Abstract
A new, simple, sensitive, and inexpensive method for simultaneous determination of binary mixtures of food colorants, carmoisine and ponceau 4R, which are species with highly overlapped spectra, by using H-point standard addition method (HPSAM) is proposed. Absorbances at the two pair of wavelengths, 460 and 549 nm or 490 and 541 nm, were monitored while adding standard solutions of carmoisine or ponceau 4R, respectively. The accuracy and reproducibility of the determination method for various known amounts of carmoisine and ponceau 4R in their binary mixtures were evaluated. This method was satisfactorily applied for the determination of carmoisine and ponceau 4R in synthetic binary mixtures and several commercial products. The total relative standard error for applying the HPSAM to five synthetic samples was 3.26% or 4.96% with carmoisine or ponceau 4R addition, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Color is a vital constituent of food and probably the first characteristic perceived by the human senses. Probably, for economic reasons, brightly colored and stable synthetic colorants have been widely used as food additives. These dyes are used to supplement and enhance natural colors destroyed during processing or storage and substantially increase the appeal and acceptability of food stuffs. The term color additive can be applied to any dye, pigment, or other substance artificially made or obtained from a vegetable, animal, mineral, or another natural source (Branen et al. 1989). Some synthetic colorants and their metabolic products that are consumed in large amounts may be toxic and cause cancer, deformation, etc. (Vries 1996). Therefore, safety data for every synthetic colorant food additive have been repeatedly determined and evaluated. Carmoisine (E 122) and ponceau 4R (E124) are synthetic dyes that contain azo and aromatic ring structures and are often used in dyeing food, drink, medicine, and cosmetics. Chromatographic methods have been used for colorant analysis in food, and they are recommended when the mixture contains many different colorants (Dossi et al. 2006; García-Falcón and Simal-Gándara 2005). Several simultaneous determinations of colorants in foods have been also reported by electro-analytical methods and capillary electrophoresis (Dossi et al. 2007; Prado et al. 2006). Generally, spectrophotometry is used in determining these colorants, and due to serious spectral overlap, separation is often required. To avoid time-consuming clean-up procedures, attempts to resolve complex spectra by using instrumental approaches or various chemometric methods have been made. Derivative techniques (Altionz and Toptan 2002; Altinoz and Toptan 2003; Kiroglu and Ozdemir 2000) and multivariate statistical analysis (Koyuncu et al. 2003; Oveisi et al. 2003; Dinc et al. 2002; Lopez-de-Alba et al. 2002; Pravdova et al. 2002; Berzas Nevado et al. 1999) have been successfully applied to the multi-component analysis of mixtures by UV/Vis molecular absorption spectrophotometry. All these approaches are useful for the reduction of band-overlapping errors in quantitative analysis, but they are often used for more complex mixtures. In 1988, Bosch-Reig and Campins-Falco (1988) presented a new technique, H-point standard addition method (HPSAM), that was based on the principle of dual wavelength spectrophotometry and the standard addition method. They further studied its principle and application (Bosch-Reig et al. 1991, 1993; Campins-Falco et al. 1992a, b, c, 1995). The greatest advantage of HPSAM is that it can remove the errors resulting from the presence of an interfering and blank reagent (Bosch-Reig et al. 1994). HPSAM was applied for the resolution of binary mixtures (Eskandari and Bagherian Dehaghi 2004) and then has been developed for ternary samples (Ni et al. 2001).
In the first publications, the HPSAM was applied to UV-Vis spectrophotometry; later, it was also extended to liquid chromatography with diode array detection (Bosch-Reig and Campins-Falco 1988) and spectrofluorimetry (Yang et al. 1996). Very accurate results are reported even for extensively overlapping in UV-visible spectrophotometry (Hund et al. 1999).
In this present work, a selective, sensitive, simple, and low-cost procedure for simultaneous determination of carmoisine and ponceau 4R by using HPSAM is suggested.
Materials and Methods
Apparatus
A UV-Visible Cintra 40 double-beam spectrophotometer equipped with a 1.0-cm path length glass cell and connected to an IBM Pentium 100 computer was used. All spectra were recorded with a scan speed of 1,000 nm min−1 at 0.427-nm intervals against a blank of deionized water containing acetate buffer solution (pH = 4.5) in the ratio of 4:1. The pH of the solutions were measured with Metrohm 713 pH meter (Metrohm, Switzerland).
