Abstract
Spirulina platensis, when grown under stress, may alter its metabolic pathways, leading to carbohydrate accumulation. Research on the combined effects of stress factors such as UV radiation, photoperiod, and light, and the influence of micronutrients (Ca, Fe, and Mg) on microalgae carbohydrate composition is scarce. The aim of the present study was to evaluate the effects of combined cell stress factors on the microalgal growth and biochemical composition of S. platensis, to apply the biomass in the context of biorefineries. Assays were performed at two cultivation stages: in the first stage, Zarrouk medium (50%) was used until the end of the exponential growth phase (18 days) and, in the second stage, the cells obtained in the first stage were centrifuged and recultivated in 20% Zarrouk’s medium under physical (UV, photoperiod/light, NaCl) and nutritional stress (limitation of Ca, Fe, and Mg concentrations in 20% Zarrouk). The highest carbohydrate yields (27.84 mg L−1 day−1) were obtained in the photoperiod/light intensity-stressed cultures in the medium with nutrient limitation. The study outcomes showed the methods to increase carbohydrate synthesis in cultures under optimized conditions. Our results showed that nutrient limitation by 20% Zarrouk combined with higher light intensity (67.5 μmol photons m−2 s−1) and photoperiod (18-h/6-h light/dark cycles) is an efficient strategy to achieve higher intracellular concentrations (59.71%) and carbohydrate yields (55.85 mg L−1 day−1) in a discontinuous culture method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustainability is a fundamental principle in natural resource management, environmental impact minimization, and socioeconomic growth [1]. For this reason, there is a need for independence from fossil fuels, given the problems generated by the increasing global demand for energy coupled with the fact that there is a worldwide trend of non-utilization of fossil resources due to their impacts on greenhouse gas emissions [2,3,4,5].
Microalgae have been evaluated as an alternative feedstock for biofuel production due to their high growth rates, high CO2 fixation abilities, renewability, and low environmental impact, in addition to their ability to accumulate biocomposites of interest such as carbohydrates, which can be converted into bioethanol through saccharification processes [6,7,8]. As it is one of the most produced biofuels in countries such as the USA and Brazil, the production of bioethanol causes competition for land and food. Therefore, the use of microalgae can assist in meeting the demand for biofuel production, thereby maintaining the global demand for biofuels without requiring large amounts of land and food resources [9]. Among the species of microalgae studied, Spirulina platensis has been highlighted as a potential candidate for bioethanol production, mainly due to its high growth rate and commercial importance [10,11,12,13].
Microalgae cultures for biofuel production require favorable growth conditions. However, these conditions are not always compatible with the appropriate conditions for carbohydrate synthesis for biofuel production, as these compounds tend to accumulate under conditions of cellular stress [14] such as nutrient limitations [15, 16], exposure to high salinity concentrations [17], ultraviolet radiation [18, 19], light intensity [20, 21], and photoperiod variation [22]. While beneficial for carbohydrate production, these conditions have the disadvantage of limiting biomass productivity [23, 24]. In this context, two-stage cultivation processes can be used to maximize biomass production under favorable conditions in the first stage and to obtain maximum carbohydrate yield by applying stress conditions in the second stage, enabling the production of bioethanol [25, 26]. As the use of this technique can require greater energy usage due to the additional centrifugation step, it is necessary to carefully evaluate energy balances and environmental and economic impacts, taking industrial-scale implementation into account [27]. Thus, choosing suitable reactors in the first and second stages can contribute significantly to the reduction of production costs [28].
Changing the cultivation conditions is a commonly employed strategy for increasing carbohydrate synthesis in microalgae. However, responses regarding the use of micronutrients such as Ca, Fe, and Mg for carbohydrate stimulation have not been extensively reported [29,30,31,32,33,34], nor have those regarding the use of mutagenesis by radiation UVC, which has a significant advantage over genetic engineering and can be used to improve strains without the need for biochemical information [19, 35]. In addition, most studies involving the application of these stresses have been demonstrated for species such as Chlorella and Scenedesmus, with a shortage of research related to Spirulina. In this context, the aim of the present work was to evaluate the effects of combined physical and nutritional stresses on the synthesis of compounds of interest in Spirulina platensis from two-stage cultivations.
Materials and Methods
Microorganism and Cultivation Conditions
The microalga Spirulina platensis was used in this study. The inoculum was kept in a greenhouse, with a luminosity of 44.55 μmol photons m−2 s−1 obtained by fluorescent lamps, agitation from constant air injection obtained by diaphragm pumps, a 12-h/12-h photoperiod, and a temperature of 30 °C [11, 36]. The assays were performed in Zarrouk’s medium [37] under sterile conditions, with a modified concentration of nutrients (50% concentrations of all nutrients), with the following components: NaHCO3 (8.4 g L−1); K2HPO4 (0.25 g L−1); NaNO3 (1.25 g L−1); K2SO4 (0.5 g L−1); MgSO4.7H2O (0.10 g L−1); CaCl2 (0.02 g L−1); FeSO4.7H2O (0.005 g L−1); EDTA (0.04 g L−1). Two stages of cultivation were performed:
-
a)
In the first stage, the microalgae were cultivated in 50% Zarrouk’s medium under the same inoculum maintenance conditions, with an initial inoculum concentration of 0.20 g L−1, in 1000-mL Erlenmeyer flasks with useable volumes of 800 mL;
-
b)
In the second stage, the cells were subjected to centrifugation and inserted into the culture medium again, this time with the introduction of physical or nutritional stresses. Therefore, a volume of 800 mL of medium containing Spirulina at the beginning of the stationary growth phase (18 days) was centrifuged at 3500 rpm for 10 min, except for the condition of stress by ultraviolet radiation, in which the application of stress occurred before the process of cell separation. After centrifugation, the biomass was reinoculated in 800 mL of 20% Zarrouk’s medium, which, due to the lower concentration of nutrients, may induce the intracellular synthesis of carbohydrates [11].
