Abstract
Mutagenesis and selection of microalgae can be used for accelerated breeding of elite strains, providing a significant advantage over genetic engineering as prior biochemical and genetic information is not required. Ultraviolet (UV)-C-induced mutagenesis combined with fluorescence-activated cell sorting (FACS) and microtiter plate reader cell density screening was used to produce Tetraselmis suecica strains with increased lipid contents without compromising on cell growth. After five rounds of mutation-selection, two dosages of UV-C (50 and >98 % lethality) yielded two improved strains (M5 and M24) that produced significantly more neutral lipids (increases of 114 and 123 %, respectively). This study highlights that repeated UV-C mutagenesis and high-throughput selection for cell growth can be a viable combined approach to improve lipid productivity in microalgae. These maybe used as elite strains for future breeding programs and as potential feedstock for biodiesel production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As fossil fuel resources diminish and cause environmental damage, there is a rapidly growing global demand for lipids, particularly triacylglycerides (TAGs) for the biofuel, aquaculture, and pharmaceutical industry. The need to develop sustainable lipid sources is now widely apparent. Traditional crop-based plants are increasingly being used for oil and biofuel production, but these cannot reasonably meet the growing demand [1]. Photosynthetic microalgae have repeatedly been proposed as a more viable lipid source due to their high productivity, environmental benefits, and ability to produce different kinds of oils [2–4]. Theoretically, microalgae can produce 10 to 20 times more lipids than oil palms [5], corn, and soybean [6–8] while achieving CO2 capture efficiencies of up to 99 % [9]. Furthermore, the production of microalgal biomass can be carried out without competing for valuable resources, such as arable land, biodiverse landscapes (e.g., rainforests), and freshwater [5]. However, the industry is still in its infancy and the cost of microalgal lipid production is still too high to achieve full commercialization of microalgal lipid feedstocks. Having the ideal algal strain with elevated lipid content, high growth rate and robust environmental tolerance remains one of the most important factors to improve algae economics [10, 11]. While many studies have focused on species selection and characterization to identify strains that contain two seemingly antagonistic traits, namely, fast growth and high-lipid content, genetic and metabolic engineering provide opportunities to create potential elite strains that meet these requirements. At present, induced mutagenesis provides a significant advantage over genetic engineering, as little biochemical or genetic information regarding the chosen organisms is needed [12, 13]. This approach requires relatively little technical manipulation. Improved non-transgenic microorganisms can be bred by incorporating mutagenesis and high-throughput selection, including microalgal strains with enhanced lipid performance.
Mutation studies involving ultraviolet (UV), ethyl methane sulfonate (EMS), and nitrosomethylguanidine (NTG) have been performed on a range of microalgal species (Phaeodactylum tricornutum, Pavlova lutheri, Nannochloropsis oculata, Haematococcus pluvialis, Schizochytrium sp., Chlorella sorokiana, Scenedesmus obliquus, Isochrysis galbana, Dunaliella salina) to produce mutants that exhibited increased/modified lipid content and growth rate [14–23]. Many of these studies, however, rely upon time-consuming techniques like metabolite antibiotic response to select for mutants and lipid extraction techniques to analyze their lipid content. Lately, the combination of lipophilic dyes (e.g., Nile red) and high-throughput technologies such as microplate readers and flow cytometry provided a powerful tool to isolate potential mutants from a complex population based on specific fluorescence cell properties. A study using the Nile red fluorochrome in conjunction with microplate reader technology has been shown to be able to select C. sorokiniana and S. obliquus mutants with elevated lipid contents [21]. Automated fluorescence-assisted cell sorting (FACS) can be more efficient than a microplate reader-based selection, as much larger populations of single cells can be handled, resulting in the recovery of a high number of candidate cells with the desired lipid content. The use of FACS in combination with mutagenesis has been described in mutation-selection studies that produced high-lipid content strains of I. galbana [23] and carotenoid-hyperproducing D. salina strains [22]. Even without mutagenesis, the use of FACS has been successful in generating higher-lipid content strains of Nannochloropsis sp. and T. suecica through selection alone [1, 24]. While most studies involve only one screening step, the present study combines a mutation-selection approach using UV-C-induced mutagenesis [19–21, 23] with FACS [1, 24] and microplate reader screening [21] to mutate, identify, and isolate T. suecica cells with a higher-lipid content without reduced growth rate.
Tetraselmis suecica is a flagellate green microalga commonly used as aquaculture feedstock and that is also considered a good candidate for biofuel production. This species is known to have a high-lipid content as well as being robust enough to tolerate a range of environmental conditions [25, 26]. Moreover, Tetraselmis cells have recently been shown to lose their flagella during stressful conditions, quickly settling and thus reducing harvesting/dewatering cost [27]. The growth characteristics of the T. suecica strain, used in the present study, have been previously described, displaying one of the highest comparative microalgal growth rates, although with a slightly lower TAG content compared to other strains tested [28]. Therefore, this microalga was chosen as a suitable candidate to generate an improved strain with fast growth and high-lipid content properties.