Reagent
All reagents were of analytical-reagent grade, and double-distilled water was used throughout. Carmoisine (E 122, C.I. 14720, Food Red 3), also named Azorubin or Chromotrope FB, and Ponceau 4R (E124, C.I. 16255, Food Red 7), also named New Coccine or Cochineal Red, were obtained from the Institute of Standard of Industrial Research of Iran. The crystalline of colorants was dried at 65 °C for 6 h. Stock solutions of each colorant were prepared at a concentration of 1 mg ml−1 in buffer that is stable approximately for 1 month and diluted to 100 μg ml−1 for use. A buffer solution was prepared from acetic acid and sodium acetate (pH = 4.5, 0.2 M). MN-polyamide-SC 6 adsorbent and TLC plastic sheets (Silica gel 60 F254) were purchased from Merck (Darmstadt, Germany). Phosphate buffer solutions (pH 1–10, with step one, 0.2 M) were used for evaluating the pH influence on color absorption spectra. The ammonia solution (pH = 11) was used for desorbing colorants.
Individual Calibration
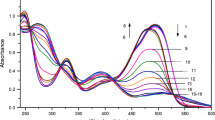
Each of two colorant solutions (100 μg ml−1) was prepared from stock solutions of each colorant in 25 ml volumetric flask individually. The spectra of carmoisine shows a maximum at 515 nm, and ponceau 4R shows a maximum at 508 nm (Fig. 1). To verify the governing Beer’s law, 2.0 ml of acetate buffer solution (pH 4.5) and adequate volumes of individual considered colorant solution (100 μg ml−1) were placed into a 10-ml volumetric flask, made up to the mark with double-distilled water, and the calibration curves were constructed over the λ = 515 nm for carmoisine and 508 nm for ponceau 4R. These calibration curves were prepared for each colorant separately and in the presence of a fixed amount of other colorant. The appropriate correlation coefficients obtained (in the range 0.9981–0.9999) indicate that the interaction between the two colorants dose not exist. Individual calibration curves were constructed with several points, as absorbance versus colorant concentration in the range of 1–50 μg ml−1 for carmoisine and 1–40 μg ml−1 for ponceau 4R and evaluated by linear regression. The intercepts on the ordinates were negligible in the calibration curves. For simultaneous calibration, a set of calibration solutions containing 2.0 ml acetate buffer (pH = 4.5) and appropriate volume of carmoisine and ponceau 4R were prepared. Simultaneous determination of carmoisine and ponceau 4R with HPSAM was performed by measuring the absorbances at 460 and 549 nm (when standards of carmoisine were added) or 490 and 541 nm (when standards of ponceau 4R were added) for each sample solution. For validation procedure, five synthetic samples (Table 1) containing different concentration ratios of carmoisine and ponceau 4R were prepared, and standard additions of carmoisine and ponceau 4R were made. The concentration range of carmoisine and ponceau 4R for construction of HPSA calibration graph were 1–50 μg ml−1 and 1–40 μg ml−1, respectively. For the analysis of real samples, the same procedure was repeated.
Commercial food sample preparations
Treatment of drink
Twenty-five milliliters of the sample was transferred to a 50-ml volumetric flask and diluted to the volume with deionized water. The pH of this solution was then adjusted to 4.5 with 20% citric acid.
Treatment of candy
The candy was ground with a mortar and pestle, 45 g of this sample dissolved in 100 ml of deionized distilled water, and gently warmed to dissolve completely. The pH of the solution was then adjusted to 4.5 with 20% citric acid.
Treatment of chocolate in crisp sugar shell
Five grams of this sample was transferred into a baker. For dissolving dye without dissolving titanium dioxide (the shell), 6 ml acetone was added; after 1 min, 2 ml of distilled deionized water was added and shook slowly. A solution of acetone and water (3:1) was prepared and added several times into the baker to dissolve the remained color. The obtained solution was filtered and warmed to remove the acetone by a rotary evaporator. The pH of the solution was then adjusted to 4.5 with 20% citric acid.
Treatment of pediatric grip syrup
Fifty milliliters of the sample was transferred in to a 100-ml volumetric flask. The volume was adjusted to 100 ml with deionized distilled water. The pH of the solution was then adjusted to 4.5 with 20% citric acid.