The definitions of the stresses used are listed in Table 1. Each stress was applied separately, at different levels or concentrations. Two control tests were performed to verify behavior under normal cultivation conditions. The first stage of both control cultures was performed in 50% Zarrouk’s medium over 18 days. Afterwards, the cells were collected by centrifugation and reinoculated in a medium containing 50% Zarrouk’s medium (control 1) or 20% Zarrouk’s medium (control 2).
For the stresses involving ultraviolet radiation, the cells were cultured in 50% Zarrouk’s medium until the end of the growth phase and subsequently added to Petri dishes (5 mL in 10-cm diameter plates) and exposed to ultraviolet radiation in a laminar flow chamber with a 30-W lamp (λ = 254 nm), at a distance of 53 cm from the surface, for the time outlined in Table 1. The medium exposed to UV radiation was kept in dark for 24 h to avoid photoreactivation [18]. Subsequently, the cells were centrifuged at 3500 rpm and inoculated into a new culture medium with limited nutrients (20% Zarrouk’s medium) for 15 days.
Micronutrient stresses (Fe, Mg, and Ca) were introduced by changes in FeSO4·7H2O, MgSO4·7H2O, and CaCl2 concentrations in the Zarrouk medium. To verify the influence of photoperiod and light on S. platensis, three experiments were performed in duplicate. S. platensis was inoculated in 50% Zarrouk’s medium until the beginning of the stationary phase of growth at 30 °C, 12-h light/dark photoperiod, luminosity 44.55 μmol photons m−2 s−1 through fluorescent lamps, agitation by constant air injection, and an initial inoculum concentration of 0.20 g L−1. After 18 days of culture in 50% Zarrouk’s medium, the cells were collected by centrifugation at 3500 rpm and reinoculated in 20% Zarrouk’s medium, with the light/dark period changed to three different levels (18 h/06 h, 06 h/18 h, and 12 h/12 h) and the light intensity changed to 67.5 μmol photons m−2 s−1 provided by LED lamps. The temperature was maintained at 30 °C.
All tests were performed in duplicate. At the end of the cultures, the cells were collected by centrifugation at 3500 rpm for 10 min and dried in an oven at a temperature of 50 °C to determine the carbohydrate and protein contents of the biomass.
Optimization of Conditions for Accumulation of Carbohydrates
To optimize the production of carbohydrates, cultures were performed in the absence of single-stage centrifugation. The cultures were performed in 1000-mL Erlenmeyer flasks with 800 mL of useable volume in an incubator provided with LED lamps with an intensity of 67.5 μmol photons m−2 s−1, temperature of 30 °C, photoperiod of 18 h/06 h (light/dark), and nutrient limitation (20% Zarrouk’s medium). The assays were performed in duplicate until the stationary growth phase was identified.
Analytical Determinations
The microalgal biomass concentration was determined every 48 h by measuring the optical density in a spectrophotometer at 670 nm, and the biomass concentrations were calculated using standard biomass curves.
The parameters evaluated included the productivity at the end of the first and second stages, and the final productivity, including the two stages (Pfinal, g L−1 day−1) obtained through Eq. (1). The maximum specific speed of growth (μmax, day−1) (Eq. (2)) and the generation time (tg, day) were also calculated (Eq. (3)).
where X is the biomass concentration (g L−1) at time t (day) and X0 is the biomass concentration (g L−1) at time t0 (day).
where X1 is the initial biomass concentration (g L−1), X2 is the biomass concentration at the end of the cultivation, and Δt is the time (day).
For the quantification of proteins and carbohydrates, cell rupture was performed in the autoclave. For this, 5 mg of biomass was added to 10 mL of distilled water and autoclaved for 20 min at 121 °C. The content of carbohydrates was determined by the phenol-sulfuric method [38]. The carbohydrate production levels in the cultivations (mg L−1 day−1) were obtained through Eq. (4) [39].
where Xf is the final biomass concentration in each culture, CHO is the carbohydrate concentration in biomass, and t is the cultivation time.
The protein content was determined as proposed by Lowry et al. [40]. The levels of carbohydrates and proteins were expressed on a dry basis. Moisture was determined by the official methods of AOAC [41].
Data Analysis
The differences between the means of the cultures were evaluated by the analysis of variance at a 95% confidence level, with subsequent comparison by the Tukey test.
Results and Discussions
Effects of Cultivation Conditions on Growth, Production, and Composition of Carbohydrates and Proteins
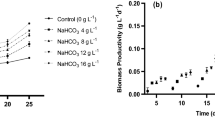
Figure 1 shows the growth curves of microalgae during the first and second stages of cultivation, corresponding to tests with physical and nutritional stresses. The maximum specific growth rate (μmax), generation time (tg), log phase interval, and final productivity (Pfinal) of the biomass of each stage are presented in Table 2.
Effects of stress conditions on S. platensis cell growth. Controls (a); stresses by NaCl (200, 300, and 400 mM) (b); limitation of calcium (0.006, 0.004, and 0.002 g L−1) (c); limitation of iron (0.0015, 0.001, and 0.0005 g L−1) (d); limitation of magnesium (0.03, 0.02, and 0.01 g L−1) (e); ultraviolet radiation (2, 4, and 6 min) (f)
The mean value of biomass concentration in the first cultivation stage, considering all tests, was 1.69 ± 0.19 g L−1. The centrifugation process performed during the transition from the first to the second stage resulted in a biomass loss of approximately 0.51 ± 0.26 g L−1. The cultures did not experience adaptation phases at the beginning of the first stage (Fig. 1 since the microalgae were previously adapted to the 50% Zarrouk’s medium, which is commonly used in the maintenance of inoculum. This situation occurs when there are not many differences between the inoculum and the culture media [42].
The mean time of logarithmic growth of the biomass cultivated in the first stage was 14 ± 1.28 days (generation time of 5 ± 0.4 days). After the centrifugation process and insertion of the cells into 20% Zarrouk’s medium, the generation times were longer than those of the first stage (15 ± 5.5 days).
