Abstract
The production of second-generation (2G) ethanol remains an interesting proposition for the implementation of sustainable and net carbon–neutral energy systems. To be economically viable, 2G biorefineries must make use of all processing streams, including the less desirable pentose (C5) sugar stream. In this work, a strategy of sequential dilute acid and alkaline pretreatment of the lignocellulosic feedstock, switchgrass, was implemented for improving the fermentable sugar yield. The hemicellulose-enriched hydrolysate obtained after dilute acid pretreatment was fermented by a newly isolated wild Scheffersomyces parashehatae strain—UFMG-HM-60.1b; the corresponding ethanol yield (YPS) and volumetric productivity (QP) were 0.19 g/g and 0.16 g/L h, respectively. The remaining switchgrass cellulignin fraction was subjected to optimized alkaline delignification at 152 ºC for 30 min. Then, the delignified solid fraction was subjected to contiguous enzymatic saccharification and fermentation releasing a glucose (C6) sugar stream. The control yeast strain, Saccharomyces cerevisiae 174, displayed an ethanol YPS of 0.46 g/g and QP of 0.70 g/L h for the C6 sugar stream, whereas the above-mentioned wild strain presented YPS and QP of 0.29 g/g and 0.38 g/L h, respectively. Upon combining the conversion of hemicellulose (37%) and cellulose-derived sugars (57%), the wild S. parashehatae strain provided higher yield (94%) than the generic S. cerevisiae (90%). Henceforth, our sequential two-stage pretreatment and fermentation of C5 and C6 sugar streams provides a pathway for maximum utilization of switchgrass carbohydrates for 2G ethanol production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioethanol production is an economical and environment-friendly alternative to fossil fuels as well as contributing towards energy independence. It can also offer socio-economic advantages, such as increase in farming income for developing countries and reduction of greenhouse gas emissions [1]. Bioethanol has long established itself as the world’s main biofuel source and the global bioethanol market is valued at $34 billion in 2020, which is expected to grow at a compound annual growth rate of 14% and reach $65 billion by 2025 [2]. In 2020, the world bioethanol production was about 26 billion gallons; the USA leads the industry at 14 billion gallons, followed by Brazil at 8 billion gallons [3].
Bioethanol can be obtained from different raw materials including starches, lignocellulose, and algae, which are accordingly named as first-generation (1G), second-generation (2G), and third-generation (3G) biofuels. Lignocellulosic biomass does not compete with food production [4], and ethanol production from lignocellulosic feedstocks, such as switchgrass, would increase the productivity per hectare of marginal lands [5], and improve biodiversity and the potential for carbon sequestration [6]. Despite these advantages, large-scale implementation of 2G ethanol production is still under development and is faced with multiple challenges in achieving economic viability. Complete availability and use of carbohydrate monomers remain a barrier, in addition to technological difficulties in microbial strain development for pentose (C5) fermentation, unit integration, and system optimization for higher fractionation efficiency and co-product utilization [7, 8]. Hence, development of strategies for feasible deconstruction of switchgrass biomass will benefit the 2G biofuel industry.
The major components of switchgrass are cellulose (32–34%), hemicellulose (26–27%), and lignin (17–18%) [9]. Pretreatments are usually applied to reduce biomass recalcitrance and to increase the hydrolytic efficiency of cellulose and hemicellulose polymeric fractions into fermentable sugars (i.e., glucose, xylose, galactose, and arabinose). Lignin is recovered from the pretreatment hydrolysates and commonly used as a fuel source in biorefineries. The resulting sugars are fermented to ethanol, which is then distilled for fuel purposes [10]. Among the different biomass pretreatment techniques, including organosolv and ionic liquids fractionation, dilute acid and alkaline pretreatments are more promising because they require lower capital investment and are technologically undemanding [11]. Dilute acid pretreatment facilitates deacetylation and subsequent fractionation of hemicellulose from lignocellulosic feedstocks, whereas the alkaline pretreatment facilitates lignin removal (and to a certain extent hemicellulose) via de-esterification of lignin-carbohydrate linkages [11]. When implemented in tandem, these two techniques could complement each other and facilitate systematic deconstruction of lignocellulosic biomass.
Our previous optimization of dilute acid pretreatment for switchgrass showed that, at 140 °C and 40 min, using 1% (v/v) of sulfuric acid as catalyst, we could maximize xylose recovery at a concentration of 22 g/L in the liquid hydrolysates [12]. However, subsequent enzymatic saccharification efficiency of the cellulignin fraction was low, at only 49%. When the dilute acid pretreatment temperature was increased to ≥ 160 °C, it improved the enzymatic saccharification efficiency to up to 96%, but also caused significant degradation of xylose and produced fermentation inhibitors like furfural [13]. Hence, to improve xylose recovery, we adopted less severe dilute acid conditions but followed suit with alkaline delignification for enhancing the subsequent cellulose conversion. Past research has shown that, due to its recalcitrant nature, switchgrass requires severe alkaline pretreatment with high NaOH loading (15.4%) at 130 °C for 30 min to achieve saccharification efficiencies exceeding 90% [14]. Another study conducted relatively mild alkaline pretreatment at 5.5% NaOH loading and 100 °C, but required 6 h to achieve 63% conversion of switchgrass biomass [15]. Thus, the process efficiency of previously reported alkaline pretreatment conditions does not meet the required cost benefits, making it difficult to implement on a large scale. In our study, we propose to use sequential dilute acid and alkaline pretreatment such that we could adopt milder conditions and reduce chemical use, while simultaneously maximizing glucose and xylose yields.
