CS had been grafted onto the MWCNT-OX surface. The results confirmed that the CS/MWCNTs-OX had been synthesized successfully.
The surface microstructures of MWCNT-OX and CS/MWCNTs-OX were visualized using TEM, and the images are shown in Fig. 2. The TEM images showed that the average diameter of MWCNT-OX (Fig. 2a) was obviously smaller than that of CS/MWCNT-OX (Fig. 2b), indicating that some of the CS might have been successfully grafted onto the MWCNT-OX surface.
Typical thermogravimetric analysis (TGA) curves of MWCNTs, MWCNT-OX, MWCNT-OX-CS, and CS are depicted in Fig. 3. The TGA curve of the MWCNTs (Fig. 3a) showed a weight loss of less than 3 % in the temperature range 25–600 °C. The TGA curve of MWCNT-OX (Fig. 3b) showed a weight loss of about 5 % when heated from 25 to 600 °C. The TGA curve of CS/MWCNT-OX (Fig. 3c) showed that a 5 % weight loss occurred at 25–150 °C, which was caused by evaporation of absorbed water. A rapid weight loss of 25 % was observed in the range 150–275 °C; this mainly resulted from decomposition of cross-linked CS. The weight percentage of CS in MWCNT-OX-CS was about 35 %. The TGA analysis results indicated that MWCNT-OX was successfully wrapped by the cross-linked CS, and the prepared MWCNT-OX-CS material had high thermal stability.
The XRD patterns of MWCNTs (a), MWCNT-OX (b), CS/MWCNT-OX (c), and CS (d) were investigated (Fig. 4). For MWCNTs, MWCNT-OX, and CS/MWCNT-OX, a diffraction peak at 2θ = 25.7° was observed, indicating the presence of MWCNTs, in good agreement with previous reports (Hernández-Ferrer et al. 2014). The XRD patterns of MWCNT-OX and CS/MWCNT-OX were similar to those of the MWCNTs. We can conclude that the purifying and CS grafting reactions had no significant effect on the basic crystalline structure of the MWCNTs, and the CS/MWCNT-OX retained the characteristic peaks of MWCNTs. In the case of MWCNT-OX, the diffraction peak background was lower than that for the MWCNTs, possibly because of removal of Co–Mo/MgO catalysts in the MWCNTs by the concentrated acid. Compared with those of MWCNT-OX, the diffraction peaks of CS/MWCNT-OX in the XRD pattern shifted slightly to lower angles, indicating that interactions between CS and MWCNT-OX had occurred, and CS/MWCNT-OX had been successfully synthesized.
Adsorption Ability Characterization
The adsorption capacity (Q) of CS/MWCNT-OX was calculated as following equation:
where C 0 and C 1 are the acrylamide concentrations before and after adsorption, V is the volume of acrylamide solution, and M is the mass of CS/MWCNT-OX.
The adsorption performances of CS/MWCNT-OX and MWCNTs toward acrylamide at 20–160 mg/L are shown in Fig. 5a. The results showed that the amounts of acrylamide molecules adsorbed by the adsorbent materials all increased with the acrylamide concentration increasing. However, CS/MWCNT-OX had a higher adsorption capacity for acrylamide than the MWCNTs, and an adsorption capacity of 9.3 mg/g was obtained at 160 mg/L, which was more than three times that of the MWCNTs (3.0 mg/g). CS grafting technique therefore improved the adsorption ability of the MWCNTs.
To evaluate the uptake kinetics, the adsorption properties of CS/MWCNT-OX toward acrylamide at 40 mg/L was examined (Fig. 5b). The results indicated that this novel material had rapid uptake kinetics. An adsorption capacity of 2.9 mg/g was obtained after shaking for 30 min, which was 68.3 % of the equilibrated adsorption capacity, and the adsorption almost reached equilibrium within 120 min. The results also indicated that the adsorption of acrylamide by CS/MWCNT-OX could be divided into three stages. In the first stage (5–30 min), the adsorption capacity increased rapidly. In this stage, the acrylamide might be adsorbed onto the surface of the functionalized material by physical adsorption, as a result of its large specific surface area. In the second stage (30–90 min), the acrylamide was absorbed into the interior by affinity binding sites through chemical bonds, and the adsorption capacity increased slowly. In the third stage (90–140 min), the adsorption increased more slowly, and then reached equilibrium.