Three factors may explain the generation time increase in the transition between stages I and II. The first factor is the insertion of stress conditions in the second stage. The second factor is that at the beginning of the second stage, the initial cell concentrations were higher than those of the first stage, causing the shading effect, which has already been reported in the literature to result in specific growth rate reductions. The phenomenon is known as photo-limitation and occurs due to the shading of cells inside the medium by the surface cells [43]. The third factor is the centrifugation process used for cell harvesting, which consists of the collision, aggregation, and sedimentation of biomass by a centripetal force, exposing the cells to high gravitational and shear forces and potentially damaging their structure [44].
In control test 2 (50% Zarrouk’s medium in the first stage and 20% Zarrouk’s medium in the second stage, Fig. 1a), it was verified that the reduction in the concentration of the culture medium did not reduce the final biomass production or the kinetic parameters of S. platensis. The μmax of control culture 1 was 0.038 ± 0.01 day−1 with a tg of 19 days, compared with a μmax of 0.040 ± 0.01 day−1 and a tg of 18 days in control 2, in 20% Zarrouk’s medium (p > 0.05).
Assays performed with the addition of NaCl stress in nutrient-limited media (20% Zarrouk’s medium) (Fig. 1b) showed the μmax and tg to be statistically equal (p > 0.05) to those of control assays in the second stage of cultivation. Salinity stress did not affect biomass yield compared with controls, indicating that higher NaCl concentrations can be added to the medium to induce higher stresses in microalgae. In a similar study, Álvarez-Díaz et al. [31] performed two-stage mode cultivation, showing a maximum growth of 1.03 g L−1 for S. obliquus microalgae in the first stage. The second stage, in which salinity stress (256 mM) was applied, did not present microalgal growth.
In the study proposed by Álvarez-Díaz et al. [45], centrifugation processes were not performed, and no loss of biomass occurred before the addition of stress. However, even if there is a small reduction in cell productivity due to NaCl stress and centrifugation, it is shown in the literature that higher carbohydrate concentrations result from cell stress, which triggers the production of reactive oxygen species such as O2, H2O2, O2-, and OH-. These reactive oxygen molecules are highly toxic and are responsible for causing damage to proteins, lipids, DNA, and other macromolecules, and also inhibit cell growth [46] and trigger carbohydrate and lipid synthesis in microalgae.
For the tests performed with the limitation of calcium as a stressor (Fig. 1c), the same behavior as in the tests performed with NaCl and the control was observed during the transition from stages I to II, but with an increase of biomass at the end of the process. Ca-limited cultures had significantly higher Pfinal values (p < 0.05) than those of the control cultures in 50% and 20% Zarrouk’s medium. Studies such as those developed by Esakkimuthu et al. [47] and Hanifzadeh et al. [34] demonstrated that optimizing the limitation of micronutrient concentrations, such as those of Ca and Mg, triggers the synthesis of compounds of interest without harming microalgal cell growth.
Iron is an essential element for the survival of all living organisms, including photosynthesizing organisms that require iron as a cofactor in multiple elements of their electron transport systems. Iron limitations can cause photoautotrophic cells to lose their photosynthetic capacity, thereby affecting microalgae growth [21, 48]. In this study, S. platensis cells were not affected by the limitation of the Fe concentration in Zarrouk’s medium (Fig. 1d), showing no differences between Pfinals and controls.
Mg (Fig. 1e), as well as Fe and Ca, when added in low concentrations, caused an increase in biomass concentration at the end of culture, resulting in increased biomass yields. Although magnesium is an essential element for microalgal biomass production as it participates in vital processes such as ATP reactions for carbon fixation and is a constituent part of the chlorophyll photosynthetic and particulate apparatus [49], its scarcity did not influence S. platensis kinetics negatively.
Strategies such as nitrogen and phosphorus limitation in microalgal cultures are frequently used to stimulate carbohydrate and lipid synthesis [16, 50]. However, research on the limitation of micronutrients such as Ca and Mg is scarce. Previous studies have addressed the influence of micronutrients on the lipid composition of biomass for subsequent biodiesel production, as in the study proposed by Gorain et al. (2013), where the effects of calcium, magnesium, and salinity concentrations on the growth of the microalgae Chlorella vulgaris and Scenedesmus obliquus were demonstrated. The highest cellular concentrations occurred for the highest magnesium concentrations. In both Ca and Mg depletion, microalgal growth was affected.
Cultures exposed to different ultraviolet radiation durations (Fig. 1f) showed growth within 2 min of exposure to UV light, with a μmax of 0.033 day−1 and a tg of 21 days. The lack of S. platensis growth due to UV radiation exposure can be explained by DNA damage, which impairs microalgal photosynthesis and growth [51], and also by photoinhibition (inhibition of photosystem II activity). In this mechanism, the high luminosity induces the production of reactive oxygen species that directly inactivate the chemical reaction center PSII, thereby inhibiting protein synthesis [52] and reducing photosynthetic activity.
When microalgae are cultivated under stressful conditions, they react and modify their and metabolic pathways to deal with the stress conditions. This dynamic change in metabolic strategy affects the composition of biomass [8].
Carbohydrate and protein quantifications were performed at the end of the second cultivation stage. The carbohydrate and protein contents and their respective production levels are presented in Figs. 2 and 3, respectively.