Aside from an efficient pretreatment process, the concomitant fermentation of pentose (C5) and hexose (C6) sugars is an important strategy to improve the commercial viability of 2G biorefineries [16, 17]. In this context, the physiological characteristics expected in an ideal fermentation microorganism are broad-spectrum substrate uptake, ability to withstand high sugar and alcohol concentration, resilience to lower pH, resistance to inhibitory compounds, and minimal byproducts formation [18]. Although Saccharomyces cerevisiae is largely employed in industrial alcohol fermentations because of its outstanding performance, it lacks the ability to assimilate C5 sugars. New yeast strains capable of metabolizing C5 sugars are under development by the use of genetic engineering tools [19,20,21]. Scheffersomyces stipitis NRRL Y-7124 has been reported to convert C5 sugars through a xylose-to-xylulose redox pathway, whereas S. cerevisiae strains have been engineered to transport xylose through heterologous gene expression [8, 22]. However, the engineered yeast strains experience drawbacks because of their susceptibility to inhibitors, which are generated during biomass pretreatment [8]. Moreover, it is also important to develop differential strategies apart from genetic engineering to enhance the productivity of yeasts. Hence, there is a need to seek wild yeast strains that are naturally robust. Few reports on wild strains have shown that they can assimilate both C5 and C6 sugars and synthesize ethanol with robust process efficiencies and scale-up properties [23]. In our previous work, immobilized Scheffersomyces parashehatae UFMG-HM-52.2 was reported to metabolize C5 sugarcane hydrolysate [23]. Recently, another novel wild strain isolated from the tropical fauna of Brazil, classified as S. parashehatae UFMG-HM-60.1b, has shown the potential to metabolize switchgrass C5 hydrolysates for the first time.
In this study, we have compared the fermentation of switchgrass sugars by the conventional S. cerevisiae as well as the novel S. parashehatae UFMG-HM-60.1b strain. This approach aims to evaluate the feasibility of harnessing a natural C5-metabolizer for ethanol fermentation by utilizing lignocellulosic hydrolysates as the sole carbon source. Henceforth, the switchgrass biomass was initially subjected to dilute acid pretreatment for separating the hemicellulose fraction and the remaining cellulignin fraction was subjected to alkaline delignification to enhance cellulose conversion. To maximize C6 sugar yields, a 22 full factorial optimization was carried out by varying the time and temperature of alkaline pretreatment. Ethanol production from S. cerevisiae 174 and S. parashehatae UFMG-HM-60.1b strains was evaluated using the switchgrass enzymatic hydrolysates. In a parallel approach, ethanol production from switchgrass hemicellulosic hydrolysates was evaluated solely by using the wild strain. This work was performed with the impetus of maximizing switchgrass sugar utilization, which would eventually increase biorefinery profitability.
Materials and Methods
Biomass
Switchgrass biomass (Panicum virgatum L. var. Alamo) was supplied by the Department of Biological & Agricultural Engineering at the University of Arkansas, in Fayetteville, USA. The switchgrass biomass composed of leaves and stalk was dried and ground in a Wiley mini-blade mill such that the biomass particle size was reduced below 20 mesh (0.84 mm).
Microorganisms
S. cerevisiae 174 was obtained from the culture collection of Department of Biotechnology in Lorena School of Engineering—SP-Brazil, while S. parashehatae UFMG-HM 60.1b was kindly provided by the culture collection of Yeast Ecology and Biotechnology Laboratory at the Federal University of Minas Gerais, in Minas Gerais, Brazil. Stock cultures of both S. cerevisiae and S. parashehatae were prepared by transferring a loopful of cells from the slant into 125-mL Erlenmeyer flasks containing 50 mL of medium (pH 5.5) composed of glucose (30 g/L), yeast extract (10 g/L), and peptone (20 g/L). The flasks were incubated in a Innova 4000 rotatory shaker-incubator (New Brunswick Scientific, CT, USA) at 30 °C for 24 h, at 100 rpm for S. cerevisiae and 200 rpm for S. parashehatae. After cultivation, both yeasts were separated via centrifugation at 2777 × g for 10 min at room temperature, washed with sterilized water, re-suspended, and used as inoculum in the batch fermentation processes [24].
Dilute Acid Pretreatment
About 20 g of milled switchgrass biomass and 1% (w/v) sulfuric acid solution were mixed in a 1 L Parr 4525 reactor (Moline, IL, USA) at 1:10 solid-to-liquid ratio and pretreated at 140 °C for 40 min at 144 rpm. Pretreatment time was measured after the reactor reached the desired temperature and the process was stopped by circulating cold water through the cooling coils. Pretreated slurry was separated into liquid and solid fractions using a Buchner funnel lined with Whatman #1 filter paper. The liquid fraction, termed here as “hemicellulosic hydrolysate,” was recovered from several runs, combined, and then concentrated under low vacuum and no heat using a Savant SPD 1010 SpeedVac concentrator (Thermo Scientific, Ashley, NC). The final volume of the concentrate was 400 mL. Composition of the concentrated hemicellulosic hydrolysate is provided in Table 1.
The concentrated hemicellulosic hydrolysate was detoxified according to the method adapted from [25] in order to reduce the concentration of fermentation inhibitory compounds generated during the dilute acid pretreatment. In short, the detoxification process consisted of the following: (i) increasing the pH from 0.5 to 7 with NaOH solution; (ii) reducing the pH to 5.5 with phosphoric acid and addition of activated charcoal at 2.5% (w/v); and finally (iii) incubating at 30 °C for 1 h and 200 rpm. After detoxification, the hydrolysate was filtered under vacuum in order to separate the liquid fraction and autoclaved at 0.5 atm (110 °C) for 20 min before being used in the fermentation process.