Material and Methods
Microalgae Culturing Conditions
The parent culture of wild-type (wt) T. suecica was originally collected by Queensland Sea Scallops (Bundaberg, Australia; [28]) and cultured in autoclaved artificial seawater with Guillard F medium [29] and maintained aerobically in 100-mL Erlenmeyer flasks with constant orbital shaking (100 rpm) at 25 °C, under a 12:12-h light/dark photoperiod of fluorescent white light (120 μmol photons/m2/s).
Mutagenesis
The mutation program used UV-C (100–280 nm) as the mutagenic agent. First, the appropriate dosage of UV-C treatment was determined in order to obtain an equivalent of the rate of non-lethal mutations. To achieve this, kill curves were produced by two methods, to establish 50 % (LD50) and >98 % lethal dosage. Mutagenesis was achieved by placing a 2-cm-deep culture of T. suecica (1 × 106 cells/mL; early starvation phase) in a 140-mm Petri dish under the lamps of a Bio-Rad GS Gene Linker UV Chamber and exposure to 15, 25, 50, and 100 mJ of UV-C. Cultures were then left in the dark for 24 h to prevent DNA repair by photo-reactivation. The first kill curve was obtained by plating 200 μL aliquots of 1/1000 diluted mutagenized cells onto a 1 % agar plate containing F medium in artificial seawater, which were then allowed to grow for 3 weeks before algal colonies from control and UV-exposed cells were counted. The second kill curve was carried out in 96-well plates inoculated with mutagenized cells after serial dilution down to 1 cell per well [22]. Wells with surviving microalgae that multiplied were then counted after 3 weeks of growth. Both, agar plates and 96-well plates were grown under fluorescent white lights (50 μmol photons/m2/s; 16:8-h light:dark photoperiod) and maintained at 24 °C. Further stages of this study used UV-C dosages of 25 and 100 mJ to induce mutagenesis, as these provided a survival rate of 50 and <2 %, respectively.
FACS and Lipid Quantification by Nile Red Fluorescence
Nile red (Sigma Inc.) was used to stain lipids for (i) FACS and (ii) quantification of lipid contents via a 96-well microplate reader. First, the appropriate amount of Nile red working solution required to produce the best lipid staining, while maintaining a high FACS survival rate, had to be determined. Cell samples in starvation phase (1 mL at 0.8–1 × 106 cells/mL) were treated with 1, 2, and 3 μL of a working solution of Nile red in acetone or dimethyl sulfoxide (both 1 mg/mL). Samples were then gently mixed and incubated in the dark for 10 min. Single cells were sorted using a BD FACSVantage SE (Becton Dickinson) cell sorter with a 485-nm argon laser and 100-μm nozzle into 96-well plates using F in seawater medium. Cell fluorescence was measured at 585 nm for yellow-gold fluorescence, indicative of neutral lipid content. Approximately 10,000 cells were analyzed, with dot plots of yellow-gold fluorescence (PE-A) vs. forward light scatter (FSC-A, cell size). Cell sorting regions were positioned to include cells presenting increased fluorescence and size compared to the general population of cells. FACS survival rates were then determined after 2 weeks of growth.
To enable quantification of neutral lipid contents of T. suecica cells in a microtiter plate reader, the ability of Nile red (in acetone working solution) to stain neutral lipids without killing the cells was first established. Two populations of microalgal cells (106 cells/mL), namely, (i) cells in late starvation phase and (ii) cells in exponential growth phase, were mixed to produce a population of 0, 25, 50, 75, and 100 % of starved cells, as a proxy for increasing lipid content within a given volume. A total of 1 mL of these samples was then stained with 2 μL of a working solution of Nile red in acetone (1 mg/mL). Samples were then gently mixed and incubated in the dark for 10 min. A total of 100 μL from each sample was then loaded into a 96-well microtiter plate (Sarsted) in triplicates. Yellow-gold fluorescence was measured on a POLARstar OPTIMA (BMG Labtech) plate reader using fluorescence intensity mode. Gain was set at 3000, with excitation and emission wavelengths of 485 and 590 nm, respectively. These settings were used for further fluorescence intensity measurements in this study.