Intracellular carbohydrate and protein concentrations (% m/m) in Spirulina platensis biomass at the end of the second stage of cultivation in the control experiments and with nutritional and physical stresses. Mean values ± standard deviations. Equal letters in equal color bars indicate statistical equality at a 95% confidence level (p > 0.05). Capital letters refer to carbohydrate production levels and lowercase letters refer to protein production levels. Control (a); NaCl (b); calcium limitation (c); iron limitation (d); magnesium limitation (e); ultraviolet radiation (f). Carbohydrates (black square); proteins (grey square)
Carbohydrate and protein yields (mg L−1 day−1) in S. platensis biomass at the end of the second stage of cultivation in the control experiments and with nutritional and environmental stresses. Mean values ± standard deviations. Equal letters in equal color bars indicate statistical equality at a 95% confidence level (p > 0.05). Capital letters refer to carbohydrate production levels and lowercase letters refer to protein production levels. a control; b NaCl; c calcium; d iron; e magnesium; f ultraviolet radiation. Carbohydrates (black square); proteins (grey square)
Control 1 and 2 (Fig. 2a) showed no statistical difference (p > 0.05) between carbohydrate contents (7.97 ± 0.39% to 9.93 ± 0.03%). Although nutrient limitation is widely reported to promote increased carbohydrate levels in microalgal cells, in the present study, the light intensity was more efficient at stimulating the growth of microalgae and carbohydrate production.
The highest carbohydrate concentrations for this stage were observed in the experiments with NaCl stress (Fig. 2b) at 200 mM (20.39 ± 0.21%), 300 mM (21.62 ± 0.16%), and 400 mM (23.36 ± 1.10%). There was no significant difference (p > 0.05) in carbohydrate content among these groups.
The combination of nutrient restriction and salinity, as performed in this study, causes metabolic changes in microalgae, resulting in the conversion of protein content to energy storage compounds such as carbohydrates [53]. Using S. platensis LEB 52, Margarites et al. [39] identified an increase in carbohydrate content using 50% fewer nutrients and 20% more NaCl (10 mM) in Zarrouk medium. A 400 mM NaCl concentration in the culture medium increased the lipid and carbohydrate contents in the microalgae Scenedesmus sp. CCMN 1077, according to Pancha et al. [54], and A. dimorphus accumulated 60% more carbohydrates (53.30 ± 2.76%) than the control cultivation when 200 mM NaCl was used in the culture medium [17].
Salinity alters the physiological and biochemical compositions of microalgae and is responsible for increasing lipid and carbohydrate levels in some species of microalgae [54]. Parallel to this, the limitation of nitrogen sources can also be responsible for increasing carbohydrate and lipid contents in S. platensis, as demonstrated by Salla et al. [11].
Among the main metallic nutrients, calcium was chosen for study, mainly due to its high solubility, low toxicity in high concentrations, and beneficial effects in many aspects of plant physiology. Detailed metabolic responses to Ca nutrition and metallic stress, as well as the direct impact of Ca nutrition and its toxicity, have not been studied in detail. Studies have indicated the effects of calcium supplementation, proposing that this element may affect the accumulation of metals and metabolic parameters in algae [55]. Responses regarding its use for carbohydrate stimulation have not been sufficiently reported, demonstrating the need for further study of the effects of calcium on microalgal metabolism and biosynthesis. In our study, Ca micronutrient limitation at the lowest proposed condition (0.002 g L−1) was responsible for increasing carbohydrate production (8.48 ± 1.72 mg L−1 day−1, Fig. 3c).
Following iron limitation, no significant effect was observed on carbohydrate and protein levels (Fig. 2d), either on the productivity (Fig. 3d) or in comparison with the results of the control experiments. In a similar study developed by Sun et al. [21] that was performed with different iron concentrations (0.056 g L−1), there were also no significant differences found in carbohydrate production between Neochloris oleoabundans HK-129 cultures with varying Fe3+ concentrations when cultivated under nitrogen limiting conditions and in two stages.
The three levels of magnesium limitation proposed in this study had similar effects on carbohydrate levels and yields. The carbohydrate yield obtained from the culture condition with the lowest Mg concentration was one of the highest in this study (9.75 mg L−1 day−1), but it was lower than that from conditions with NaCl (10.27 mg L−1 day−1) and UV radiation for 6 min (9.80 mg L−1 day−1).
Regarding the other micronutrients used as limiting sources in this study, Mg has the highest concentration in the Zarrouk medium, followed by Ca and Fe (Mg > Ca > Fe). Therefore, it can be suggested that nutrients and micronutrients that have higher concentrations in the culture medium, when limited, result in more significant stress to the microalgae, as has been observed in other studies, especially regarding nitrogen and phosphorus.
Most studies involving the use of UV radiation for the synthesis of compounds of interest have focused on the use of UVB; however, it is believed that higher light intensities such as UVC, which was used in this study, yield better results in microalgae. Thus, mutagenesis via UVC can be combined with other stresses, such as the limitation of nutrients, to stimulate the synthesis of biocomposites, as demonstrated by Sharma et al. [56].
The exposure time to ultraviolet radiation was fundamental for carbohydrate accumulation in S. platensis. It was found that in the longest exposure time (6 min), the highest carbohydrate contents were obtained (Fig. 2f), at 19.83 ± 1.18%, as was one of the highest carbohydrate production levels, at 9.80 ± 0.58 mg L−1 day−1 (Fig. 3f). The severity of cell DNA damage depends on the exposure time to UV rays, while the DNA self-repair mechanism is interrupted when kept in the dark for 24 h [18].
The effects of light and photoperiods are shown in Fig. 4 and Table 3. The highest growth rates were observed in the 18-h/6-h light/dark photoperiod during the second growth stage. This is due to higher microalgae photosynthesis activity, which leads to increased cell growth. The μmax of the second stage was higher than that of the first stage (0.159 ± 0.01 day−1), and the generation time was 4 days. The production level in the second stage was 0.13 ± 0.02 g L−1 day−1. Thus, increased light photoperiods resulted in faster growth rates and higher cell densities. The effect of higher light intensity on the biochemical composition of algal photosynthesis is controlled by processes known as photoadaptation, in which dynamic changes in metabolic pathways facilitate increased cell growth [57].
