Abstract
An efficient microextraction procedure based on modified ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction (M-IL-CIA-DLLME) was developed for trace determination of chromium in water and food samples by flame atomic absorption spectrometry (FAAS), and it was used for speciation of Cr(III) and Cr(VI) in water samples by using Na2SO3 as the reducing agent. A mixture of water-immiscible 1-hexyl-3-methylimidazolium hexafluorophosphate ([Hmim][PF6]) ionic liquid (IL) (microextraction solvent) and ethanol (disperser solvent) were directly injected into a heated aqueous solution containing bis(2-methoxy benzaldehyde) ethylene diimine as a Schiff’s base ligand (chelating agent), hexafluorophosphate (NaPF6; as a common ion) and Cr(III). Afterwards, the solution was placed in an ice-water bath and a cloudy solution was formed due to a considerable decrease of IL solubility. After centrifuging, the sedimented phase containing enriched analyte was determined by FAAS. Under the optimum conditions, the calibration graph was linear over the range of 2–50 μg L−1 with limit of detection of 0.7 μg L−1. The accuracy of the present methodology was tested by recovery experiments and by analyzing a certified reference material. Relative standard deviation (RSD %) was 2.7 % for Cr(III). The proposed method was successfully applied for trace determination of chromium in water and food samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium shows various oxidation states from 0 to +6, but the most stable forms are +3, and +6, which exist in equilibrium in water. Cr(III) and Cr(VI) show different properties and toxicities. Cr(VI) is 100 times more toxic than Cr(III) and there are no specific antidotes. Cr(VI) is very carcinogenic and causes skin allergy and some diseases, such as dermatitis, dermal necrosis and dermal corrosion (Gad 1989), while Cr(III) is a necessary nutrient and has a significant task in transferring sugar, proteins and fat (Anderson 1989). Heavy metals are potentially toxic and may cause health problems to humans via the food chain. Maximum permissible levels (MCL) in drinking water as recommended by United States Environmental Protection Agency (US-EPA) and World Health Organization (WHO) are 50 μg L−1.
In recent years, speciation methods are becoming more and more popular. Different methods have been used for speciation of chromium and other heavy metals, such as precipitation (Duran et al. 2011; Soylak et al. 2011; Uluozlu et al. 2009), liquid–liquid extraction (Agrawal and Sharma 2005), cloud point extraction (Matos et al. 2009), dispersive liquid–liquid microextraction (DLLME; Ying et al. 2011; Chen et al. 2010), and solid phase extraction (SPE; Tuzen et al. 2006; Ghaedi et al. 2008; Monasterio et al. 2009). Different analytical methods have been applied for recognizing chromium, such as flame atomic absorption spectrometry (FAAS; Shemirani et al. 2003a), inductively coupled plasma-atomic emission spectrometry (Liang et al. 2006), inductively coupled plasma-mass spectrometry (Sun et al. 2006; Pantsar-Kallio and Manninen 1999), electrothermal-atomic absorption spectrometry (Hao et al. 2010), electrochemistry (Kiptoo et al. 2004; Kuban et al. 2003), fluorometry (Hosseini and Belador 2009), chemiluminescence (Yuan et al. 2008), and UV–Vis spectrophotometry (Mahmoud et al. 2008).