Because of its good adsorption capacity and fast uptake kinetics, the functionalized CS/MWCNT-OX has the potential to be used as a sorbent in the pretreatment procedure for rapid extraction of acrylamide in food samples.
Optimization of MSPD Conditions
Using the prepared CS/MWCNT-OX as a sorbent, an MSPD-HPLC for the determination of trace acrylamide was developed. To achieve good sensitivity and precision, the MSPD conditions, including the ratio of matrix to sorbent, drip washing solution, and the composition and volume of the elution solution, were optimized.
The ratio of matrix to sorbent is an important parameter in MSPD and can affect the extraction efficiency. In this study, four different matrix/CS/MWCNT-OX ratios (1:1, 1:2, 1:3, and 1:4, m/m) were investigated. The results showed that the acrylamide peak area increased when the proportion of matrix to CS/MWCNT-OX was increased from 1:1 to 1:2. The peak area did not change significantly when the ratio was increased to 1:3, and the impurity peak increased when the proportion was 1:4. Good recovery was obtained when the ratio was 1:2. Therefore, 1.0 g of matrix (sample) and 2.0 g of CS/MWCNT-OX were blended in the MSPD procedure.
To reduce the matrix effect, the drip washing conditions were optimized. The results showed that impurities in the samples could be removed using 2.0 mL of n-hexane and 2.0 mL of methanol/DDW (40:60, v/v), without affecting the analysis.
In the MSPD procedure, selection of a solvent that can effectively elute the target analytes from the MSPD cartridge is very important. In this study, 4.0 mL of different ratios of methanol/DDW was investigated as elution solutions. The results indicated that with the proportion of DDW increasing, the acrylamide peak area decreased. When a solution consisting of methanol/DDW (90:10, v/v) was used, the maximum acrylamide peak area was obtained. Acetic acid can increase the eluting strength; so, the effect of the addition level of acetic acid (17.5 mol/L) was studied by varying the amount from 0.5 to 3.0 mL. The results showed that as the level of acetic acid gradually increased from 0.5 to 2.0 mL, the peak area increased, and then eventually stabilized in the range 2.0–3.0 mL. A mixture of methanol/DDW/acetic acid (90:10:2, v/v/v) was therefore selected as the eluting solution for subsequent experiments.
To obtain the optimal elution volume, various elution volumes in the range 1.0–6.0 mL were tested. As the eluting solvent volume increased from 1.0 to 4.0 mL, the chromatographic peak of acrylamide increased, and then leveled off in the range 5.0–6.0 mL, indicating that the adsorbed acrylamide could be fully eluted with 4.0 mL of methanol/DDW/acetic acid (90:10:2, v/v/v). Furthermore, too much elution solution would need a long concentration time. Therefore, 4.0 mL of methanol/DDW/acetic acid (90:10:2, v/v/v) was selected for the next experiments.
Analytical Parameters of MSPD-HPLC Method
The adsorptions of acrylamide by CS/MWCNT-OX (a) and MWCNTs (b) sorbent in MSPD-HPLC were tested (Fig. 6). The acrylamide signal obviously appeared in the chromatogram (Fig. 6a) after elution. However, the acrylamide peak in the chromatogram b was lower. These results indicated that CS/MWCNT-OX had a higher adsorption capacity for acrylamide than MWCNTs under the same conditions.
The analytical parameters of the developed method were evaluated and tested under the optimal conditions. The limit of detection (LOD, S/N = 3) of this method was 1.5 μg/L, and the linear range of the calibration graph was 0.005–50 mg/L. The peak area precision (relative standard deviation) for five replicate extractions of 50 μg/L acrylamide was 4.3 %.