Light intensity and photoperiod play important roles in the growth and biochemical composition of microalgae. Under high light intensities (100 μmol m−2 s−1) and photoperiods (18-h/6-h light/dark), the cell concentration (6.5 × 107 cells mL−1) and lipid content (31.3%) of Nannochloropsis sp. increased [58]. High light intensities (150 μmol m−2 s−1) were also responsible for high growth rates and biomass and carbohydrate yields in Ankistrodesmus falcatus. Due to the period of exposure to light, it was observed that the increase in the photoperiod from the 12 h/12 h regimes resulted in a decrease in the growth rate [59].
Even in the experiments with the shortest period of light exposure, 6 h/18 h, a high μmax (0.073 ± 0.003 day−1) was obtained when compared with the tests performed in the first experimental phase (μmax 0.040 ± 0.011 day−1 for the control 2). This fact evidences the influence of higher light intensity on the cultivation of S. platensis.
Many studies show the effects of light intensity on the synthesis of compounds of interest, with mixed results. By investigating the effects of light intensity (21, 42, and 63 μmol photons m−2 s−1) and photoperiod (8-h/16-h, 12-h/12-h, and 16-h/8-h light/dark) on biomass production and the biochemical composition of the N. calcicola microalgae, Khajepour et al. [22] found that microalgae prefer low light. However, most light intensity and photoperiod levels showed no significant impact on biomass production in this study. An increase in light intensity correlated with a reduction in protein content and an increase in carbohydrate content. The same was observed when the light duration (photoperiod) increased.
Intracellular carbohydrate and protein concentrations, as well as the yields of these compounds during cultivation, are shown in Fig. 5. Results show higher carbohydrate concentrations (31 ± 0.20%) and carbohydrate yields (27.84 ± 0.18 mg L−1 day−1) for the photoperiod condition with 18-h/6-h light/dark, and this was the largest change observed of all experiments performed in this study. These concentrations showed statistical differences when compared with the cultures subjected to the other photoperiod variations.
Intracellular carbohydrate and protein concentrations (% m/m) in Spirulina platensis biomass at the end of the second stage, and respective carbohydrate and protein yields (mg L−1 day−1) of cultures subjected to higher light intensities and photoperiod intervals. Carbohydrate production level (black square); protein production level (grey square)
Regarding other studies using S. platensis, high carbohydrate yields were found using organic sources in the culture medium, as in the study proposed by Salla et al. [11], who reported a carbohydrate production level of 60 mg L−1 day−1 using whey ultrafiltration and nanofiltration residues. High yields were also found with the use of nitrogen source limitation (8.4 g NaHCO3) or 0.3 vvm CO2 (5 min) in the study proposed by Braga et al. [60], which resulted in carbohydrate concentrations of 59.1% p p−1 and 49.3% p p−1, respectively.
Increasing the production of carbohydrates at higher light intensities may be a method by which S. platensis controls carbon pools and energy storage to adjust its growth under varying environmental conditions, such as the nutrient limitation used in the second stage of cultivation. Markou et al. [16] studied light intensity (24, 42, and 60 μE m−2 s−1) and demonstrated no effect on S. platensis carbohydrate content, except when other stressors were applied in combination, such as phosphorus limitation. These results may indicate that combined stressors must be utilized to accumulate higher carbohydrate levels in cells.
In contrast to the conditions that facilitated carbohydrate accumulation, the highest protein concentration was obtained with 6-h/18-h light/dark conditions. However, the highest yields were obtained in experiments with longer periods of light exposure (18 h/6 h and 12 h/12 h), due to the effect of light exposure on cell concentration. In general, protein content decreases, whereas intracellular carbohydrate concentrations increase in response to an increase in light intensity.
Optimization of Cultivation Parameters
In the application phase of cell stress, biomass losses were verified during the transition from the first to the second stage of cultivation. In relation to this, multi-stage cultivation can consume more energy compared with single-stage systems, especially when harvesting is essential to perform the subsequent stage(s) [61]. Considering that the energy consumption of the centrifugation process significantly impacts the total production costs and that this process may also cause a decline in cellular and carbohydrate production levels, the following tests were performed in one stage only.
It was also verified by control assays in 50% and 20% diluted Zarrouk medium that nutrient limitation at 20% did not affect cell productivity; this restriction can be introduced during the first stage of cultivation. Microalgae such as S. platensis can adjust their nutrient absorption according to availability. Therefore, they can store large amounts of a nutrient and grow under limiting conditions [49].
Regarding carbohydrate concentrations, the use of higher light intensities and longer periods of light exposure positively influenced the kinetic parameters and carbohydrate yields. Cultures subjected to the longest period of light exposure (photoperiod 18-h/6-h light/dark) exhibited the maximum cell growth, due to more time available to microalgae for photosynthesis, which also led to the highest concentrations (31%) and carbohydrate yields (27.84 mg L−1 day−1).
With the evaluated parameters taken into consideration, subsequent assays were performed in Zarrouk 20% medium with a light intensity of 67.5 μmol m−2 s−1 and a photoperiod of 18-h/6-h light/dark in a BOD incubator.
S. platensis obtained a high growth rate, approximately 2 g L−1, with a final yield of 0.079 ± 0.002 g L−1 day−1, a μmax of 0.114 ± 0.009 day−1, and a generation time of 6.10 ± 0.50 day after 24 days of cultivation (Fig. 6). Compared with the results of the control trials of the first stage of this study, productivity increased by approximately 0.030 g L−1 day−1, which was accompanied by reductions in generation time and cultivation time. The carbohydrate and protein determinations were made after 18 days (end of the log phase, Fig. 7) and 24 days (end of stationary phase) of culture.