Mutation-Selection Cycles
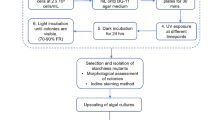
Mutation and selection cycles are summarized in Fig. 1. Cultures were grown until late log phase and then mutagenized according to the optimized method at 25 and 100 mJ. Mutagenized cultures were then left in the dark for 24 h and then cultured for 2 weeks to allow for culture recovery (addition of fresh F medium every 7 days). Two days prior to FACS, nutrient deprivation to stimulate lipid production was achieved by removal of previous medium by centrifugation (1200×g, 5 min) and replacement with only seawater (without F medium). FACS was then carried out according to the optimized methodology, with mutagenized single cells sorted into 96-well plates. Plates were incubated at 24 °C under a 16:8-h light/dark photoperiod of fluorescent white lights (50 μmol photons/m2/s). For generations (cycles of mutagenesis and selection rounds) 1, 2, and 3, the plates after FACS were monitored daily, and the first 6 wells that showed visual signs of algal growth were selected and scaled up for subsequent rounds of mutagenesis and cell sorting. For generations 4 and 5, the top 24 wells from the 96-well plates after FACS that showed visual signs of growth were scaled up (1:10 dilution) in a 24-well plate. After 7 days of growth at 24 °C under a 16:8-h light/dark photoperiod of fluorescent white light (50 μmol photons/m2/s), the absorbance value (450 nm) and fluorescence intensity of the wells were measured. Cell counts were performed on the top 12 wells that showed the highest fluorescence/absorbance ratio using a hemocytometer. The top three clones that showed the highest fluorescence/cell count ratio were selected and scaled up for subsequent rounds of mutagenesis. In all cycles of the program, selected strains were allowed to grow for 3 to 4 weeks to ensure a genetically stable population before mutagenesis.
Standard Protocol for Culture Growth Analysis, Lipid Induction Phase, Sampling for Fluorescence, and Lipid Analysis
A standard protocol was designed to allow direct comparison of growth rates, fluorescence intensity, and fatty acid (FA) profile between selected strains and wt based on a modified method by Lim et al. [28]. Briefly, a total of 5 mL of selected strain or wt culture in late log phase was used as inoculum (8 h after start of the light cycle) for 50-mL artificial seawater complemented with F medium in 100-mL Erlenmeyer flasks and grown under constant orbital shaking (100 rpm) at 25 °C, under a 12:12-h light/dark photoperiod of fluorescent white light (120 μmol photons/m2/s). After day 7, nutrient deprivation to stimulate lipid production was achieved by centrifugation (1200×g, 5 min) and replacement with only seawater (without F medium). Cultures were then grown for another 5 days post starvation. Cell counts were performed on days 0, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, and 13, while fluorescence intensities were measured on days 0, 2, 3, 4, 5, and 6 post starvation. The fatty acid methyl ester (FAME) contents of the cultures were analyzed on day 4 post starvation.
Fatty Acid Methyl Ester Analysis
For FAME analyses, 4 mL of algal culture was collected from each replicate and centrifuged at 16,000×g for 3 min. After the supernatant was discarded, lipids in the algal pellet were hydrolyzed and methyl-esterified by shaking (1200 rpm) with 300 μL of a 2 % H2SO4/methanol solution for 2 h at 80 °C. Prior to the reaction, 50 mg of heneicosanoic acid (Sigma, USA) was added as internal standard to the pellet. A total of 300 μL of 0.9 % (w/v) NaCl and 300 μL of hexane was then added followed by mixing for 20 s. Subsequently, phase separation was performed by centrifugation at 16,000×g for 3 min. A total of 1 mL of the hexane layer was then injected splitless into an Agilent 6890 Gas Chromatograph coupled to a 5975 MSD Mass Spectrometer. A DB-Wax column (Agilent, 122–7032) was used with running conditions as described for Agilent’s RTL DBWax method (Application note 5988–5871EN). Quantification of FAMEs was carried out by taking the ratio of the integral of each FAME’s total ion current peak to that of the internal standard (50 mg), with the molecular mass of each FAME also factored into the equation. FAMEs were then identified based on mass spectral profiles, in comparison to standards and expected retention times from Agilent’s RTL DBWax method (Application note 5988–5871EN).
Analytical Methods
Growth rates and doubling times were calculated from day 0 to day 7 to measure the growth rate during growth phase, day 0 to day 10 to measure the overall growth rate, and from day 7 to day 10 to measure the growth rate during starvation phase.
Calculations for growth rate and doubling time are based on the following equations:
with Ny and Nx being the number of cells from the selected days of analysis.
Results
Mutagenesis Survival Rate
The appropriate dosage of UV-C exposure to obtain a 50 and <2 % survival rate was determined by treating microalgal cells with a range of UV-C dosages (0–100 mJ), followed by growth on agar (solid media) and in 96-well plates (liquid media). Survival rate (Fig. 2a) was found to be dosage dependent, with the survival rate decreasing as UV-C dosage increased. The growth method also had an effect of post-UV-C exposure survival rates. When grown on agar plates, the LD50 was found to be at 16 mJ, while <2 % survival rate was found to be at 63 mJ. In liquid medium, the 50 % survival rate and <2 % survival rate were at 26 and 92 mJ, respectively (Fig. 2a), and this dosage was used during the subsequent experiments.