*Concentrated composition from ten runs; #average and standard deviations for N = 3.
Alkaline Pretreatment of Cellulignin and Enzymatic Hydrolysis
The cellulignin fraction obtained after dilute acid pretreatment was subjected to alkaline delignification in a 1 L digester (B03-1L, PHD Equipamentos para Laboratório, Piracicaba-SP, Brazil), according to the maximum feasible ranges for the independent variables defined in this methodology [26,27,28]. Sodium hydroxide solution (1% w/v) was used as catalyst and the cellulignin fraction was loaded at 1:10 solid-to-liquid ratio [29]. A 22 full factorial design was implemented, using the Statistica software for Windows (v5.0 Stat Soft Inc., Tulsa, OK, USA), to investigate the effects of two independent process variables: temperature (90 and 152 °C) and time (0.5 and 1.5 h) on the glucose yield (%) resulting from the enzymatic saccharification of the alkaline pretreated biomass. After treatment, the solid fraction was recovered by filtration using muslin cloth and washed with running tap water until neutral pH and dried at 45 °C. Three replicates of the alkaline pretreatment were implemented at the center point and one run per block was implemented for the four factorial points (Table 2). The response surface model predicting the interaction between the input variables and their relationship with the output response was depicted by a first-order polynomial equation. An analysis of variance (ANOVA), conducted by pure error analysis, was performed to validate the regression model and coefficients [30]. Any statistical significance was established for p < 0.05.
†Determined using enzymatic saccharification of sequentially pretreated switchgrass; (0) = center point, (-) = lower level, and ( +) = higher level in this full factorial design.
The enzymatic hydrolysis of sequentially pretreated switchgrass was performed in a 125-mL Erlenmeyer flask containing 3 g (dry wt.) of pretreated solids and 40 mL of citrate buffer (50 mM, pH 4.8). The substrate soaked in citrate buffer was supplemented with a cellulase enzyme cocktail obtained from Dyadic International, Inc. (Cellulase CP CONC; 20 FPU/g of the dry substrate) and surfactant (Tween 20 at 0.1 g/g). Enzymatic hydrolysis was performed at 50 °C and 150 rpm in the Innova 4000 shaking incubator for 48 h. Samples were collected at different time intervals, centrifuged, and analyzed to determine the sugars released. Glucose concentration after enzymatic hydrolysis step was used to calculate the digestibility (% of cellulose converted to glucose per gram of biomass), by using a correction factor of 0.9 according to the equation proposed by Lu et al. [31]. Enzymatic hydrolysis was performed for the optimized treatment conditions.
Fermentation of Switchgrass Enzymatic Hydrolysate
S. cerevisiae and the wild S. parashehatae strains were used for the fermentation of switchgrass enzymatic hydrolysate. For S. cerevisiae, the enzymatic hydrolysate was supplemented with yeast extract (1 g/L), peptone (1 g/L), diammonium hydrogen phosphate (1 g/L), dipotassium hydrogen phosphate (1 g/L), and magnesium sulfate and manganese sulfate (each at 0.5 g/L) and adjusted to pH 5.5 [23]. Fermentation assays were carried out in 125-mL Erlenmeyer flasks containing 50 mL of supplemented hydrolysate medium and 0.5 g/L of yeast suspension. The experiments were performed in the shaking incubator at 30 °C, 150 rpm for 96 h. In another set of fermentation, S. parashehatae–inoculated enzymatic hydrolysate was supplemented with ammonia sulfate (5 g/L), malt extract (3 g/L), and yeast extract (3 g/L). Fermentation assays were carried out in a similar fashion at 30 °C and 200 rpm for 96 h. In both experiments, samples were collected periodically to determine the sugar consumption and production of ethanol.
Fermentation of Switchgrass Hemicellulosic Hydrolysate
Switchgrass hemicellulosic hydrolysate was diluted to achieve xylose concentration of 23 g/L and then was supplemented with ammonium sulfate (5 g/L), malt extract (3 g/L), and yeast extract (3 g/L) [23]. Fermentation assays were carried out in 125-mL Erlenmeyer flasks containing 50 mL of supplemented hemicellulosic hydrolysate medium and 0.5 g/L of S. parashehatae cell suspension [23]. The experiments were performed in the shaking incubator shaker at 30 °C and 200 rpm for 96 h. In both experiments, samples were collected periodically to determine the sugars consumption and production of ethanol. Both C5 and C6 fermentation assays were carried out in triplicate and the statistical significance was evaluated for α = 0.05.
Analytical Methods
Glucose, xylose, and ethanol concentrations were determined using a Shimadzu LC- 10AD (Kyoto, Japan) high-performance liquid chromatography system, coupled to a refractive index detector, and equipped with a Aminex HPX-87H analytical column (300 × 7.8 mm; Bio-Rad, Hercules, CA, USA). Sulfuric acid at 0.01 N was used as an eluent at a flow rate of 0.6 mL/min, column temperature of 45 °C, and injection volume of 20 μL.
Scanning electron microscopy (SEM) analysis of samples was performed as previously described in [32]. The X-ray diffraction (XRD) analysis was performed by using a Seifert ISO-Debyeflex 3003 (Germany) instrument. The crystallinity was analyzed by regulating the diffractometer at 40 kV, 30 mA; radiation wavelength was Cu Kα (λ = 1.54 Å). A 2θ range of 10 to 50 degrees was used with a step size of 0.05° for scanning all samples. The crystallinity index (CrI) of biomass powder was calculated by the empirical peak height method described by Segal et al. [33].