In order to increase the concentration of analyte and reduce matrix effects, development of sample pretreatment methods prior to analysis is necessary. It is well documented that DLLME belongs to one of the best microextraction procedures (Bidari et al. 2007; Jahromi et al. 2007; Zeeb et al. 2010). But the usage of toxic organic solvents in this sample pretreatment procedure is an important problem. In order to completely remove the toxic extraction solvents in preconcentration methods, several sample preparation techniques based on ionic liquids (ILs), such as ionic liquid-based liquid–liquid microextraction (Hirayama et al. 2005; Berton et al. 2009), ionic liquid-based dispersive liquid–liquid microextraction (IL-DLLME; Yao and Anderson 2009; Yao et al. 2011; Gharehbaghi et al. 2009a; Abdolmohammad-Zadeh and Sadeghi 2010; Yousefi and Shemirani 2010), cold-induced aggregation microextraction (CIAME; Baghdadi and Shemirani 2008; Gharehbaghi et al. 2009b), ionic liquid-based single-drop microextraction (Vidal et al. 2010; Aguilera-Herrador et al. 2008; Zhou and Ye 2008), temperature-controlled ionic liquid dispersive liquid phase microextraction (Zhou et al. 2008), have been applied. In recent years, CIAME and IL-DLLME have shown excellent results in the case of preconcentration and isolation. In CIAME procedure, IL is dispersed through the sample using a relatively high temperature and afterwards, the IL is aggregated by applying a low temperature. On the contrary, in IL-DLLME method the microextraction solvent is dispersed into the sample by applying a disperser solvent. In comparison with CIAME, IL-DLLME is more efficient for dispersing the microextraction phase through the sample thus significantly improves the extraction time and the extraction efficiency. It is well established that the solubility of ILs in aqueous phase depends on temperature changes. As a result, CIAME requires less amount of IL to complete the extraction process. Therefore, combination of these two microextraction methods (ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction) can offer all the advantages mentioned above (Zhang et al. 2010). Based on the results obtained in our previous studies, performance of microextraction procedures based on ILs depends on variations in the ionic strength of the sample solution (Zeeb and Sadeghi 2011; Zeeb et al. 2011). It is well documented that solubility of ILs increases as the ionic strength of the aqueous solution increases. To solve this problem, a common ion of the IL was dissolved in the sample solution. As a result, solubility of the IL significantly decreased and volume of the settled phase was not affected by variations of the salt content of the sample. In the present work, bis(2-methoxybenzaldehyde) ethylene diimine is a Schiff’s base ligand, which acts as a complexing agent. The structure of this ligand is shown in (Fig. 1).
In the present study, combination of M-IL-CIA-DLLME and FAAS was applied for trace determination of chromium in water and food samples, and it was used for speciation and trace determination of two oxidation states of Cr in water samples with satisfactory results.
Experimental
Apparatus
A Shimadzu AA-6300 (Kyoto, Japan, www.shimadzu.com) flame atomic absorption spectrometer equipped with deuterium background correction, chromium hollow-cathode lamp (Hamamatsu Photonics, Shizuoka, Japan, www.hamamatsu.com) and air-acetylene flame was used for the measurement of chromium. The operational conditions are summarized in Table 1. The pH values were measured with a Metrohm pH meter (Herisau, Switzerland, www.metrohm-ag.com). Test tubes with a conic bottom were used for extraction and centrifuging vessels in order to trap and remove the sedimented phase. A 1-mL Hamilton syringe (Reno, NV, USA, www.hamiltoncompany.com) was used. A centrifuge (centurion scientific Ltd model: 1020D, West Sussex, England, www.centurionscientific.net) was used to accelerate separation of the phases. An adjustable 1,000 μL micropipette (Hamilton, Germany) was used to take an appropriate amount of organic solvent.
Reagents and Materials
All used reagents were of analytical grade with high purity. 1-Hexyl-3-methylimidazolium hexafluorophosphate [Hmim][PF6], acetone, acetonitrile, methanol, ethanol, sodium hexafluorophosphate (NaPF6), Na2SO3, ascorbic acid, and all used salts were purchased from Merck Co. (Darmstadt, Germany, www.merck.de). Cr(III) and Cr(VI) stock solutions (1,000 mg L−1) were prepared from Cr(NO3)3 · 9H2O and K2Cr2O7, respectively. The working standard solutions were prepared daily by a suitable stepwise dilution of the stock solution with deionized water. Double deionized water was used throughout the procedure. A solution of 250 mg mL−1 NaPF6 was prepared by dissolving an appropriate amount of NaPF6 in deionized water. The pH of the solution was adjusted to 10 using NH3/NH4 + buffer (0.1 M). In order to reduce loss of adsorption, since small amounts of chromium may be adsorbed on the surface of the vessel, the glassware were soaked in 10–20 % nitric acid solution overnight, and then rinsed with deionized water. To avoid a source of contamination, high quality nitric acid was used. Stock solutions of interfering ions were prepared from their high purity compounds. Bis(2-methoxybenzaldehyde) ethylene diimine and reducing agent were dissolved in ethanol and double deionized water, respectively. The chelating-agent solution was prepared by dissolving 0.458 g of bis(2-methoxybenzaldehyde) ethylene diimine in 100 mL of ethanol (the molecular weight of chelating agent is 296 g/mol).