Accuracy and Applicability of MSPD-HPLC Method
The accuracy of this method was evaluated by extraction and analysis of flour and potato samples spiked with acrylamide at different levels, i.e., 2.25, 4.50, and 6.75 μg/kg. The data are summarized in Table 1. For each concentration, triple measurements were performed, and good recoveries ranging from 85.3 to 94.6 % were achieved.
Twisted crullers, potato chips, and toast are typical starch-based foods, and large amounts of acrylamide are produced during heat processing. The applicability of the developed method was evaluated by extraction and determination of acrylamide in twisted cruller, potato chip, and toast samples; the chromatograms are displayed in Fig. 7. The acrylamide amounts in potato chip (a), toast (b), and twisted cruller (c) samples were quantitatively determined to be 530.0 ± 5.4, 209.0 ± 3.0, and 233.0 ± 3.2 μg/kg, respectively. The high levels of acrylamide in these samples would seriously harm human health. Further research and food production should therefore be devoted to the control of acrylamide formation during heating of food products.
Merits of Developed Method
Some methods, including LC-MS/MS (Arisseto et al. 2008; Liu et al. 2008), SPE-HPLC (Xu et al. 2012; Zhao et al. 2013; Xu et al. 2013), GC (Paleologos and Kontominas 2005), and GC-MS (Tareke et al. 2002; Russo et al. 2014), have been reported for the extraction and determination of acrylamide. Each method has advantage and limitation in terms of sensitivity and interference of matrix compounds.
Because of the good adsorption ability of the CS/MWCNT-OX sorbent, the HPLC sensitivity improved. The LOD of the developed method is lower than that of HPLC-UV (Paleologos and Kontominas 2005) and is almost the same as that of GC-MS and LC-MS/MS methods (Liu et al. 2008; Russo et al. 2014). Moreover, compared with LC-MS and LC-MS-MS methods, this presented MSPD-HPLC method requires relatively low-cost instrumentation. More importantly, traditional extraction methods such as SPE are usually time consuming and use large amounts of chemical solutions, which are harmful to the environment. In contrast, the MSPD method is a simple and fast pretreatment procedure, reducing the cost per analysis.
Conclusion
In this study, the functional material CS/MWCNT-OX was successfully synthesized. It had a good adsorption capacity and rapid kinetics in acrylamide adsorption. A sensitive and simple MSPD-HPLC method was developed using CS/MWCNT-OX as the sorbent in the MSPD pretreatment. This method was used for the extraction and determination of acrylamide in foodstuffs, with good accuracy. This study provides a new tool for the rapid screening and detection of trace acrylamide in complex food samples.
References
Arisseto AP, Figueiredo Toledo MCF, Govaert Y, Van Loco J, Fraselle S, Degroodt JM (2008) A modified sample preparation for acrylamide determination in cocoa and coffee products. Food Anal Method 1:49–55
Bacsa RR, Laurent C, Peigney A, Bacsa WS, Vaugien T, Rousset A (2000) High specific surface area carbon nanotubes from catalytic chemical vapor deposition process. Chem Phys Lett 323:566–571
Barker SA (2007) Matrix solid dispersion (MSPD). J Biochem Biophys Methods 70:151–162
Bogialli S, Di Corcia A (2007) Matrix solid-phase dispersion as a valuable tool for extracting contaminants from foodstuffs. J Biochem Biophys Methods 70:163–179
Casella IG, Pierri M, Contursi M (2006) Determination of acrylamide and acrylic acid by isocratic liquid chromatography with pulsed electrochemical detection. J Chromatogr A 1107:198–203
Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164:1012–1018
Costa LG, Deng H, Gregotti C, Manzo L, Faustman EM, Bergmark E, Calleman CJ (1991) Comparative studies on the neuro-and reproductive toxicity of acrylamide and its epoxide metabolite glycidamide in the rat. Neurotoxicology 13:219–224