Ethanol yield (YPS) and volumetric productivity (QP) were considered response variables for analysis of significance and were calculated at the time corresponding to a consumption of more than 90% of fermentable sugars. YPS (g/g) is defined as the ratio between ethanol production (g/L) and sugar consumption (g/L), whereas QP (g/L h) is the ratio between ethanol production (g/L) and fermentation time (h).
Results and Discussion
Effect of Sequential Pretreatments on Switchgrass Chemical Composition
The in natura chemical composition of switchgrass biomass was 40 ± 0.5% glucan, 24 ± 1% xylan, 7 ± 0.7% arabinan, 21 ± 0.5% total lignin, 5 ± 0% total extractives, and 2 ± 0% ash, on oven dry weight basis. The scheme for sequential pretreatment, enzymatic saccharification, and fermentation of switchgrass is provided in Fig. 1; changes in the chemical composition of pretreated solid fractions were compared and conversions of xylose and glucose sugars by the conventional and wild yeast strains were evaluated. After dilute acid pretreatment, cellulose and lignin content of switchgrass biomass increased by 20% and 76%, respectively, while the hemicellulose content decreased by 68%. These results are aligned with previous reports [34], where under similar severity of dilute acid pretreatment, switchgrass cellulose and lignin content increased by 20% and 50%, respectively, while the hemicellulose content decreased by 73%. Dilute acid hydrolysis breaks down glycosidic bonds, releases acetyl groups, and depolymerizes hemicellulose [35], resulting in xylan solubilization, which in turn increases biomass cellulose and lignin content.
The switchgrass cellulignin obtained from dilute acid pretreatment was subsequently subjected to alkali pretreatment and the response was roughly a 1.7-fold increase in cellulose content with simultaneous reduction of lignin and hemicellulose content (Fig. 1). The alkali catalyst serves as delignifying agent via disruption of structural interunit linkages and reduction of lignin’s degree of polymerization [36]. The two-stage pretreatment was demonstrated to be effective with only 12% (w/w) of lignin remaining in the pretreated solids. Although several strategies could be implemented to achieve similar results, chemically mediated biomass pretreatments represent low-cost methods that are promising for industrial implementation [37].
Optimization of Alkaline Pretreatment of Switchgrass
The time and temperature conditions for alkaline pretreatment were chosen based on previous reports [26,27,28], where a non-pressurized low-level severity was set at 90 ºC, center point was set at 1 atm pressure (121 ºC), and the maximum severity was set at 152 ºC. The lowest and highest time variables were 0.5 h and 1 h, respectively. Statistical significance of the studied variables was evaluated using analysis of variance (ANOVA), where glucose yield (%) from enzymatic saccharification was set as the response variable (Table 3).
R2 = 0.95967; pure error mean square = 1.068233.
*Significant at α0.05; DF degrees of freedom.
As shown in Table 3, only the temperature variable had a significant effect on glucose yield, at 95% confidence level, indicating that severe alkaline pretreatment temperatures are required for switchgrass cellulignin. Since time was not a significant factor affecting enzymatic saccharification, the low-level setting (0.5 h) was selected for optimized conditions aiming for operating cost reductions. Thus, the final selected conditions for alkaline pretreatment of switchgrass cellulignin were 0.5 h and 152 ºC. For this study, we did not consider temperatures above 152 ºC because it would increase the energy costs and compromise the process viability. As shown in Fig. 2, optimization of alkaline pretreatment was centered on a linear model with R2 = 0.95967 (which implies that the response factor was effectively satisfied by the proposed model) without curvature (p < 0.05). The model for glucose yield, as a function of pretreatment time and temperature, is represented by using real values in Eq. (1):
Effect of Sequential Pretreatment and Saccharification on Biomass Structure
Switchgrass biomass, in natura, after sequential pretreatments and enzymatic hydrolysis was evaluated using SEM (Fig. 3) and X-ray diffraction analyses. Through SEM analysis, it was determined that the natural integrity of original biomass was intact, where the fibers were aligned and pores undetectable, implying that cellulose is likely coated and protected by hemicellulose and lignin (Fig. 3A). After dilute acid pretreatment (Fig. 3B), evident disruption of the surface layer was observed, owing to the depolymerization of hemicellulose and to some extent the damage resulting from pressure and temperature. However, the deeper layers and the fiber core were undisturbed. By contrast, the subsequent alkaline pretreatment (Fig. 3C) caused a clear rupture of fibers and disruption of compactness, which was visualized as voids and surface disorganization, and attributed to lignin removal. Results presented in Fig. 3 show that the strategy of using dilute acid followed by alkaline pretreatment was effective for breaching the tightly woven switchgrass cell walls, as compared to solely dilute acid pretreated biomass.
The results obtained from X-ray diffraction demonstrated that partial removal of lignin and hemicellulose led to increase in CrI of pretreated biomass. Switchgrass in natura presented a CrI of 48%, whereas after dilute acid and subsequent alkaline pretreatments, the respective CrI increased to 51% and 55%. It is well known that, structurally, hemicellulose and lignin can be classified as heteropolymers with a hyperbranched structure [38, 39]. Therefore, they possibly comprised the amorphous moiety of the complex lignocellulosic molecular arrangement. When sequential pretreatments are employed, removal of lignin and most of hemicellulose leads to the reduction of amorphous constituents and increases the overall crystallinity. Similar results have been reported for sugarcane bagasse, where sequential pretreatments led to successive increase in CrI [40, 41]. Following the enzymatic saccharification, CrI of leftover solid residues decreased to 37.5% which is to be expected after biologically catalyzed depolymerization of crystalline cellulose moieties of switchgrass.