Synthesis of Schiff’s Base [(CH3)2Salen]
Bis(2-methoxybenzaldehyde) ethylene diimine was synthesized according to the following procedure. 0.3 g of ethylenediamine was dissolved in 40 mL of ethanol. Afterwards, the obtained solution was transferred into a 250-mL flask. In the next section, 1.36 g of 2-methoxybenzaldehyde was dissolved in 30 mL of ethanol and then this solution was added dropwise to the first obtained solution, which was under reflux condition. After cooling the resulting solution, it was filtered and then recrystallized with ethanol for its purification. Afterwards, the solution was vacuum-dried for 12 h (Shemirani et al. 2003b).
Sample Preparation
Preparation of Water Samples
Tap water was freshly collected from our laboratory, after allowing it to flow for 1 min. River water was collected from Karaj River (Alborz, Iran) and Chamran River (Shiraz, Iran). These samples were collected in PTFE containers, which were soaked in concentrated HNO3 and washed with deionized water. In order to remove suspended particles, they were centrifuged for 10 min at 2,000 rpm and then filtered through 0.45 μm membrane filter. To avoid the losses of chromium, since chromium can be adsorbed onto container’s wall, the samples and solutions were kept in diluted nitric or hydrochloric acid (0.05 M) below pH 1.5. In acidic conditions, reduction of Cr(VI) to Cr(III) is favored, which is an unpleasant reaction while performing the speciation. Therefore, all the samples were analyzed as soon as possible (maximum recommended interval of time for storage is 8 h).
Preparation of Food Samples
Non-fat long life liquid cow’s milk was purchased from the local market. The liquid sample (0.50 mL) and 5 mL of HNO3 were mixed in a glass beaker at a fairly low temperature and then heated to dryness. Then it was left at room temperature in order to cool down. Then 3.0 mL of H2O2 (30 %) was added and the above described procedure was repeated to obtain nearly 0.50 mL of the sample solution. After cooling down to room temperature, the solution was transferred into a 50-mL volumetric flask and diluted to the mark with deionized water. The filtration of the digested solution is not necessary because there is no particle in the suspension to cause any difficulty in the absorption measurement by FAAS. Black tea (0.5 g) and green tea (0.5 g) were weighted and then transferred into a ceramic vessel. Concentrated nitric acid (6 mL) was added to this sample, and after 15 min it was heated for 5 min at 150 °C. Afterward, the resulting solution was cooled and 3 mL of hydrogen peroxide was added. Then, this solution was heated to dryness at 200 °C, and the residue was dissolved in nitric acid. The solution was transferred into a flask and neutralized with NaOH. After neutralization step, the obtained sample was diluted to 50 mL and analyzed using the proposed procedure. 1 g of wheat flour was dried to stable weight at 200 °C, put into a muffle furnace, heated to 400 °C and kept at this temperature for 8 h. The residue was then cooled, treated by 8.0 mL of concentrated nitric acid and 2 mL of 30 % (w/v) H2O2. The obtained solution was kept in furnace for 4 h at 400 °C. Afterwards, 4 mL of concentrated hydrochloric acid and 3 mL of 70 % (w/v) perchloric acid were added to this and evaporated to fumes. Finally, the resulting material was transferred to a volumetric flask, and diluted to 50 mL.
General Analytical Procedure
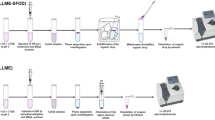
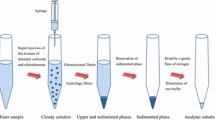
In M-IL-CIA-DLLME method, 30 mL of the sample or standard solution containing Cr(III) and 10−4 M bis(2-methoxybenzaldehyde) ethylene diimine (Schiff’s base ligand, as a chelating agent) was adjusted to pH 10.0 in a test tube. 0.8 mL of NaPF6 (250 mg mL−1) was then added to this. Afterwards, the tube was transferred into a thermo stated bath and kept at 40 °C for 5 min. A solution containing 500 μL of ethanol and 90 mg of 1-hexyl-3-methylimidazolium hexafluorophosphate [Hmim][PF6] was injected into the sample using a 1.0-mL syringe. Afterward, the resulting solution was cooled in an ice-water bath for 5 min and a cloudy condition was formed due to a considerable decrease of IL solubility. The solution was centrifuged for 5 min at 4,000 rpm. After removal of the aqueous solution, the sedimented phase (about 9 μL) in the glass test tube was dissolved in a diluting agent (0.5 mL of 0.1 M HNO3 in ethanol) and introduced into the FAAS by conventional aspiration.