Dai BY, Cao MR, Fang GZ, Liu B, Dong X, Pan MF, Wang S (2012) Schiff base-chitosan grafted multiwalled carbon nanotubes as a novel solid-phase extraction adsorbent for determination of heavy metal by ICP-MS. J Hazard Mater 219:103–110
Fernandes JO, Soares C (2007) Application of matrix solid-phase dispersion in the determination of acrylamide in potato chips. J Chromatogr A 1175:1–6
Gao ZM, Bandosz TJ, Zhao ZB, Han M, Qiu JS (2009) Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes. J Hazard Mater 167:357–365
Hernández-Ferrer J, Laporta P, Gutiérrez F, Rubianes MD, Rivas G, Martínez MT (2014) Multi-walled carbon nanotubes/graphene nanoribbons hybrid materials with superior electrochemical performance. J Electrochem Soc 39:26–29
IARC (1994) Monographs on the evaluation of carcinogenic risk of chemicals to humans. IARC, Lyon, pp 389–433
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Kim SH, Hwang JH, Lee KG (2011) Analysis of acrylamide using gas chromatography-nitrogen phosphorus detector (GC-NPD). Food Sci Biotechnol 20:835–839
Kristenson EM, Brinkman UAT, Ramos L (2006) Recent advances in matrix solid-phase dispersion. TrAC Trends Anal Chem 25:96–111
Kumar MNVR (2000) Nano and microparticles as controlled drug delivery devices. J Pharm Sci 3:234–258
Li YH, Wang SG, Wei JQ, Zhang X, Xu C, Luan ZK, Wei BQ (2002) Lead adsorption on carbon nanotubes. Chem Phys Lett 357:263–266
Li L, Huang YM, Wang Y, Wang WD (2009) Hemimicelle capped functionalized carbon nanotubes-based nanosized solid-phase extraction of arsenic from environmental water samples. Anal Chim Acta 631:182–188
Liang P, Zhao E, Ding Q, Du D (2008) Multiwalled carbon nanotubes microcolumn preconcentration and determination of gold in geological and water samples by flame atomic absorption spectrometry. Spectrochim Acta B 63:714–717
Liu J, Zhao GH, Yuan Y, Chen F, Hu XS (2008) Quantitative analysis of acrylamide in tea by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Food Chem 108:760–767
Long RQ, Yang RT (2001) Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc 123:2058–2059
Min G, Wang S, Zhu HP, Fang GZ, Zhang Y (2008) Multi-walled carbon nanotubes as solid-phase extraction adsorbents for determination of atrazine and its principal metabolites in water and soil samples by gas chromatography-mass spectrometry. Sci Total Environ 396:79–85
Mottram DS, Wedzicha BL, Dodson AT (2002) Food chemistry: acrylamide is formed in the maillard reaction. Nature 419:448–449
Muzzarelli RAA, Muzzarelli C (2005) Chitosan chemistry: relevance to the biomedical sciences. Adv Polym Sci 186:151–209
Paleologos EK, Kontominas MG (2005) Determination of acrylamide and methacrylamide by normal phase high performance liquid chromatography and UV detection. J Chromatogr A 1077:128–135
Pedersen JR, Olsson JO (2003) Soxhlet extraction of acrylamide from potato chips. Analyst 128:332–334
Peng XJ, Li YH, Luan ZK, Di ZH, Wang HY, Tian BH, Jia ZP (2003) Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem Phys Lett 376:154–158
Russo MV, Avino P, Centola A, Notardonato I, Cinelli G (2014) Rapid and simple determination of acrylamide in conventional cereal-based foods and potato chips through conversion to 3-[bis (trifluoroethanoyl) amino]-3-oxopropyl trifluoroacetate by gas chromatography coupled with electron capture and ion trap mass spectrometry detectors. Food Chem 146:204–211
Sheng GD, Li JX, Shao DD, Hu J, Chen CL, Chen YX, Wang XK (2010) Adsorption of copper (II) on multiwalled carbon nanotubes in the absence and presence of humic or fulvic acids. J Hazard Mater 178:333–340
Shvartzman-Cohen R, Levi-Kalisman Y, Nativ-Roth E, Yerushalmi-Rozen R (2004) Generic approach for dispersing single-walled carbon nanotubes: the strength of a weak interaction. Langmuir 20:6085–6088