Ethanol Production from Hemicellulosic and Enzymatic Hydrolysates
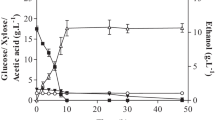
The liquid hydrolysate generated after enzymatic saccharification was employed as carbon feedstock for ethanol production by the benchmark S. cerevisiae strain, establishing parameters for comparison of fermentation performance with the wild strain, S. parashehatae. The fermentation yield and kinetics of sugar consumption of S. cerevisiae are displayed in Fig. 4; about 100% of the initial glucose concentration was depleted within 10 h, while ethanol concentration reached 12 g/L within 17 h. The yield factor (YPS), which was estimated based on the sugars consumed during the fermentation process, was 0.46 g ethanol/g glucose, while the productivity rate was 0.70 g/L h. Since the theoretical maximum yield of ethanol from glucose or xylose is 0.51 g/g, this S. cerevisiae strain provided a 90% conversion thereby justifying its current popularity in the bioethanol industry [42].
Unfortunately, S. cerevisiae cannot metabolize xylose; thus, no changes occurred in xylose concentrations during the course of fermentation (Fig. 4). No lag phase was detected, indicating that the presence of xylose did not repress the anabolic pathway or glucose catabolism to any extent. Early investigations have documented a noticeable catabolic repression of an engineered S. cerevisiae strain whenever cultivated in the presence of xylose [43]. Furthermore, when an engineered pentose metabolizing S. cerevisiae YRH400 strain was investigated for the conversion of pretreated switchgrass biomass into ethanol, a 11.4% increase was reported when compared to the S. cerevisiae D5A control [44]. Interestingly, S. cerevisiae YRH400 did not completely exhaust the xylose concentrations as 6.3 g/L of residual xylose was detected in the media at the end of the cycle. The current ethanol concentration at 12.5 g/L is comparable to that of a similar S. cerevisiae D5A strain reported in the previous study.
The search for new microorganisms capable of metabolizing pentoses through the pentose phosphate pathway (PPP) is still open. The novel-wild strain S. parashehatae UFMG-HM-60.1b was isolated from the Brazilian fauna and was previously identified to be capable of synthesizing ethanol from xylose sugars. Hence, for the first time, this novel yeast strain has been tested for its capability to utilize switchgrass enzymatic hydrolysates, the results of which are displayed in Fig. 5. The glucose concentration in the hydrolysate decreased to 0 g/L within 24 h. However, due to the ease of hexose assimilation in the first 18 h, xylose which is normally metabolized through the PPP pathway was inhibited and catabolically repressed [45]. Organisms with natural pentose fermentation capabilities have the tendency to assimilate glucose readily over any other available carbon sources [46]. As expected, after glucose exhaustion, the xylose concentration of enzymatic hydrolysate declined sharply to 0.5 g/L between 24 and 36 h. Ethanol production reached its peak at 9 g/L within 24 h, corresponding to YPS and QP of 0.29 g/g (glucose + xylose) and 0.38 g/L h, respectively. In comparison to the fermentation performance of the generic S. cerevisiae strain, the wild S. parashehatae showed lower efficiency despite its ability to assimilate C5 sugars. Nonetheless, these results may indicate that the wild strain is capable of continuously consuming C5 sugars even after the depletion of C6 sugars. These results are similar to the conversion performance of S. stipitis NRRL Y-7124 which was reported to produce an ethanol yield and productivity of 0.33 g/g and 0.34 g/L h, respectively, for a medium containing both pentoses and hexoses [22].
The low ethanol yield determined for S. parashehatae could be attributed to the fact that ethanol was metabolized during the final stage of fermentation, likely due to the overaccumulation of oxidized cofactor NAD+ and ensuing need for regeneration. After xylose transport to the cell plasma, reduction to xylitol occurs through stepwise reactions mediated by xylose reductase with the assistance of the cofactor NAD(P)H. The xylose reductase enzyme can be either be NADPH or NADH dependent, suggesting that cofactors in the reduced forms are required to initiate xylose consumption. It is possible that, in this wild yeast strain, xylose reductase required NADH over NADPH due to its constitutive affinity. Therefore, lack of NAD+ regeneration would have prevented this yeast strain from effectively utilizing xylose. It is important to note that, concomitant to the decrease in xylose concentration, the ethanol concentration also started falling supporting the fact that the wild yeast’s metabolism showed flexibility under redox stress. Capacity of consuming ethanol in situations of carbohydrate scarcity has been reported before with other strains [40]; therefore, this wild yeast strain will require controlled feeding rate as well as metabolite removal during fermentation to prevent ethanol losses.