Intracellular carbohydrate and protein concentrations (% m/m) in Spirulina platensis biomass during the stationary phase of growth and their carbohydrate and protein yields (mg L−1 day−1). Carbohydrates (black square), proteins (gray square); carbohydrate and protein concentrations (a); carbohydrate and protein production levels (b). Mean values ± standard deviations. Equal letters in equal color bars indicate statistical equality at a 95% confidence level (p > 0.05). Capital letters refer to carbohydrate production levels and lowercase letters refer to protein production levels
The highest carbohydrate concentrations were obtained at the beginning of the stationary growth phase, reaching ~ 60%. The carbohydrate content decreased throughout this growth phase, as did the carbohydrate yields. A study developed by Magro et al. [10] showed higher carbohydrate concentrations in the declining phase of S. platensis (72%).
The carbohydrate yield obtained in the optimized cultivation was 55.85 mg L−1 day−1. Regarding the stress selection step, an approximately 6-fold increase in carbohydrate levels was observed in relation to the control cultivation in modified 20% Zarrouk, and a 10.4-fold increase was observed in carbohydrate yields for this same culture.
Protein concentrations remained the same throughout the stationary phase of cultivation. Regarding the first stage of this study, there was a reduction in protein content. Other studies have demonstrated the effects of increased light intensity, which gives rise to an increase in carbohydrate concentration and a reduction in protein content, as described by Markou et al. [62].
The application of multiple stressors has been described in the literature. Chentir et al. [63] studied an isolated strain of S. platensis, demonstrating the metabolic flexibility of microalgae when exposed to different light intensity ranges, NaCl levels, and nitrogen and phosphorus concentrations. The combined culture conditions significantly modified the composition of S. platensis. Under high light intensity (120 μmol photons m−2 s−1) and high concentrations of NaCl (40 g L−1), NaNO3 (0.5 g L−1), and K2HPO4 (0.5 g L−1), the carbohydrate content was maximized to 31.62%. The study has shown that the use of multiple stressors can be a promising strategy for producing enriched biomass from various compounds with high added values.
The reduction of production costs is still a parameter that remains to be achieved in microalgal cultures, along with improvements in productivity. As the Zarrouk medium is one of the most widely accepted and traditionally used methods for cultivating Spirulina [13], cost reduction can be achieved through a combination of limited nutrients and greater light intensities and photoperiods. Cultivating microalgae under stress-optimized conditions can reduce the cost of cultivation and maximize the accumulation of biocompounds of interest, thus offering a sustainable strategy to improve biofuel production [14]. In addition, another strategy to be considered in future research is the use of wastewater, which, aside from being used for the accumulation of biocomposites such as carbohydrates, lipids, and pigments, can perform treatment of effluents rich in organic compounds and nutrients [64,64,65,67].
Conclusions
Nutrient limitation from 50 to 20% of Zarrouk’s medium did not affect cell growth and could be used as a tool to stimulate carbohydrate accumulation pathways. NaCl stress and exposure to UV radiation under nutrient-limited conditions improved carbohydrate yields in S. platensis, but nutrient limitation (Zarrouk 20%) combined with higher light intensity (67.5 μmol m−2 s−1) and photoperiod (18-h/6-h light/dark cycle) was a more effective strategy for this purpose. The combination of these stresses resulted in a 6-fold increase in carbohydrate content and a 10.4-fold increase in carbohydrate yields when compared with the 20% Zarrouk control cultivation in the first stage of the study. From the combination of stresses, higher carbohydrate yields were verified at the end time of the first stage (~ 60% of carbohydrates in the biomass with a production level of 55 mg L−1 day−1), and it was not necessary to prolong the cultivation until the end of the stationary phase of growth.
References
Brennan L, Owende P (2010) Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577. https://doi.org/10.1016/j.rser.2009.10.009
Deprá MC, dos Santos AM, Severo IA, Santos AB, Zepka LQ, Jacob-Lopes E (2018) Microalgal biorefineries for bioenergy production: can we move from concept to industrial reality? Bioenergy Res 11:727–747
Fan J, Lvhong Z (2017) Acclimation to NaCl and light stress of heterotrophic Chlamydomonas reinhardtii for lipid accumulation. J Biosci Bioeng 124:302–308. https://doi.org/10.1016/j.jbiosc.2017.04.009
Shuba ES, Kifle D (2018) Microalgae to biofuels: ‘promising’ alternative and renewable energy, review. Renew Sust Energ Rev 81:743–755. https://doi.org/10.1016/j.rser.2017.08.042
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197. https://doi.org/10.1016/j.rser.2015.12.150
Rempel A, Machado T, Treichel H, Colla E, Margarites AC, Colla LM (2018) Saccharification of Spirulina platensis biomass using free and immobilized amylolytic enzymes. Bioresour Technol 263:163–171. https://doi.org/10.1016/j.biortech.2018.04.114
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:1–21
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Silva CEF, Sforza E (2016) Carbohydrate productivity in continuous reactor under nitrogen limitation: effect of light and residence time on nutrient uptake in Chlorella vulgaris. Process Biochem 51:2112–2118. https://doi.org/10.1016/j.procbio.2016.09.015
Magro FG, Margarites AC, Reinehr CO, Gonçalves GC, Rodigheri G, Costa JAV, Colla LM (2018) Spirulina platensis biomass composition is influenced by the light availability and harvest phase in raceway ponds. Environ Technol 39:1868–1877. https://doi.org/10.1080/09593330.2017.1340352