Mutation-selection cycle for the production of T. suecica cells for improved lipid production. Cycles 1-3: the top 6 clones with the fastest growth after each sorting run were selected for subsequent mutagenesis. Cycle 4 & 5: the top 24 fastest growers were scaled up and their lipid production performance evaluated. The top 3 lipid producing cultures were selected for subsequent cycles
Optimization of Nile Red Staining for Fluorescence-Activated Cell Sorting
The use of appropriate Nile red staining solution was required to ensure maximum fluorescence while maintaining a high recovery of viable clones. While the increase in staining solution did not affect fluorescence intensities or clone recovery, it was found that cells stained with Nile red dissolved in acetone produced fluorescence intensities markedly higher than cells stained with Nile red in DMSO (Fig. 3). Unstained populations achieved a recovery rate of 78 % viable cells, while both DMSO and acetone solvents achieved 40–50 % recovery. Therefore, for this study, the addition of 1 μL Nile red in acetone working solution was chosen for FACS. The ability of Nile red staining in acetone to determine varying levels of neutral lipids in T. suecica was also established (Fig. 4). This study also found that 2 mg/mL of Nile red was suitable to detect populations of T. suecica with >12.5 % of cells containing neutral lipids, demonstrating a strong linear correlation (r 2 = 0.98, n = 6) between the percentage of starved cells and fluorescence intensity.
Optimization of UV-C lethal dosage and Nile red staining. a Survival rate of T. suecica in agar plates and 96-well plates after exposure to varying UV-C dosages. The 10-mJ survival rates in 96-well plates were not measured. b Fluorescence units of mixed starved and unstarved T. suecica population demonstrating the ability of Nile red staining in acetone to determine varying levels of neutral lipids in T. suecica. Data represent mean ± SEM from three independent replicates
Growth Rates and Cell Density
After the fifth cycle of mutation-selection, two strains with one of the best fluorescence/cell count ratio (one from each UV dosage) were chosen for further analyses: (i) M5 (originating from 25 mJ UV-C (50 % survival rate) mutagenesis) and (ii) M24 (100 mJ UV-C (<2 % survival rate)) were compared with wt T. suecica to determine their growth and lipid production performance. During the first 7 days of growth, all cultures exhibited similar growth rates and cell densities. After starvation was induced, both M5 (0.2 day−1; P = 0.06) and M24 (0.2 day−1; P < 0.05) exhibited higher growth rates than wt. The strains also achieved significantly higher (P < 0.05) cell densities than wt (1.25 × 106 cells/mL) on day 10 and reached 1.82 × 106 and 1.71 × 106 cells/mL, respectively. Overall growth rates (μ10 days) of the selected strains were also found to be slightly higher than wt, although not at a significant rate (Fig. 5).
Cell density of T. suecica selected strains and wild-type over the span of 13 days with induced starvation on day 7. Boxed data points indicate significant differences from wild-type (P<0.05). Inserted table: Growth rates and doubling time for the first 7 days, first 10 days and from day 7 to day 10. Asterisks indicate significant differences from wild-type (P<0.05). Data represent mean ± SEM from two independent replicates
Neutral Lipid Content
After 7 days of growth, nutrient deplete conditions were used to induce lipid production, and the neutral lipid content of selected T. suecica strains and wt were determined. The total neutral lipid content of wt increased until day 3 and then plateaued, while the total neutral lipid content of M5 and M24 continued to increase. From day 4 onwards, total neutral lipid production of the selected strains was significantly higher than wt (P < 0.05), with M5 and M24 achieving maximum total neutral lipids on day 5 (114 % increase from wt) and day 6 (123 % increase from wt), respectively (Fig. 5a). On a per-cell basis, wt cells accumulated lipids from day 2 to day 3 and then stopped, while selected strains cells continued to accumulate lipids until day 6. M5 cells had 80–90 % more neutral lipids than wt on days 4 and 5 (P < 0.05), while M24 cells contained 96–100 % more neutral lipids than wt on days 5 and 6 (P < 0.05; Fig. 5b).
GC/MS analyses revealed an overall reduction in monounsaturated FAs (MUFAs; P < 0.05) in the selected strains on day 5 (Fig. 6). This trend was observed for C16, C16:1, C18, C18:1, C20, and C20:1 FAs, where significant reductions were accompanied by significant increases in polyunsaturated FAs (PUFAs), such as C16:4, C18:4, and C20:5. The selected strains also exhibited an increase in C14 and a decrease in C18:2, although this was only significant (P < 0.05) in the M24 strain.