The wild strain was also used to ferment the hemicellulosic hydrolysate, whose initial xylose and glucose concentration was adjusted to 23 g/L and 9 g/L, respectively. As denoted previously, despite the high concentration of xylose, glucose was easier to assimilate due to lower enzymatic and transport requirements. However, distinct behavior was observed in this case regarding the concomitant metabolism of C5 and C6 sugars. As shown in Fig. 6, glucose was rapidly consumed at a higher rate in the beginning, but did not trigger an acute catabolic repression of xylose which was continuously assimilated albeit at a relatively lower rate. Ethanol concentration peaked at roughly 6 g/L within 38 h of fermentation, wherein glucose and xylose were fully depleted at approximately 25 and 35 h, respectively. The calculated ethanol yield and productivity corresponded to 0.19 g/g (glucose + xylose) and 0.16 g/L h, respectively. Similar to the enzymatic hydrolysate, after xylose depletion, ethanol started to be metabolized as a substrate from the hemicellulosic hydrolysate for cell survival. A small concentration of ethanol (~ 1 g/L) was also found to be relegated for xylitol production (data not shown). As a result, it was assumed that xylose was mainly directed towards ethanol synthesis. S. stipitis has been reported to utilize xylose-rich hydrolysates producing a maximum ethanol concentration of 4.9 g/L after 24 h [47]. The course of fermentation by S. stipitis showed great resemblance to the current study and similarly displayed low levels of xylitol production throughout the process [47]. By analyzing the results obtained with cellulosic and hemicellulosic hydrolysates, it could be inferred that glucose, to some extent, was mainly used to produce ethanol and xylose served as a carbon source for cellular maintenance. The diverse effects from different sugar-based mediums may also indicate that the wild yeast presented a versatile metabolism and could broaden the spectrum of yeast sugar utilization. When the substrate was glucose rich, ethanol production by the wild yeast achieved higher conversion (Fig. 5); however, in case of hemicellulose hydrolysates, its response was sub-optimal. Interestingly, the conditions and parameters of the fermentation processes were identical, except for the predominant sugar composition, which leads to the assumption that the cellular requirements for ethanol fermentation must be different for the wild yeast. Hence in this case, despite xylose consumption, the ethanol production was poor. Further investigations are needed to trace possible nutritional parameters influencing C5 fermentation in this wild yeast strain.
It is well known that assimilation of xylose contributes to the consumption of NAD+ and accumulation of NADH, by the action of NAD+-dependent xylitol dehydrogenase, which mediates the conversion of xylitol into D-xylulose and enters the PPP as a “shunt” [48]. This overaccumulation of reduced cofactor NADH may stimulate ethanol production, regeneration of NAD + and subsequently alleviate NAD + /NADH cofactor imbalance. By the acknowledgement of this mechanism, inexhaustible efforts have been applied on the task of constructing microbial strains capable of efficiently assimilating different sugar types simultaneously. Genetic engineering tools allowed the aggregation of exogenous genes in recombinant microorganisms, especially S. cerevisiae, to overcome this challenge [49]. The involvement of natural wild strains with the ability to metabolize pentoses plays a crucial role in this trend, by providing the genes as well as metabolomic insights. In this context, the strain exploited in this work may also be categorized as a competitive alternative for consolidating ethanol production in biorefineries due to low xylitol accumulation and minimal catabolite repression in addition to the ability to consume both C5 and C6 sugars.
The novel, wild Brazilian S. parashehatae strain has potential for further research to assess the optimal conditions for ethanol fermentation. Wild xylose-consuming yeast strains that do not require genetic modifications could be more profitable and cost-effective. The bottleneck of modern biorefineries lies in the search for efficient microorganisms that can “readily” harness lignocellulosic materials as a whole for the synthesis of a variety of bio-based products [50]. In that context, it will be beneficial to investigate the ample metabolic pathways that reside in the newly isolated yeast strain. Also, combining the current tailored fermentation processes with the emerging metabolomics technology will make high productivity of biorefineries achievable and reproducible.
Conclusions
This work demonstrated that a sequential dilute acid and alkaline pretreatment process could be employed to maximize C5 and C6 fermentable sugar recovery from switchgrass biomass. Physico-chemical characterization of pretreated solids highlighted the importance of sequential pretreatment in reducing recalcitrance and increasing enzymatic saccharification yield. A newly isolated wild yeast strain, S. parashehatae strain—UFMG-HM-60.1b, was shown to metabolize both the hemicellulosic and enzymatic hydrolysates of switchgrass and thus improved the overall ethanol yield. One of the bottlenecks of industrial-scale 2G ethanol production is the lack of efficient and readily available microorganisms capable of metabolizing both C5 and C6 fractions. Henceforth, the current investigation highlights a strain that could aid in the design of sustainable and profitable biorefineries.
Data availability
Data available on request from the authors: The data that support the findings of this study are available from the corresponding author, on request.