Salla ACV, Margarites AC, Seibel FI, Holz LC, Brião VB, Bertolin TE, Colla LM, Costa JAV (2016) Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour Technol 209:133–141. https://doi.org/10.1016/j.biortech.2016.02.069
Li X, Li W, Zhai J, Wei H (2018) Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour Technol 263:555–561. https://doi.org/10.1016/j.biortech.2018.05.046
Costa JAV, Freitas BCB, Rosa GM, Moraes L, Morais MG, Mitchell BG (2019) Operational and economic aspects of Spirulina-based biorefinery. Bioresour Technol 292:121946. https://doi.org/10.1016/j.biortech.2019.121946
Cheng D, He Q (2014) Assessment of environmental stresses for enhanced microalgal biofuel production - an overview. Front Energy Res 2:26. https://doi.org/10.3389/fenrg.2014.00026
Dragone G, Fernandes BD, Abreu AP, Vicente AA, Teixeira JA (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335. https://doi.org/10.1016/j.apenergy.2011.03.012
Markou G, Chatzipavlidis I, Georgakakis D (2012) Effects of phosphorus concentration and light intensity on the biomass composition of Arthrospira (Spirulina) platensis. World J Microbiol Biotechnol 28:2661–2670. https://doi.org/10.1007/s11274-012-1076-4
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour Technol 244:1376–1383. https://doi.org/10.1016/j.biortech.2017.05.003
Liu S, Zhao Y, Liu L, Ao X, Ma L, Wu M, Ma F (2015) Improving cell growth and lipid accumulation in green microalgae Chlorella sp. via UV irradiation. Appl Biochem Biotechnol 175:3507–3518. https://doi.org/10.1007/s12010-015-1521-6
Sharma KK, Li Y, Schenk PM (2015) Rapid lipid induction in Chlorella sp. by UV-C radiation. Bioenergy Res 8:1824–1830. https://doi.org/10.1007/s12155-015-9633-y
Mandotra SK, Kumar P, Suseela MR, Nayaka S, Ramteke PW (2016) Evaluation of fatty acid profile and biodiesel properties of microalga Scenedesmus abundans under the influence of phosphorus, pH and light intensities. Bioresour Technol 201:222–229. https://doi.org/10.1016/j.biortech.2015.11.042
Sun X, Cao Y, Xu H, Liu Y, Sun J, Qiao D, Cao Y (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour Technol 155:204–212. https://doi.org/10.1016/j.biortech.2013.12.109
Khajepour F, Hosseini SA, Ghorbani Nasrabadi R, Markou G (2015) Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl Biochem Biotechnol 176:2279–2289. https://doi.org/10.1007/s12010-015-1717-9
Wang X, Ruan Z, Sheridan P, Boileau D, Liu Y, Liao W (2015) Two-stage photoautotrophic cultivation to improve carbohydrate production in Chlamydomonas reinhardtii. Biomass Bioenergy 74:280–287. https://doi.org/10.1016/j.biombioe.2015.01.024
Aziz MMA, Kassim KA, Shokravi Z, Jakarni FM, Lieu HY, Zaini N, Tan LS, Islam S, Shokravi H (2020) Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: a review. Renew Sust Energ Rev 119:109621. https://doi.org/10.1016/j.rser.2019.109621
Ho SH, Ye X, Hasunuma T, Chang J, Kondo A (2014) Perspectives on engineering strategies for improving biofuel production from microalgae - a critical review. Biotechnol Adv 32:1448–1459. https://doi.org/10.1016/j.biotechadv.2014.09.002
Zhu L (2015) Microalgal culture strategies for biofuel production: a review. Biofuels Bioprod Biorefin 9:801–814. https://doi.org/10.1002/bbb.1576
Ferreira AF, Ribeiro LA, Batista AP, Marques PASS, Nobre BP, Palavra AMF, Silva PP, Gouveia L, Silva C (2013) A biorefinery from Nannochloropsis sp. microalga - energy and CO2 emission and economic analyses. Bioresour Technol 138:235–244. https://doi.org/10.1016/j.biortech.2013.03.168
Nagappan S, Devendran S, Tsai PC, Dahms HU, Ponnusamy VK (2019) Potential of two-stage cultivation in microalgae biofuel production. Fuel 252:339–349. https://doi.org/10.1016/j.fuel.2019.04.138
Polat E, Yüksel E, Altınbaş M (2020) Mutual effect of sodium and magnesium on the cultivation of microalgae Auxenochlorella protothecoides. Biomass Bioenergy 132:105441. https://doi.org/10.1016/j.biombioe.2019.105441
Di Caprio F, Altimari P, Pagnanelli F (2018) Effect of Ca2+ concentration on Scenedesmus sp. growth in heterotrophic and photoautotrophic cultivation. New Biotechnol 40:228–235. https://doi.org/10.1016/j.nbt.2017.09.003
Gorain PC, Bagchi SK, Mallick N (2013) Effects of calcium, magnesium and sodium chloride in enhancing lipid accumulation in two green microalgae. Environ Technol 34:1887–1894. https://doi.org/10.1080/09593330.2013.812668
Ren HY, Liu BF, Kong F, Zhao L, Xie GJ, Ren NQ (2014) Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour Technol 169:763–767. https://doi.org/10.1016/j.biortech.2014.06.062
Islami HR, Assareh R (2020) Enhancement effects of ferric ion concentrations on growth and lipid characteristics of freshwater microalga Chlorococcum oleofaciens KF584224.1 for biodiesel production. Renew Energy 149:264–272. https://doi.org/10.1016/j.renene.2019.12.067
Hanifzadeh MM, Garcia EC, Viamajala S (2018) Production of lipid and carbohydrate from microalgae without compromising biomass productivities: role of Ca and Mg. Renew Energy 127:989–997. https://doi.org/10.1016/j.renene.2018.05.012
Lim DKY, Schuhmann H, Sharma K, Schenk PM (2015) Isolation of high-lipid Tetraselmis suecica strains following repeated UV-C mutagenesis, FACS, and high-throughput growth selection. Bioenergy Res 8:750–759. https://doi.org/10.1007/s12155-014-9553-2
Morais MG, Costa JAV (2007) Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol Lett 29:1349–1352. https://doi.org/10.1007/s10529-007-9394-6
Zarrouk C (1966) Contribution à l’étude d’une cyanophycée: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima. Thesis, Université of Paris. (In French)
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Margarites AC, Volpato N, Araújo E, Cardoso LG, Bertolin TE, Colla LM, Costa JAV (2016) Spirulina platensis is more efficient than Chlorella homosphaera in carbohydrate productivity. Environ Technol 38:2209–2216. https://doi.org/10.1080/09593330.2016.1254685