Speciation Procedure
Total chromium was determined by addition of 10−3 M Na2SO3 to the sample solution and therefore Cr(VI) was converted to Cr(III). After 40 min, the solution was diluted with ultra pure water in a calibrated flask, and then the pH was adjusted to 10 with NaOH. Finally, Cr(III) was determined as indicated in the general procedure. The concentration of Cr(VI) was obtained as the difference between the total chromium and Cr(III).
Results and Discussion
Elemental Analysis of Chelating Agent
Elemental analysis of bis(2-methoxybenzaldehyde) ethylene diimine gave the following percentages C 72.90 %, H 6.71 %, and N 9.44 %.
Selection of Reducing Agent
In order to examine the effect of reducing agent, 10−3 M Na2SO3 and ascorbic acid (10 % w/v) were chosen as reducing reagents to reduce Cr(VI) to Cr(III). One hundred percent efficiency was obtained by Na2SO3, while a reduction of 67 % was observed when ascorbic acid solution was used as reducing agent. Therefore, Na2SO3 was used as reducing agent in the following experiment.
Selection of IL and Disperser Solvent
The IL applied in this microextraction must be liquid and show suitable hydrophobicity. In this type of microextraction procedures, the density of IL must be high enough to let the dispersed droplets of IL settle to the bottom of the test tube. Short alkyl chain imidazolium-based ILs containing Cl−, BF4 −, and CF3SO3 − show hydrophilic behavior and ILs containing PF6 − and (CF3SO2)2N− show hydrophobic behavior. IL must be inexpensive. ILs containing (CF3SO2)2N− are relatively expensive and those containing PF6 − are fairly inexpensive. According to the above criteria and the cost of ILs, [Hmim][PF6] was selected as an extraction phase. Miscibility of the dispersive solvent in the extraction phase and the aqueous sample is a critical point for the selection of the disperser solvent in M-IL-CIA-DLLME procedure. Various disperser solvents including acetone, ethanol, acetonitrile, and methanol were chosen as disperser solvents. The results revealed that the type of the organic solvent had no significant effect on the performance of the microextraction method. Due to less toxicity of ethanol, it was selected for all experiments.
Effect of pH
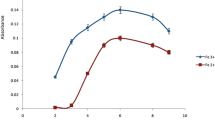
Type and concentration of chromium species are dependent on different processes, such as hydrolysis, complexation, redox and adsorption reactions. Bis(2-methoxybenzaldehyde) ethylene diimine forms a selective complex with Cr(III). Stability of a metal–chelate complex and extraction of Cr(III) is highly dependent on pH. The influence of pH on the M-IL-CIA-DLLME was studied over the pH range of 3-13. At low pH protons can compete with the metal for the coordination sites of a ligand, and in basic media of the sample solution, more stable complex formation and displacement reaction can occur. In the absence of complexing agent, Cr(III) exists as hexa-aqua chromium (III), in basic media Cr(OH)2 + is converted to Cr(OH)3, which is the dominant form in pH 4–10. At pH 10, the highest absorbance was obtained (Fig. 2), because formation of Cr(OH)3 was complete and selective hydrophobic complex was formed between bis(2-methoxy benzaldehyde) ethylene diimine and Cr(III). Therefore Cr(III) can be extracted into the fine droplets of IL and consequently, Cr(III) ions are separated and preconcentration takes place. Cr(III) shows amphoteric behavior in pH ≥ 10 and forms a soluble complex [Cr(OH)4 −]. While the extraction efficiency of Cr(III) is quantitative at pH 10, the extraction efficiency of Cr(VI) is rather low. In order to separate Cr(III) from Cr(VI) and determine the concentration of Cr(III), pH = 10 was selected for further studies.
Effect of Chelating Agent Concentration
The effect of different concentrations of bis(2-methoxybenzaldehyde) ethylene diimine on the recovery of Cr(III) was examined. Low concentration of the chelating agent was not enough to react with chromium. By increasing the concentration of chelating agent, the recovery increased. In concentrations greater than 10−4 M, the signal decreased because the excess amount of ligand dissolves the hydrophobic metal–chelate (Fig. 3). The optimum concentration of the chelating agent was 10−4 M.