References
Hamelinck CN, van Hooijdonk G, Faaij APC (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 28(4):384–410. https://doi.org/10.1016/j.biombioe.2004.09.002
Narayanan L (2020) Bioethanol market by feedstock, end-use industry, fuel blend and region: global forecast to 2025. https://www.marketsandmarkets.com/Market-Reports/bioethanol-market-131222570. Accessed 21 Jan 2021
Sönnichsen N, (2021) Ethanol fuel production in top countries 2020. https://www.statista.com/statistics/281606/ethanol-production-in-selected-countries. Accessed 11 June 2021
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37(1):52–68. https://doi.org/10.1016/j.pecs.2010.01.003
Pereira SC, Maehara L, Machado CMM, Farinas CS (2015) 2G Ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol Biofuels. https://doi.org/10.1186/s13068-015-0224-0
Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100(4):1515–1523. https://doi.org/10.1016/j.biortech.2008.09.035
Taha M, Foda M, Shahsavari E, Aburto-Medina A, Adetutu E, Ball A (2016) Commercial feasibility of lignocellulose biodegradation: possibilities and challenges. Curr Opin Biotechnol 38:190–197. https://doi.org/10.1016/j.copbio.2016.02.012
Liu C-G, Xiao Y, Xia X-X, Zhao X-Q, Peng L, Srinophakun P, Bai F-W (2019) Cellulosic ethanol production: progress, challenges and strategies for solutions. Biotechnol Adv 37(3):491–504. https://doi.org/10.1016/j.biotechadv.2019.03.002
Mohammed YA (2015) Nutrient sources and harvesting frequency on quality biomass production of switchgrass (Panicum virgatum L.) for biofuel. Biomass Bioenergy 81:242–248. https://doi.org/10.1016/j.biombioe.2015.06.027
Cardona CA, Quintero JA, Paz IC (2010) Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour Technol 101(13):4754–4766. https://doi.org/10.1016/j.biortech.2009.10.097
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res. https://doi.org/10.3389/fenrg.2018.00141
Djioleu A, Carrier DJ (2016) Effects of dilute acid pretreatment parameters on sugar production during biochemical conversion of switchgrass using a full factorial design. ACS Sustain Chem Eng 4(8):4124–4130. https://doi.org/10.1021/acssuschemeng.6b00441
Djioleu A, Carrier DJ (2018) Statistical approach for the identification of cellulolytic enzyme inhibitors using switchgrass dilute acid prehydrolyzates as a model system. ACS Sustain Chem Eng 6(2):3443–3452. https://doi.org/10.1021/acssuschemeng.7b03686
Karp EM, Resch MG, Donohoe BS, Ciesielski PN, O’Brien MH, Nill JE, Mittal A, Biddy MJ, Beckham GT (2015) Alkaline pretreatment of switchgrass. ACS Sustain Chem Eng 3(7):1479–1491. https://doi.org/10.1021/acssuschemeng.5b00201
Jin G, Bierma T, Walker PM (2014) Low-heat, mild alkaline pretreatment of switchgrass for anaerobic digestion. J Environ Sci Health A Tox Hazard Subst Environ Eng 49(5):565–574. https://doi.org/10.1080/10934529.2014.859453
Biswas R, Uellendahl H, Ahring BK (2013) Conversion of C6 and C5 sugars in undetoxified wet exploded bagasse hydrolysates using Scheffersomyces (Pichia) stipitis CBS6054. AMB Express. https://doi.org/10.1186/2191-0855-3-42
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5(4):337–353. https://doi.org/10.1007/s13205-014-0246-5
Banerjee S, Mudliar S, Sen R, Giri B, Satpute D, Chakrabarti T, Pandey RA (2010) Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuel Bioprod Biorefin. https://doi.org/10.1002/bbb.188
Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF (2007) Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol 74:937–953. https://doi.org/10.1007/s00253-006-0827-2
Sanchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99(13):5270–5295. https://doi.org/10.1016/j.biortech.2007.11.013
Wackett LP (2011) Engineering microbes to produce biofuels. Curr Opin Biotechnol 22(3):388–393. https://doi.org/10.1016/j.copbio.2010.10.010
Branco RH, Amândio MS, Serafim LS, Xavier AM (2020) Ethanol production from hydrolyzed kraft pulp by mono-and co-cultures of yeasts: the challenge of C6 and C5 sugars consumption. Energies 13(3):744. https://doi.org/10.3390/en13030744
Antunes FAF, Santos JC, Chandel AK, Carrier DJ, Peres GFD, Milessi TSS, da Silva SS (2019) Repeated batches as a feasible industrial process for hemicellulosic ethanol production from sugarcane bagasse by using immobilized yeast cells. Cellulose 26:3787–3800. https://doi.org/10.1007/s10570-019-02341-z
Chandel AK, Antunes FFA, Anjos V, Bell MJV, Rodrigues LN, Singh OV, da Silva SS (2013) Ultra-structural mapping of sugarcane bagasse after oxalic acid fiber expansion (OAFEX) and ethanol production by Candida shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-6-4
Alves LA, Felipe MGA, Silva JBA, Silva SS, Prata AMR (1998) Pretreatment of sugarcane bagasse hemicellulose hydrolysate for xylitol production by Candida guilliermondii. In: Finkelstein M, Davison BH (eds) Biotechnology for fuels and chemicals. Humana Press, Totowa, NJ, Appl Biochem Biotechnol, pp 89–98
Pietrobon VC, Monteiro RTR, Pompeu GB, Borges EP, Lopes ML, Amorim HV, Cruz SH, Viégas EKD (2011) Enzymatic hydrolysis of sugarcane bagasse pretreated with acid or alkali. Braz Arch Biol Technol. https://doi.org/10.1590/S1516-89132011000200002
Akhtar MS, Saleem M, Ruby G (2001) Enzymatic saccharification of lignocellulosic materials by the xylanase of Bacillus subtilis. J Biol Sci 1(5):398–400. https://doi.org/10.3923/jbs.2001.398.400
Kosseva M, Tjutju NA, Tantra BD (2017) Enzymatic hydrolysis of cellulose in coconut coir: pretreated via sonication. Proc Int Conf BioSci Biotechnol 2:65–71. https://doi.org/10.17501/biotech.2017.2107
Ascencio JJ, Chandel AK, Philippini RR, da Silva SS (2019) Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Conv Bioref 10:813–822. https://doi.org/10.1007/s13399-019-00547-6