Lowry OH, Rosebrough NJ, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem:265–276
Association of Official Analytical Chemists (2000) Official Methods of Analysis of AOAC International 17th
Lourenco S de O (2006) Cultivo de microalgas marinhas : princípios e aplicações. RiMa, São Carlos. (In Portuguese)
Fernandes BD, Mota A, Ferreira A, Dragone G, Teixeira JA, Vicente AA (2014) Characterization of split cylinder airlift photobioreactors for efficient microalgae cultivation. Chem Eng Sci 117:445–454. https://doi.org/10.1016/j.ces.2014.06.043
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simões M (2012) Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew Sust Energ Rev 16:3043–3053. https://doi.org/10.1016/j.rser.2012.02.055
Álvarez-Díaz PD, Ruiz J, Arbib Z, Barragán J, Garrido-Pérez MC, Perales JA (2015) Wastewater treatment and biodiesel production by Scenedesmus obliquus in a two-stage cultivation process. Bioresour Technol 181:90–96. https://doi.org/10.1016/j.biortech.2015.01.018
Chokshi K, Pancha I, Trivedi K, George B, Maurya R, Ghosh A, Mishra S (2015) Biofuel potential of the newly isolated microalgae Acutodesmus dimorphus under temperature induced oxidative stress conditions. Bioresour Technol 180:162–171. https://doi.org/10.1016/j.biortech.2014.12.102
Esakkimuthu S, Krishnamurthy V, Govindarajan R, Swaminathan K (2016) Augmentation and starvation of calcium, magnesium, phosphate on lipid production of Scenedesmus obliquus. Biomass Bioenergy 88:126–134. https://doi.org/10.1016/j.biombioe.2016.03.019
Wan M, Jin X, Xia J, Rosenberg JN, Yu G, Nie Z, Oyler GA, Betenbaugh MJ (2014) The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl Microbiol Biotechnol 98:9473–9481. https://doi.org/10.1007/s00253-014-6088-6
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Margarites ACF, Costa JAV (2014) Increment of carbohydrate concentration of Chlorella minutissima microalgae for bioethanol production. J Eng Res Appl 4:80–86
Zhang X, Tang X, Wang M, Zhang W, Zhou B, Wang Y (2017) ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J Photochem Photobiol B 173:360–367. https://doi.org/10.1016/j.jphotobiol.2017.05.038
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421. https://doi.org/10.1016/j.bbabio.2006.11.019
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19:235–240. https://doi.org/10.1016/j.copbio.2008.05.007
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348. https://doi.org/10.1016/j.biortech.2015.04.017
Kováčik J, Dresler S (2018) Calcium availability but not its content modulates metal toxicity in Scenedesmus quadricauda. Ecotoxicol Environ Saf 147:664–669. https://doi.org/10.1016/j.ecoenv.2017.09.022
Sharma K, Li Y, Schenk PM (2014) UV-C-mediated lipid induction and settling, a step change towards economical microalgal biodiesel production. Green Chem 16:3539–3548. https://doi.org/10.1039/c4gc00552j
Hu Q (2004) Environmental effects on cell composition. In: Richmond A (ed) Handbook of 560 microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 83–93
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11. https://doi.org/10.1016/j.biortech.2012.11.032
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus - a potential strain for bio-fuel production. Bioresour Technol 171:367–374. https://doi.org/10.1016/j.biortech.2014.08.086
Braga V d S, Mastrantonio DJ d S, Costa JAV, Morais MG d (2018) Cultivation strategy to stimulate high carbohydrate content in Spirulina biomass. Bioresour Technol 269:221–226. https://doi.org/10.1016/j.biortech.2018.08.105
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina-Grima E, Guerrero MG (2008) Efficiency assessment of the one-step production of astaxanthin by the microalga Haematococcus pluvialis. Biotechnol Bioeng 100:397–402. https://doi.org/10.1002/bit.21770
Markou G, Chatzipavlidis I, Georgakakis D (2012) Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: improvements through phosphorus limitation process. Bioenergy Res 5:915–925. https://doi.org/10.1007/s12155-012-9205-3
Chentir I, Doumandji A, Ammar J, Zili F, Jridi M, Markou G, Ouada HB (2018) Induced change in Arthrospira sp. (Spirulina) intracellular and extracellular metabolites using multifactor stress combination approach. J Appl Phycol 30:1563–1574. https://doi.org/10.1007/s10811-017-1348-3
Iasimone F, Panico A, De Felice V, Fantasma F, Iorizzi M, Pirozzi F (2018) Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: biomass production, lipids accumulation and settleability characteristics. J Environ Manag 223:1078–1085. https://doi.org/10.1016/j.jenvman.2018.07.024
Wen Q, Chen Z, Li P, Han Y, Feng Y, Ren N (2013) Lipid production for biofuels from effluent-based culture by heterotrophic Chlorella protothecoides. Bioenergy Res 6:877–882. https://doi.org/10.1007/s12155-013-9308-5
Ferreira AF, Ferreira A, Dias APS, Gouveia L (2020) Pyrolysis of Scenedesmus obliquus biomass following the treatment of different wastewaters. Bioenergy Res:1–11. https://doi.org/10.1007/s12155-020-10102-1
Aketo T, Hoshikawa Y, Nojima D, Yabu Y, Maeda Y, Yoshino T, Takano H, Tanaka T (2020) Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J Biosci Bioeng 129:565–572. https://doi.org/10.1016/j.jbiosc.2019.12.004
Funding
The authors are pleased to acknowledge the National Council for Scientific and Technological Development (CNPq) and Coordination of Improvement of Higher Education Personnel (CAPES) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaparoli, M., Ziemniczak, F.G., Mantovani, L. et al. Cellular Stress Conditions as a Strategy to Increase Carbohydrate Productivity in Spirulina platensis. Bioenerg. Res. 13, 1221–1234 (2020). https://doi.org/10.1007/s12155-020-10133-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10133-8