Further treatment of food samples
The solutions obtained above were then warmed on a water-bath, and the temperature was let to increase to 70 °C. A 0.5 g of polyamide adsorbent was added, and the mixture was stirred vigorously to adsorb all the colorants in the solution (if the solution still had some color, a small further amount of polyamide adsorbent should be added). The mixture was filtered, and the adsorbent was washed thrice with 20 ml citric acid solution (20% v/v, pH = 4.5) at 70 °C and then several times with distilled water of 70 °C. The prepared adsorbent was then washed by solution of alkaline-ammonia (pH = 11) several times to desorb all the colorants. The solution collected was warmed on a water-bath to draw out all the ammonia and then transferred in to a volumetric flask with appropriate volume (due to the amount of color); 2 ml of acetate buffer solution (pH = 4.5) was added and diluted with distilled deionoized water. The colorants type in these food samples were detected by thin-layer chromatography method (butanol/acetic acid/water, 4:1:1) and then analyzed by the proposed method.
Results and Discussion
HPSAM is a modification of the standard addition method that permits transformation of the indeterminate error resulting from the presence of a direct interferent in the determination of an analyte into a systematic constant error that can be evaluated free from bias error. The basis of the method permits the determination of two species with extensively overlapped spectra (Bosch-Reig and Campins-Falco 1988) and corrects both proportional and constant errors originated from matrix of the sample and interferent. The method is based on selecting two pairs of independent variables in such a way that in each pair, the analytical signal, due to one of the species, is constant and for another one as different as possible. Two pairs of standard addition plots can be obtained by plotting the analytical signals in two pairs of selected independent variables versus the analyte concentrations that are added to the sample solution simultaneously and in a constant ratio. Each pair of plots has an intersection point that its coordinate on the concentration axis is −CA.
Influence of pH
The influence of pH on the absorption spectra for solutions of ponceau 4R and carmoisine (20 μg ml−1) was studied. To establish a suitable pH for this study, a range between 1.0 and 10.0 pH values with one step was examined obtaining the following results: carmoisine shows a maximum at 515 nm whose absorbance decrease over pH 9.0 values but no significant change was observed for ponceau 4R in the 1.0-10.0 pH range. Therefore, there was not any significant difference between pH ranges, and the pH value of 4.5 was selected in this research.
Wavelength Selection
When caimoisine is selected as an analyte, some pairs of wavelengths showing the same absorbances for the ponceau 4R are possible. When selecting one pair of wavelengths for obtaining good accuracy, absorbance differences at the two wavelengths for the carmoisine must be considered as high as possible. Based on the spectra of the carmoisine and poncea 4R (Fig. 1), one of the best pairs of wavelengths are 460 and 549 nm. With these wavelengths, almost maximum sensitivity differences are attainable. HPSAM can also be applied by addition of standard solutions of ponceau 4R. For this purpose, one pair of wavelengths must be selected as analytical for ponceau 4R. The selected wavelengths are 490 and 541 nm. Figure 2 shows the H-point standard addition plot for the simultaneous determination of carmoisine and ponceau 4R using the selected wavelengths when carmoisine was added.
Accuracy of the Method
Several experiments for evaluating HPSAM on the determination of carmoisine and ponceau 4R in a series of samples containing fixed amounts of carmoisine with different amounts of ponceau 4R (Fig. 3) or fixed amounts of ponceau 4R with different amounts of carmoisine (Fig. 4) were carried out by the addition of carmoisine and ponceau 4R, respectively. The accuracy of the method for both colorants addition was evaluated using 490 and 549 nm wavelength when carmoisine was added and the two other wavelength (490 and 541 nm) when ponceau 4R was added. The proposed HPSAM also was applied to the resolution of five synthetic mixtures of the two colorants. The composition of the mixtures is shown in Table 1. The prediction ability of HPSAM for each colorant can be described in terms of the relative standard error (RSE), which is the square root of the mean square of the error in the prediction.