Chakraborty S, Dasgupta J, Farooq U, Sikder J, Drioli E, Curcio S (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Memb Sci 456:139–154. https://doi.org/10.1016/j.memsci.2014.01.016
Lu J, Li X, Zhao J, Qu Y (2012) Enzymatic saccharification and ethanol fermentation of reed pretreated with liquid hot water. J Biomed Biotechnol. https://doi.org/10.1155/2012/276278
Kristensen JB, Thygesen LG, Felby C, Jørgensen H, Elder T (2008) Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-1-5
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Samuel R, Pu Y, Raman B, Ragauskas AJ (2010) Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl Biochem Biotechnol 162:62–74. https://doi.org/10.1007/s12010-009-8749-y
Seidl PR, Goulart AK (2016) Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr Opin Green Sustain Chem 2:48–53. https://doi.org/10.1016/j.cogsc.2016.09.003
Xu J, Cheng JJ, Sharma-Shivappa RR, Burns JC (2010) Sodium hydroxide pretreatment of switchgrass for ethanol production. Energy Fuels 24(3):2113–2119. https://doi.org/10.1021/ef9014718
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38(4):449–467. https://doi.org/10.1016/j.pecs.2012.03.002
de Gonzalo G, Colpa DI, Habib MHM, Fraaije MW (2016) Bacterial enzymes involved in lignin degradation. J Biotechnol 236:110–119. https://doi.org/10.1016/j.jbiotec.2016.08.011
Chandel AK, Antunes FAF, Anjos V, Bell MJ, Rodrigues LN, Polikarpov I, da Silva SS (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels. https://doi.org/10.1186/1754-6834-7-63
Velmurugan R, Muthukumar K (2011) Utilization of sugarcane bagasse for bioethanol production: sono-assisted acid hydrolysis approach. Bioresour Technol 102(14):7119–7123. https://doi.org/10.1016/j.biortech.2011.04.045
Dien BS (2010) Mass balances and analytical methods for biomass pretreatment experiments. In: Vertès AA, Qureshi N, Blaschek HP, Yukawa H (eds) Biomass to biofuels: strategies for global industries. Wiley and Sons, United Kingdom, pp 213–231
Belinchón MM, Gancedo JM (2003) Xylose and some non-sugar carbon sources cause catabolite repression in Saccharomyces cerevisiae. Arch Clin Microbiol 180:293–297. https://doi.org/10.1007/s00203-003-0593-9
Dien BS, O’Bryan PJ, Hector RE, Iten LB, Mitchell RB, Qureshi N, Sarath G, Vogel KP, Cotta MA (2013) Conversion of switchgrass to ethanol using dilute ammonium hydroxide pretreatment: influence of ecotype and harvest maturity. Environ Technol 34:1837–1848. https://doi.org/10.1080/09593330.2013.833640
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34. https://doi.org/10.1007/s002530100624
Luo Y, Zhang T, Wu H (2014) The transport and mediation mechanisms of the common sugars in Escherichia coli. Biotechnol Adv 32(5):905–919. https://doi.org/10.1016/j.biotechadv.2014.04.009
Mesa L, Martínez Y, de Armas AC, González E (2020) Ethanol production from sugarcane straw using different configurations of fermentation and techno-economical evaluation of the best schemes. Renew Energy 156:377–388. https://doi.org/10.1016/j.renene.2020.04.091
Bruinenberg PM, de Bot PH, van Dijken JP, Scheffers WA (1984) NADH-linked aldose reductase: the key to anaerobic alcoholic fermentation of xylose by yeasts. Appl Microbiol Biotechnol 19:256–260. https://doi.org/10.1007/BF00251847
Gao M, Ploessl D, Shao Z (2019) Enhancing the co-utilization of biomass-derived mixed sugars by yeasts. Front Microbiol. https://doi.org/10.3389/fmicb.2018.03264
Parada MP, Osseweijer P, Duque JAP (2017) Sustainable biorefineries, an analysis of practices for incorporating sustainability in biorefinery design. Ind Crop Prod 106:105–123. https://doi.org/10.1016/j.indcrop.2016.08.052
Acknowledgements
The authors acknowledge the Department of Biological and Agricultural Engineering, University of Arkansas, Fayetteville, AR, USA, for their infrastructure support. YCSM is also grateful to FAPESP for providing financial assistance for the scientific initiation scholarship program (Process No. 2019/19695-5).
Funding
This research was funded by (i) the National Science Foundation award (#0822275), United States Department of Energy award (#GO88036), and the Plant Powered Production (P3) Center. P3 is funded through the RII: Arkansas ASSET Initiatives (AR EPSCoR) I (EPS-0701890) 323 and II (EPS-1003970) by the National Science Foundation and the Arkansas Science and Technology Center; (ii) the Brazilian Federal Agency for the Support and Evaluation of Graduate Education award (#154193/2018–6), Research Council for the State of São Paulo (FAPESP) award (#2014/27055–2; 2016/10636–8). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
D.J.C., K.R., and A.D. provided the biomass, conducted the dilute acid pretreatment, and isolated the switchgrass cellulignin and hemicellulosic hydrolysate fractions. F.A.F.A., T.M.R., and L.P.B. performed alkaline pretreatment, enzymatic hydrolysis, fermentation, and biomass characterization. Y.C.S.M provided support for biomass characterization and in manuscript configuration. C.A.R. provided the feasible wild yeast. J.C.S., D.J.C., and S.S.S. supervised all work. All authors contributed to the data analysis and manuscript writing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable for this study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Antunes, F.A.F., Rajan, K., Djioleu, A. et al. Sustainable Second-Generation Ethanol Production from Switchgrass Biomass via Co-fermentation of Pentoses and Hexoses Using Novel Wild Yeasts. Bioenerg. Res. 15, 1157–1168 (2022). https://doi.org/10.1007/s12155-021-10302-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10302-3