Lipid accumulation in T. suecica wild-type and selected strains (M5 & M24) during nutrient starvation phase measured by Nile red-stained fluorescence units at 485/590. (a) Total fluorescence units measured represent total lipid accumulated per mL of culture (b) Total fluorescence/cell represent total lipid accumulated per cell. Boxed data points indicate significant differences from wild-type (P<0.05), data represent mean ± SEM from two independent replicates
Discussion
This study describes a combined and repeated mutation-selection method designed to increase the neutral lipid content of T. suecica without compromising its growth rate. It also includes the optimization of key steps within the mutation-selection cycle, such as the appropriate UV-C dosage and Nile red concentration. UV-C has been successfully used to generate microalgae mutants [19, 21] and was selected as a mutagenic agent because it was practical and safer than chemical mutagens such as EMS and MTG. As chemical mutagens are more effective than UV radiation, UV-C mutagenesis requires higher dosages (with lower survival rates) [30]. However, although the frequency of mutants increases among survivors at high dosages, so does damage to the genetic background which reduces clone recovery and fitness. Therefore, two different UV-C dosages were chosen to either increase mutation probability (>98 % lethal dosage) or increase recovery of a high number of clones (50 % lethal dosage) with less genetic background damage, a survival rating similarly applied in other microalgae mutation studies [19, 22]. The recovery of UV-C-exposed cells in liquid media was more effective, as survival rates in 96 well-plates were higher compared to solid media agar plates. The poor growth of other flagellate microalgae on solid media was also demonstrated for P. lutheri as well as other filamentous and flagellate organisms, primarily due to the dehydration of the medium [31, 32].
The use of Nile red as a fluorescence probe for neutral lipid detection and quantification in microalgae has been well documented [1, 21, 22, 24, 23, 33, 34]; a strong correlation between lipid content and fluorescence intensity has already been established [34]. The addition of solvents such as acetone and DMSO has been shown to improve the transition of the dye into lipids, although their efficacy varies between species, depending on the characteristics of the individual algae species [34]. The optimization stage of this study revealed acetone as a better stain carrier than DMSO for staining T. suecica cells that displayed higher fluorescence intensities during FACS. Other flow cytometry studies involving D. salina [33], Nannochloropsis sp. [1], and I. galbana [23] also utilized acetone as a carrier. The percentage of viable cells post-sorting achieved in this study (40–50 %) was lower than the 80 % reported by Montero et al. [24], which was achieved by seawater as sheath fluid, but still higher than the 20–30 % reported by other studies involving the sorting of other phytoplankton flagellates [35]. The staining efficacy of DMSO has been shown to be better than acetone at a higher volume/volume [34], but would prove toxic and reduce FACS clone recovery. Therefore, when at similar volumes, acetone produced a higher fluorescence intensity than DMSO and was the preferred solvent in this study. This study has also established a correlation between Nile red fluorescence intensity between percentage of starved cell (a proxy of total lipid content within a volume). In T. suecica, correlations between Nile red fluorescence signal and TAG content [24] as well as neutral lipids estimated by gravimetry have been established [36]. Other studies have also found a strong correlation between fluorescence signal and total lipid content [34, 37], thus confirming the use of Nile red fluorescence to quantify lipid content in this study to allow for sorting of hyperlipidic T. suecica strains.
The overall aim of the mutation-selection program was to develop a strain that had a high-lipid content while maintaining its high growth rate. For generations 1 to 3, lipid selection was achieved during flow cytometry, while growth rate selection was carried out post-sorting during the grow-up phase of the sorted cells. While other studies pooled their sorted cells [1, 24], individual cells were sorted into individual 96-well plate wells to facilitate the selection of the top six fastest growing individuals that would be carried into the next cycle of selection. The growth screening step was introduced to maintain the growth rate of the selected strains, as there have been studies that indicated reduced growth rates in strains isolated for high-lipid content [22, 8, 38, 21]. To confirm that the selected individuals still maintained their high-lipid content, an additional screening step similar to that of Vigeolas et al. [21] was introduced in generations 4 and 5. A total of 24 instead of six of the fastest growing strains were selected and scaled up in 24-well plates before their Nile red fluorescence intensity and absorbance values were measured to obtain fluorescence/absorbance. As absorbance values do not account for cell viability and can be misleading, cell numbers were then used to confirm the top six performers with the highest fluorescence/absorbance. The final mutation-selection program therefore now incorporates FACS with a fast-growth selection step, followed by a high-lipid per-cell step.