Effect of Diluting Agent
Because of high viscosity of ILs, they must be diluted with a diluting agent prior to the introduction into the FAAS instrument. Different solvents including methanol, ethanol, acetone, and acetonitrile were examined in order to select a diluting agent that can completely dissolve the IL phase and provide the highest signals. The best analytical performance was achieved by using ethanol. Thus, in order to dissociate the complex and release Cr(III) into the solution, 0.5 mL of 0.1 M HNO3 in ethanol was used as a diluting agent in all experiments. It should be noted that appropriate dilution could decrease the matrix effect.
Effect of IL Amount and Volume of Disperser Solvent
The amount of [Hmim][PF6] IL that is used as an extractor in this sample preparation method is an important parameter to obtain high signals of chromium. Therefore, the microextraction system was carefully evaluated in order to obtain the highest analytical responses. The variation in the recovery as a function of the amount of IL that was added to 30.0 mL of the sample was studied in the range of 10-120 mg (Fig. 4). The results obtained in this experiment show that the analytical responses increase as the amount of IL increases and then start to decrease. Therefore, 90 mg of IL was selected as the optimum value. Effect of the volume of disperser solvent (ethanol) on the signals was tested in the range of 400 to 1,000 μL. In the studied range, no appreciable change in analytical signals was observed. As a result, 500 μL of ethanol was chosen for all experiments.
Effect of Common Ion Amount and Ionic Strength
Each ion of IL can be dissolved in the studied sample solution as a common ion, in order to decrease IL solubility and subsequently to increase performance of the system. In the present experiment, NaPF6 was selected as a common ion source and its influence on the analytical responses was tested in the range of 0-300 mg (Fig. 5). These results reveal that the analytical signals increase as the amount of NaPF6 increases and then remain nearly constant. Therefore, 200 mg of this agent was used for the rest of the work. Based on the results obtained in our previous studies (Zeeb and Sadeghi 2011; Zeeb et al. 2011), in sample pretreatment methods based on the application of ILs, the volume of the enriched phase depends on the salt concentration of the sample solution, which is due to the dependence of ILs’ solubility upon the ionic strength of the aqueous solution. In order to solve the mentioned problem, a common ion of IL was dissolved in the sample solution. NaCl was chosen as an electrolyte to study influence of the ionic strength on the system performance. This factor was tested within the range of 0–35 % (w/v). The results obtained in this experiment revealed that phase separation occurred up to 30 % of NaCl. It can be concluded that in the presence of common ion of IL, the performance of the presented sample pretreatment method is not affected by variations in the ionic strength of the studied sample.
Effect of Temperature
Temperature effect on the microextraction performance was tested in the range of 30–50 °C. This experiment showed that the highest signals were obtained at 40 °C. Thus, 40 °C was chosen as the optimum value for all experiments. After injection of a binary solution consisting of disperser and extraction solvent, solutions were cooled in the temperature range of 0–25 °C. The results revealed that when the temperature decreased, the sensitivity of analytical signals increased due to a decrease in [Hmim] [PF6] solubility. Thus, a temperature of 0 °C was used as the optimal one.
Interferences
In order to demonstrate selectivity of the proposed method for determining chromium and competition between interfering ions, effect of addition of transition metal ions, such as Pb2+, Zn2+, Cd2+, Co2+, Ni2+, and Cu2+, to a 50 μg L−1 Cr(III) upon complexation with complexing agent were examined at ratios of 1:1, 1:10 and 1:100. But of course, a high concentration of chromium can decrease this competition effect. Bis(2-methoxybenzaldehyde) ethylene diimine is selective enough and interfering ions with a specific tolerance limit did not change the signal more than ±5 %. In this study, chromium was quantitatively extracted and coexisting ions did not interfere with the extraction of chromium.
Analytical Figures of Merits
Several analytical parameters, such as repeatability, linearity, correlation of coefficient, and detection limit under optimized conditions were studied and a good linear regression between the absorbance and the concentration was found. The analytical characteristics of the optimized method are summarized in Table 2.