Where N is the number of samples, C j is the concentration of the colorant in the jth mixture and \(\widehat{\text{C}}_{\text{j}} \) is the estimated concentration. The total prediction error of N samples is calculated as follows:
Where C ij is the concentration of the ith colorant in the jth mixture and \(\widehat{\text{C}}_{{\text{ij}}} \) is its estimation; N is total number of samples, and M is total number of components. Table 2 shows reasonable single and total relative errors for such a system. The results show that the carmoisine and ponceau 4R concentrations in the samples have been determined accurately, but the single and total relative standard errors for ponceau 4R addition were relatively more than carmoisine (Table 2). Therefore, the question of applicability of the proposed procedure to carmoisine and ponceau 4R determination has been clarified.
Application to Real Samples
The proposed method was used to determine the amount of colorant in some commercial samples. The accuracy of the method was verified by means of recovery assay. This was accomplished by an analyzing sample solution and spiked (enriched) samples. The average recovery obtained for carmoisine and ponceau 4R were 97.55 and 97.10, respectively. The analytical results obtained from the samples show that they are quite acceptable and consistent with those of the nominal contents provided by the manufacturers (Table 3). The HPSAM was applied to determine carmoisine and ponceau 4R simultaneously. The proposed simple method offers sufficient selectivity and sensitivity reproducibility in a wide range concentration of carmoisine and ponceau 4R.
References
Altionz S, Toptan S (2002) J Food Comp Anal 5:667
Altinoz S, Toptan S (2003) J Food Comp Anal 16:517
Berzas Nevado JJ, Rodriguez Flores J, Villasenor Llerena MJ, Rodriguez Farinas N (1999) Anal Chim Acta 391:353
Bosch-Reig F, Campins-Falco P (1988) Analyst 113:1011
Bosch-Reig F, Campins-Falco P, Sevillano-cabeza A, Herraez-Herhnandaz R, Molins-legua C (1991) Anal Chem 63:2424
Bosch-Reig F, Campins-Falco P, Verdu-Andres J (1993) Anal Chim Acta 283:831
Bosch-Reig F, Verdu-Andres J, Campins- Falco P, Molins-legua C (1994) Talanta 41:39
Branen A, Davidson P, Salminen S (1989) Food additives. Academic, New York
Campins-Falco P, Bosch-Reig F, Verdu-Andres J (1992a) Talanta 39:1
Campins-Falco P, Bosch-Reig F, Verdu-Andres J (1992b) Anal Chim Acta 270:253
Campins-Falco P, Bosch-Reig F, Herraez-Hernandez R, Sevilano-Cabeza A (1992c) Anal Chim Acta 257:89
Campins-Falco P, Verdn-Adres J, Bosch-Reig F, Molins-Legua C (1995) Anal Chim 302:323
Dinc E, Baydan E, Kanbur M, Onur F (2002) Talanta 58:579
Dossi N, Toniolo R, Susmel S, Pizzariello A, Bontempelli G (2006) Chromatographia 63:557
Dossi N, Piccin E, Bontempelli G, Carrilho E, Wang J (2007) Electrophoresis 28:4240
Eskandari H, Bagherian Dehaghi G (2004) Microchim Acta 146:265
García-Falcón MS, Simal-Gándara J (2005) Food Control 16:293
Hund E, Massart DL, Smeyers-Verbeke J (1999) J Pharm Biomed Anal 21:23
Kiroglu F, Ozdemir Y (2000) Adv Food Sci 22:86
Koyuncu I, Uzgur MV, Bozdogan A (2003) Int J Chem 13:19
Lopez-de-Alba PL, Lopez-Martinez L, De-Leon-Rodriguez LM (2002) Electroanal 14:197
Ni Y, Qi M, Kokot S (2001) Anal Lett 34:2585
Oveisi MR, Hajimahmoodi M, Davami F (2003) Daru 11:1
Prado MA, Vilas Boas LF, Bronze MR, Godoy HT (2006) J Chromatogr A 1136:231
Pravdova V, Boucon C, Jong SD, Walczak B, Massart DL (2002) Anal Chim Acta 462:133
Vries J (1996) Food safety and toxicity. CRC, London
Yang J, Zhou G, Jie N, Han R, Lin C, Hu J (1996) Anal Chim Acta 325:195
Acknowledgments
The authors acknowledge Tehran University of Medical Sciences and Health Services for its support by a grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajimahmoodi, M., Oveisi, M.R., Sadeghi, N. et al. Simultaneous Determination of Carmoisine and Ponceau 4R. Food Anal. Methods 1, 214–219 (2008). https://doi.org/10.1007/s12161-008-9022-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-008-9022-7