After five cycles of mutation-selection that yielded two improved strains, namely, M5 and M24, a standard protocol to compare the growth rates, lipid content, and FA content was performed. Growth rate comparisons found the overall growth rates of improved strains to be slightly higher than wt, with significant increases occurring during the starvation period. Improved strains also achieved a significantly higher maximum cell density compared to wt. While the growth rates achieved in this study were expected to be lower than reported by Montero et al. [24] due to the lack of CO2 aeration, FACS-isolated T. suecica cells in that study exhibited lower growth rates than the original wt. Reduced growth and cell density were also reported in a mutant study involving another flagellate, D. salina, that did not incorporate a growth selection step [22], although no reduction in growth rates was reported for I. galbana after two rounds of mutation-selection [23]. Other mutation studies that reported maintained or increased growth rates in mutants were found to have incorporated a growth selection step as well [15–17, 21].
When comparing neutral lipid productivities, selected T. suecica cultures in the current study exhibited a 114–123 % increase in total fluorescence compared to wt and a 90–100 % increase on a fluorescence per cell basis (but the cultures did not show any significant differences during nutrient replete conditions on day 0; Fig. 5). These results were much higher than other UV mutation studies without FACS or Nile red fluorescence screening, which reported only an 8–35 % increase in FA content [14, 18, 19]. Nevertheless, the yield improvement of this study was more similar to studies involving microplate reader screening and FACS. Vigeolas et al. [21] screened UV-mutated cells based on Nile red fluorescence using a 96-well plate reader to develop C. sorokiana and S. obliquus strains with 50–300 % increase in fluorescence units per cell, corresponding to similar increases in TAG content per cell, while Bougaran et al. [23] combined UV mutagenesis and FACS in an I. galbana mutation-selection procedure that increased lipid productivity by 80 %. It is interesting to note that in FACS studies without mutagenesis, a T. suecica strain with up to fourfold increase in fluorescence signal was obtained after two rounds of sorting [24], while a Nannochloropsis sp. strain with a threefold increase in total lipid content was selected after three rounds of sorting [17]. While significantly higher than wt, the neutral lipid content of both improved T. suecica strains in the present study was not significantly different from each other (Fig. 5). This indicates that both 50 and >98 % lethal dosages can be used to produce viable mutants with selectable traits. Nevertheless, a 50 % lethal dosage was preferred as the recovery of clones was easier and less time-consuming.
As cell sizes between selected cells and wt cells were not significantly different during FACS analyses, it is expected that, similar to a per cell basis (Fig. 5b), lipid contents per dry weight would also be increased (although this was not directly measured here). Although selected cells were not found to be significantly smaller, it appears that both, a more rapid growth after N depletion (Fig. 4) as well as an increased cellular lipid fluorescence (Fig. 5b), contributed to an increased overall lipid fluorescence in the selected cells (Fig. 5a). This is in alignment with the selection protocol (Fig. 1) that selected for both of these traits. GC-MS data only showed a slight increase of total fatty acid contents in the selected strains (30.7 and 29.7 μg/mL for M5 and M24, respectively) compared to the wt (26.8 μg/mL), raising the question whether lipids other than fatty acids may have contributed to the higher lipid fluorescence in the selected strains. The comparison of FA profiles between improved strains and wt T. suecica revealed a decrease in MUFAs such C16:1 and C18:1, accompanied by increases in PUFAs such as C16:4, C18:4, and C20:5 (Fig. 6). While certain studies report unaltered FA composition in their mutants [1, 17, 21], there have been studies that have also reported elevated PUFAs. The increase of PUFA in mutants has been documented in P. lutheri mutants irradiated by UV [19] and Nannochloropsis sp. irradiated by EMS and N-methyl-N-nitrosourea [15, 16]. Furthermore, Chaturvedi and Fujita [15] and Chaturvedi et al. [16] also reported increases in C14 and reductions in C18:1. This decrease in the proportion of saturated FAs and MUFAs in comparison to an increase in PUFAs suggests that the mutants preferentially store lipids in the form of PUFAs. This, along with an overall increase in neutral lipids detected by elevated Nile red fluorescence, points towards mutations occurring in the gene coding for ACCase enzyme and the coding sequences of key desaturase genes. Changes in the ACCase enzyme, considered to be the rate-limiting step in FA biosynthesis [39], would increase the substrate pool of TAG production, leading to more short-chain FAs (e.g., C12, C14, C16) that become precursors for MUFAs and PUFAs as well as an increase in overall TAG accumulation [16]. Variations in PUFA contents between wt and selected strains also point towards mutations in key desaturase genes, which could explain the shift towards PUFA production [16]. Another explanation towards increased PUFA production in selected strains is the antioxidant effect of PUFAs against reactive oxygen species (ROS) generated during mutagenesis [40]. The antioxidant function of PUFAs in marine microorganisms, particularly eicosapentaenoic acid (EPA), has been reviewed and points towards their stabilizing function against oxidation by ROS. This is achieved by increased PUFA presence as membrane phospholipids to function as shield molecules [41]. Therefore, during the course of multiple rounds of mutation and selection, selected strains with increased PUFA production would have increased survivability towards UV-C radiation. Future studies should also investigate whether carbon partitioning is altered in the selected strains and whether the increased lipid content may result from a decrease of starch reserves as was found for other strains with elevated lipid contents [42].