Analytical Applications
To validate the accuracy of the proposed method, extraction efficiency of chromium in different samples was studied. Different samples, such as wheat flour, green tea, black tea, cow’s milk, tap water and river water were analyzed by the present methodology to determine chromium concentration. The accuracy of the proposed analytical method was tested by means of recovery experiments and analysis of certified reference material (GBW 07605 Tea). The obtained results are shown in Table 3. In addition, the proposed method was applied for determination and speciation of chromium in tap water and mineral water (Table 4). These results show the accuracy and validity of the combined analytical methodology for trace determination and speciation of chromium in water and food samples.
Comparison with Other Methods
The figures of merit of the presented method (i.e., LOD, LR, and RSD) were compared with some of the previously reported methods and the results are shown in Table 5. As can be seen, the method has a relatively low LOD, wide dynamic range and good reproducibility. The results obtained in this study indicate the fact that M-IL-CIA-DLLME-FAAS is a sensitive, safe, environmentally friendly, and reproducible technique. The method developed in this work is recommended as a suitable alternative to expensive instrumental methods for pre-concentration, speciation, and determination of chromium in real samples. The usage of toxic organic solvents in previously reported sample pretreatment procedure such as DLLME is an important problem. On the contrary, in the present approach, [Hmim][PF6] was selected as a green microextraction phase and an alternative to traditional volatile organic solvents. In addition, the performance of the present sample preparation method was not affected by variations of the ionic strength of the sample.
Conclusion
In this work a new method has been developed for preconcentration and speciation of trace amounts of chromium by M-IL-CIA-DLLME combined with FAAS. The proposed technique provides a simple, inexpensive, rapid, sensitive, reliable, safe, environmentally friendly, and less tedious tool for quantitation. A Schiff’s base ligand reacts selectively with Cr(III) in the presence of foreign ions. The extraction time is very short because the equilibrium state is achieved quickly. High extraction recoveries were obtained for a small volume of only 30 mL. The presented method was applied to determination of chromium in environmental water and food samples with satisfactory results.
References
Abdolmohammad-Zadeh H, Sadeghi GH (2010) Combination of ionic liquid-based dispersive liquid-liquid micro-extraction with stopped-flow spectrofluorometry for the pre-concentration and determination of aluminum in natural waters, fruit juice and food samples. Talanta 81:778–785
Agrawal YK, Sharma KR (2005) Speciation, liquid–liquid extraction, sequential separation, preconcentration, transport and ICP-AES determination of Cr(III), Mo(VI) and W(VI) with calix-crown hydroxamic acid in high purity grade materials and environmental samples. Talanta 67:112–120
Aguilera-Herrador E, Lucena R, Cardenas S, Valcarcel M (2008) Determination of trihalomethanes in waters by ionic liquid-based single drop microextraction/gas chromatographic/mass spectrometry. J Chromatogr A 1209:76–82
Anderson RA (1989) Essentiality of chromium in humans. Sci Tot Environ 86:75–81
Baghdadi MF, Shemirani A (2008) Cold-induced aggregation microextraction: A novel sample preparation technique based on ionic liquids. Anal Chim Acta 613:56–63
Bermejo-Barrera P, Barciela-Alonso MC, Perez-Fernandez B, Bermejo-Barrera A (2003) Direct speciation analysis of Cr(VI) by electrothermal atomic absorption spectrometry, based on the volatilization of Cr(III)–thenoyltrifluoracetonate from the graphite furnace. Spectrochim Acta B 58:167–173
Berton P, Martinis EM, Martinezc LD, Wuilloud RG (2009) Room temperature ionic liquid-based microextraction for vanadium species separation and determination in water samples by electrothermal atomic absorption spectrometry. Anal Chim Acta 640:40–46
Bidari A, Jahromi EZ, Assadi Y, Hosseini MRM (2007) Monitoring of selenium in water samples using dispersive liquid-liquid microextraction followed by iridium-modified tube graphite furnace atomic absorption spectrometry. Microchem J 87:6–12