Smith RM (2003) Before the injection-modern methods of sample preparation for separation techniques. J Chromatogr A 1000:3–27
Soares CMD, Fernandes JO (2009) MSPD method to determine acrylamide in food. Food Anal Methods 2:197–203
Sugimoto M, Morimoto M, Sashiwa H, Saimoto H, Shigemasa Y (1998) Preparation and characterization of water-soluble chitin and chitosan derivatives. Carbohydr Polym 36:49–59
Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Vaisman L, Wagner HD, Marom G (2006) The role of surfactants in dispersion of carbon nanotubes. Adv Colloid Interf Sci 128:37–46
Wang SF, Shen L, Zhang WD, Tong YJ (2005) Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 6:3067–3072
Wenzl T, Lachenmeier DW, Gökmen V (2007) Analysis of heat-induced contaminants (acrylamide, chloropropanols and furan) in carbohydrate-rich food. Anal Bioanal Chem 389:119–137
Wijewardane S (2009) Potential applicability of CNT and CNT/composites to implement ASEC concept: a review article. Sol Energy 83:1379–1389
Wu CH (2007) Adsorption of reactive dye onto carbon nanotubes: equilibrium, kinetics and thermodynamics. J Hazard Mater 144:93–100
Xu LH, Zhang LM, Qiao XG, Xu ZX, Song JM (2012) Determination of trace acrylamide in potato chip and bread crust based on SPE and HPLC. Chromatographia 75:269–274
Xu LH, Qiao XG, Ma Y, Zhang X, Xu ZX (2013) Preparation of a hydrophilic molecularly imprinted polymer and its application in solid-phase extraction to determine of trace acrylamide in foods coupled with high-performance liquid chromatography. Food Anal Methods 6:838–844
Zhang Y, Ren YP, Zhao HM, Zhang Y (2007) Determination of acrylamide in Chinese traditional carbohydrate-rich foods using gas chromatography with micro-electron capture detector and isotope dilution liquid chromatography combined with electrospray ionization tandem mass spectrometry. Anal Chim Acta 584:322–332
Zhao HD, Dai BY, Xu LH, Wang XL, Qiao XG, Xu ZX (2013) Preparation and application of immobilised ionic liquid in solid-phase extraction for determination of trace acrylamide in food samples coupled with high-performance liquid chromatography. J Sci Food Agric 94:1787–1793
Zhou QX, Wang WD, Xiao JP (2006) Preconcentration and determination of nicosulfuron, thifensulfuron-methyl and metsulfuron-methyl in water samples using carbon nanotubes packed cartridge in combination with high performance liquid chromatography. Anal Chim Acta 559:200–206
Zhu YH, Li GR, Duan YP, Chen SQ, Zhang C, Li YF (2008) Application of the standard addition method for the determination of acrylamide in heat-processed starchy foods by gas chromatography with electron capture detector. Food Chem 109:899–908
Acknowledgments
The authors are grateful for financial support from the National Natural Science Foundation of China (project No. 31071543), the Science & Technology Project of Shandong Province, China (project No. 2014GGB01096) and the Science and Technology Project of Tai’an, China (project No. 20123064).
Compliance with Ethics Requirements
We have no financial relationship with the organization that sponsored the research. Dr. Zhixiang Xu has received research grants from the National Natural Science Foundation of China and the Science and Technology Project of Tai’an, China.
Conflict of Interest
Handong Zhao, Ningyang Li, Jinwang Li, Xuguang Qiao, and Zhixiang Xu declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ningyang Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, H., Li, N., Li, J. et al. Preparation and Application of Chitosan-Grafted Multiwalled Carbon Nanotubes in Matrix Solid-Phase Dispersion Extraction for Determination of Trace Acrylamide in Foods Through High-Performance Liquid Chromatography. Food Anal. Methods 8, 1363–1371 (2015). https://doi.org/10.1007/s12161-014-0022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0022-5