Fatty acid composition in percentage of total FAME of wild-type and selected T. suecica strains. Inserted table: Percentage of saturated, monounsaturated and polyunsaturated FA. Arrows and asterisk indicate significant differences from wild-type (P<0.05). Data represent mean ± SEM from two independent replicates
Considering the average doubling time of 2.55 days of the selected strains, the fluorescence values obtained during the comparison to wt were obtained more than 24 cell divisions after the final selection cycle step and 36 divisions after UV radiation in cycle 5. This supports the idea of a stable genetic makeup of the obtained putative mutants that produce increased neutral lipid content. Although the nature of these putative mutations are beyond the scope of this study, the observed improvements could be a result of adaptation (e.g., by epigenetics) and not DNA mutations. It cannot be ruled out that strains adapted to, instead of mutated to, UV-C exposure. Therefore, the lipid content of these strains should be evaluated again in the future, particularly after long-term storage without selection pressure for high-lipid content. At that point, unchanged lipid content values in comparison to wt would indicate a stable genetic mutation, while epigenetic change would cause these strains to adapt back a more wt phenotype. Unlike plant crops that undergo very few reproductive cycles between harvests (typically just one), bred elite microorganisms are constantly at high risk to revert back to faster growing wt-like strains. At present, the risks of this occurring for the microalgae in this study appear reduced, as the selected strains did not compromise on their ability to grow.
References
Doan YTT, Obbard JP (2011) Enhanced intracellular lipid in Nannochloropsis sp. via random mutagenesis and flow cytometric cell sorting. Glob Chang Biol Bioenergy 3:264–270
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Malcata FX (2011) Microalgae and biofuels: a promising partnership? Trends Biotechnol 29:542–549
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1:20–43
Ahmad AL, Mat Yasin NH, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sustain Energy Rev 15:584–593
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Gouveia L, Oliveria A (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Zeiler KG, Heacox DA, Toon ST, Kadam AL, Brown LM (1995) The use of microalgae for assimilation and utlization of carbon dioxide from fossil fuel-fired power plant flue gas. Energy Convers Manag 36:702–712
Van Thang D, Li Y, Nowak E, Schenk PM (2012) Microalgae isolation and selection for prospective biodiesel production. Energies 5:1835–1849
Gallagher BJ (2011) The economics of producing biodiesel from algae. Renew Energy 36:158–162
Queener SW, Lively DH (1986) Screening and selection for strain improvement. In: Demain AL, Solomon NA (eds) Manual of industrial microbiology and biotechnology. American Society for Microbiology, Washington DC, pp 155–169
Rowlands RT (1984) Industrial strain improvement: mutagenesis and random screening procedures. Enzym Microb Technol 6:3–10
Alonso DL, Castillo CIS, Grima EM, Cohen Z (1996) First insights into improvement of eicosapentaenoic acid content in Phaeodactylum tricornutum (Bacillariophyceae) by induced mutagenesis. J Phycol 32:553–558
Chaturvedi R, Fujita Y (2006) Isolation of enhanced eicosapentanoic acid producing mutant of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol Res 54:208–219
Chaturvedi R, Uppalapati SR, Alamsjah MA, Fujita Y (2004) Isolation of quizalofop-resistant mutants of Nannochloropsis oculata (Eustigmatophyceae) with high eicosapentanoic acid following N-methyl-N-nitrosourea-induced mutagenesis. J Appl Phycol 16:135–144
Anandarajah K, Mahendraperumal G, Sommerfeld M, Hu Q (2012) Characterization of microalga Nannochloropsis sp. mutants for improved production of biofuels. Appl Energy 96:371–377
Lian M, Huang H, Ren L, Ji X, Zhu J, Jin L (2010) Increase of docosahexaenoic acid production by Schizochytrium sp. through mutagenesis and enzyme assay. Appl Microbiol Biotechnol 162:935–941
Meireles LA, Catarina Guesdes A, Xavier Malcata F (2003) Increase of the yields of eicosapentanoic and docosahexanoic acids by the microalga Pavlova lutheri following random mutagenesis. Biotechnol Bioeng 81:50–55
Kamath BE, Vidhyavathi R, Sarada R, Ravishakar GA (2008) Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour Technol 99:8867–8673
Vigeolas H, Duby F, Kaymak E, Niessen G, Motte P, Franck F, Remacle C (2012) Isolation and partial characterization of mutants with elecated lipid content in Chlorella sorokiana and Scenedesmus obliquus. J Biotechnol 162:3–12