Chen H, Du P, Chen J, Hu S, Li S, Liu H (2010) Separation and preconcentration system based on ultrasonic probe-assisted ionic liquid dispersive liquid–liquid microextraction for determination trace amount of chromium(VI) by electrothermal atomic absorption spectrometry. Talanta 81:176–179
Duran A, Tuzen M, Soylak M (2011) Speciation of Cr(III) and Cr(VI) in geological and water samples by ytterbium(III) hydroxide coprecipitation system and atomic absorption spectrometry. Food Chem Toxicol 49:1633–1637
Gad CS (1989) Acute and chronic systemic chromium toxicity. Sci Tot Environ 86:149–157
Ghaedi M, Shokrollahi A, Kianfar AH, Mirsadeghi AS, Pourfarokhi A, Soylak M (2008) The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation–preconcentration on bis (salicyl aldehyde, 1, 3-propandiimine (BSPDI) loaded on activated carbon. J Hazard Mater 154:128–134
Gharehbaghi M, Shemirani F, Baghdadi M (2009a) Dispersive liquid-liquid microextraction based on ionic liquid and spectrophotometric determination of mercury in water samples. Int J Environ Anal Chem 89:21–33
Gharehbaghi M, Shemirani F, Farahani MD (2009b) Cold-induced aggregation microextraction based on ionic liquids and fiber optic-linear array detection spectrophotometry of cobalt in water samples. J Hazard Mater 165:1049–1055
Hao C, Ping D, Jie C, Shenghua H, Shengqing L, Hanlan L (2010) Separation and preconcentration system based on ultrasonic probe-assisted ionic liquid dispersive liquid–liquid microextraction for determination trace amount of chromium(VI) by electrothermal atomic absorption spectrometry. Talanta 81:176–179
Hirayama N, Deguchi M, Kawasumi H, Honjo T (2005) Use of 1-alkyl-3-methylimidazolium hexafluorophosphate room temperature ionic liquids as chelate extraction solvent with 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione. Talanta 65:255–260
Hosseini MS, Belador F (2009) Cr (III)/Cr (VI) speciation determination of chromium in water samples by luminescence quenching of quercetin. J Hazard Mater 165:1062–1067
Jahromi EZ, Bidari A, Assadi Y, Hosseini MRM, Jamali MR (2007) Dispersive liquid-liquid microextraction combined with graphite furnace atomic absorption spectrometry - Ultra trace determination of cadmium in water samples. Anal Chim Acta 585:305–311
Kiptoo JK, Ngila JC, Sawula GM (2004) Speciation studies of nickel and chromium in wastewater from an electroplating plan. Talanta 64:54–59
Kuban P, Kuban P, Kuban V (2003) Speciation of chromium (III) and chromium (VI) by capillary electrophoresis with contactless conductometric detection and dual opposite end injection. Electrophoresis 24:1397–1403
Lapanantnoppakhun S, Kasuwas S, Ganranoo L, Jakmunee J, Grudpan K (2006) Simple Specrophotometric Flow Injection System with an In-valve Minicolumn for Enhancement during the Determination of Chromium(III) Using EDTA. Anal Sci 22:153–155
Liang P, Ding Q, Liu Y (2006) Speciation of chromium by selective separation and preconcentration of Cr(III) on an immobilized nanometer titanium dioxide microcolumn. J Sep Sci 29:242–247
Mahmoud ME, Yakout AA, Ahmed SB, Osman MM (2008) Chromium speciation, selective extraction and preconcentration by alumina-functionalised 2-pyridenecarboxyladehyde thiosemicarbazone. Int J Environ Anal Chem 88:1017–1031
Matos D, dos Reis EB, Costa ACS, Ferreira SLC (2009) Speciation of chromium in river water samples contaminated with leather effluents by flame atomic absorption spectrometry after separation/preconcentration by cloud point extraction. Microchem J 92:135–139
Monasterio RP, Lascalea GE, Martınez LD, Wuilloud RG (2009) Determination of Cr(VI) and Cr(III) species in parenteral solutions using a nanostructured material packed-microcolumn and electrothermal atomic absorption spectrometry. J Trace Elem Med Biol 23:157–166
Pantsar-Kallio M, Manninen PKG (1999) Optimizing Ion Chromatography-Inductively Coupled Plasma Mass Spectrometry for Speciation Analysis of Arsenic, Chromium and Bromine in Water Samples. Int J Environ Anal Chem 75:43–55
Shemirani F, Abkenar SD, Mirroshandel AA, Niasari MS, Kozani RR (2003a) Preconcentration and speciation of chromium in water samples by atomic absorption spectrometry after cloud-point extraction. Anal Sci 19:1453–1456