Mendoza H, de la Jara A, Presmanes KF, Duarte LC, Ramos AA, Duarte VS, Varela JCS (2008) Characterization of Dunaliella salina strains by flow cytometry: a new approach to select carotenoid hyperproducing strains. Electron J Biotechnol 11:5–6
Bougaran G, Rouxel C, Dubois N, Kaas R, Grouas S, Lukomska E, Le Coz J, Cadoret J (2012) Enhancement of neutral lipid productivity in the microalga Isochrysis affinis galbana (T-Iso) by a mutation-selection procedure. Biotechnol Bioeng 11:2737–2745
Montero MF, Aristizabal M, Reina GG (2011) Isolation of high-lipid content strains of the marine microala Tetraselmis suecica for biodiesel prodction by flow cytometry and single-cell sorting. J Appl Phycol 23:1053–1057
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Chini Zitelli G, Rodolfi L, Biondi N, Tredici MR (2006) Productivitty and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular collumns. Aquaculture 261:932–943
Sharma KS, Li Y, Schenk PM (2014) UV-C-mediated lipid induction and settling, a step change towards economical microalgal biodiesel production. Green Chem 16:3539–3548
Lim DKY, Garg S, Timmins M, Zhang ESB, Thomas-Hall SR, Schumann H, Li Y, Schenk PM (2012) Isolation and evaluation of oil-producing microalgae from subtropical coastal and brackish waters. PLoS One 7:e40751
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. 1. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Carlton BC, Brown BJ (1981) Gene mutation. In: Gerhardt P (ed) Manuals of methods for general bacteriology. American Society for Microbiology, Washington DC, pp 222–242
Droop MR (1954) A note on the isolation of small marine algae and flagellates for pure cultures. J Marine Biol Assoc 33:511–514
Williams E, Lambert J, O’Brien P, Houghton JA (1979) Evidenec for dark repair of far ultraviolet light damage in the blue-green algae Gleocapsa aplicola. Photochem Photobiol 29:543–547
Mendoza H, de la Jara A, Presmanes KF, Duarte LC (2012) Quick estimation of intraspeciific variation of fatty acid composition in Dunaliella salina using flow cytometry and Nile Red. J Appl Phycol 24:1237–1243
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–71
Sensen CW, Heimann K, Melkonian M (1993) The production of clonal and axenic cultures of microalgae using fluorescence-activated cell sorting. Eur J Phycol 28:93–97
Mendoza H, de la Jara A, Carmona L, Presmanes KF (2009) Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to cluture conditions. Aquac Int 18:189–199
de la Jara A, Mendoza H, Martel A, Molina C, Nordstro L, de la Rosa V, Diaz R (2003) Flow cytometric determination of lipid content in a marine dinoflagellate Cryptecodinium cohnii. J Appl Phycol 15:433–438
Sheehan J, Dunahay T, Benemann J, Roessler PG (1998) A look back at the US department of energy’s aquatic species program—biodiesel from algae, close out report. National Renewable Energy Laboratory
Post-Beittenmiller D, Roughan G, Ohlrogge J (1992) Regulation of plant fatty acid biosynthesis: analysis of acyl-CoA and acyl-ACP substrate pools in spinach and pea chloroplasts. Plant Physiol 100:923–930
Kocavik J, Klejdus B, Backor M (2010) Physiological responses of Scenedesmus quadricauda (Chlorophyceae) to UV-A and UV-C light. Photochem Photobiol 86:612–616
Okuyama H, Orikasa Y, Nishida T (2008) Significant of antioxidative functions of eicosapentaenoic and docosahexaenoic acids in marine microorganisms. Appl Environ Microbiol 74:570–574
Breuer G, de Jaeger L, Artus VP, Martens DE, Springer J, Draaisma RB, Eggink G, Wijffels RH, Lamers PP (2014) Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (II) evaluation of TAG yield and productivity in controlled photobioreactors. Biotech Biofuels 7:1–11
Acknowledgments
We wish to thank the Australian Research Council for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• UV-C mutagenesis and high-throughput selection technology has been combined to improve lipid productivity in Tetraselmis suecica.
• Both 50 and >98 % lethal dosage successfully produced improved strains with approximately 100 % increase in lipid accumulation.
• Growth rates of improved strains remained unchanged.
Rights and permissions
About this article
Cite this article
Lim, D.K.Y., Schuhmann, H., Sharma, K. et al. Isolation of High-Lipid Tetraselmis suecica Strains Following Repeated UV-C Mutagenesis, FACS, and High-Throughput Growth Selection. Bioenerg. Res. 8, 750–759 (2015). https://doi.org/10.1007/s12155-014-9553-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-014-9553-2