Shemirani F, Abkenar SD, Mirroshandel SS, Salavati-Niasari AA, Kozani RR (2003b) Colorimetric Determination of Benzocaine, Lignocaine and Procaine Hydrochlorides in Pure Form and in Pharmaceutical Formulations Using p-Benzoquinone. Anal Sci 19:1453–1456
Soylak M, Aydin A (2011) Determination of some heavy metals in food and environmental samples by flame atomic absorption spectrometry after coprecipitation. Food Chem Toxicol 49:1242–1248
Sun YC, Lin CY, Wu SF, Chung YT (2006) Evaluation of on-line desalter-inductively coupled plasma-mass spectrometry system for determination of Cr(III), Cr(VI), and total chromium concentrations in natural water and urine samples. Spectrochim Acta B 61:230–234
Tuzen M, Soylak M (2006) Chromium speciation in environmental samples by solid phase extraction on chromosorb 108. J Hazard Mater 129:266–273
Uluozlu OD, Tuzen M, Soylak M (2009) Speciation and separation of Cr(VI) and Cr(III) using coprecipitation with Ni2+/2-Nitroso-1-naphthol-4-sulfonic acid and determination by FAAS in water and food samples. Food Chem Toxicol 47:2601–2605
Vidal L, Chisvert A, Canals A, Salvador A (2010) Ionic liquid-based single-drop microextraction followed by liquid chromatography-ultraviolet spectrophotometry detection to determine typical UV filters in surface water samples. Talanta 81:549–555
Yao C, Anderson JL (2009) Dispersive liquid-liquid microextraction using an in situ metathesis reaction to form an ionic liquid extraction phase for the preconcentration of aromatic compounds from water. Anal Bioanal Chem 395:1491–1502
Yao C, Li T, Wu P, Pitner WR, Anderson JL (2011) Selective extraction of emerging contaminants from water samples by dispersive liquid-liquid microextraction using functionalized ionic liquids. J Chromatogr A 1218:1556–1566
Ying LY, Jiang HL, Zhou SC, Zhou Y (2011) Ionic liquid as a complexation and extraction medium combined with high-performance liquid chromatography in the evaluation of chromium(VI) and chromium(III) speciation in wastewater samples. Microchem J 98:200–203
Yousefi SR, Shemirani F (2010) Development of a robust ionic liquid-based dispersive liquid-liquid microextraction against high concentration of salt for preconcentration of trace metals in saline aqueous samples: Application to the determination of Pb and Cd. Anal Chim Acta 669:25–31
Yuan D, Fu D, Wang R, Yuan J (2008) Rapid determination of chromium(VI) in electroplating waste water by use of a spectrophotometric flow injection system. Spectrochim Acta A 71:276–279
Zeeb M, Ganjali MR, Norouzi P (2010) Dispersive liquid-liquid microextraction followed by spectrofluorimetry as a simple and accurate technique for determination of thiamine (vitamin B1). Microchim Acta 168:317–324
Zeeb M, Ganjali MR, Norouzi P, Kalaei MR (2011) Separation and preconcentration system based on microextraction with ionic liquid for determination of copper in water and food samples by stopped-flow injection spectrofluorimetry. Food Chem Toxicol 49:1086–1091
Zeeb M, Sadeghi M (2011) Modified ionic liquid cold-induced aggregation dispersive liquid-liquid microextraction followed by atomic absorption spectrometry for trace determination of zinc in water and food samples. Microchim Acta 175:159–165
Zhang H, Cheng M, Jiang X (2010) Determination of benzoic acid in water samples by ionic liquid cold-Induced aggregation dispersive LLME coupling with LC. Chromatographia 72:1195–1199
Zhou Q, Ye C (2008) Ionic liquid for improved single-drop microextraction of aromatic amines in water samples. Microchim Acta 162:153–159
Zhou QX, Bai HH, Xie GH, Xiao JP (2008) Temperature-controlled ionic liquid dispersive liquid phase micro-extraction. J Chromatogr A 1177:43–49
Acknowledgments
Support of this investigation by the Research Council of University of Tehran through grant is gratefully acknowledged, as well as proofreading by Barbora Ehrlichová.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeeb, M., Ganjali, M.R. & Norouzi, P. Preconcentration and Trace Determination of Chromium Using Modified Ionic Liquid Cold-Induced Aggregation Dispersive Liquid–Liquid Microextraction: Application to Different Water and Food Samples. Food Anal. Methods 6, 1398–1406 (2013). https://doi.org/10.1007/s12161-012-9557-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9557-5