Abstract
Thermophilic anaerobic digestion (TAD) is an efficient method for biogas production. In this study, the TAD of Arundo donax cv. Lvzhou No. 1 (ADL-1, a new kind of energy crop with high cold tolerance) at different growth stages was carried out, in order to investigate the relationship between microbial community structure and its function during the fermentation process. The results showed that the most optimal growth period of ADL-1 was 3 months, regarding the yield of biogas production. The TAD process lasted for 10 days with cumulative biogas and methane yields of 312.7 mL/g VS and 231 mL/g VS, respectively. The degradation rates of hemicellulose, cellulose, and lignin were 41.78%, 27.99%, and 14.46%, respectively. The high-throughput sequencing of 16S rRNA gene amplicons revealed that the most abundant bacterial phylum in TAD was Firmicutes with three dominant genera of Tepidiphilus, Sedimentibacter, and Gelria. Also, the main archaeal order was Methanomicrobiales, in which Methanoculleus and Methanosarcina were detected as dominant genera. Therefore, this article reveals the dynamic changes of structure and function of microbial communities during TAD of ADL-1, providing the theoretical basis for the development of energy crops with cold tolerance as potential biogas feedstock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid growth of the human population, the demand for energy is also increasing, and nonrenewable fossil fuel (mainly derived from petroleum and coal materials) has become in short supply. Worldwide, depletion of fossil fuel reserves to fuel price hike will lead to an energy crisis in the near future [1,2,3]. In addition, fossil fuel combustion will cause great damage to the environment, mainly due to various combustion products such as sulfur dioxide (SO2), nitrogen oxides (NOx), ground level ozone, particulate matter (PM), carbon monoxide (CO), carbon dioxide (CO2), volatile organic compounds (VOC), and some other heavy metals [2]. In order to meet future energy demands and address current environmental concerns relating to nonrenewable energy sources, biofuel could offer a suitable alternative energy reserve. Biofuels are the fuels derived from biological sources like plants, animals, microbes, etc., which are biodegradable, nontoxic, and environmentally friendly and can be used as a substitute for environmentally unsafe fossil fuels [2, 4, 5]. Biofuels include bioethanol, biobutanol, biodiesel, biogas, and biohydrogen [2, 5, 6]. It is also important to find a sustainable raw material which can produce biofuels.
The exploitation of energy crops as raw materials for agricultural biogas production is developing rapidly nowadays [7,8,9]. Perennial energy crops can be harvested periodically for many years with only one planting, which could greatly reduce the planting cost. Using such raw materials has been regarded as a good choice for biogas fermentation. Since perennial energy crops prefer to hot and humid environments, they are often difficult to adapt to the environmental conditions of China’s large northern area where the temperature usually drops from − 10 to − 30 °C in winter. In such area, they need to be replanted in every year, resulting in the increase of the production cost. Therefore, the energy crops with high cold tolerance would be promising for biogas fermentation industry in northern China.
The National Engineering Research Center of JUNCAO Technology has developed a new kind of energy crop with high cold tolerance, called Lvzhou No. 1, which belongs to Arundo donax L. (Arundo donax L. Lvzhou No. 1, ADL-1). Owing to its property of developed root system and sturdy stems, ADL-1 has rapid growth rate and high reproduction capacity. Under normal circumstances, the yield of mature ADL-1 can reach 105–300 t/hm2, with a height of as high as 4 to 6 m and stem diameter ranging from 2 to 3.5 cm. More importantly, ADL-1 can tolerate the severe cold of − 20 °C, and thus it is suitable for large-scale planting in northern China, providing a steady stream of raw materials for industrial production of biogas independent of climate and locality. But ADL-1, as a new energy plant that can be widely planted and can survive at low temperatures, has not been completely studied so far.
Anaerobic digestion process commonly used in biogas production is not a certain reaction but a multistage process involving microbial degradation of organic matter and gas production through various microorganisms. The whole process usually can be divided into five stages, including hydrolysis, acidogenesis, acetogenesis, methanogenesis, and stabilization [10]. In the process of anaerobic digestion, microbial communities are complex. Different microorganisms depend on each other and restrict each other to reach an equilibrium state [11]. During this process, many bacteria produce multiple enzymes to hydrolyze organic polymers, such as glucanases, hemicellulases, xylanases, amylases, lipases, and proteases [12]. The hydrolysis of organic matter could increase the contents of precursors of methane synthesis and improve the effect of gas production [13, 14]. Usually, the anaerobic digestion process for biogas production is classified into TAD (50–60 °C), mesophilic anaerobic digestion (30–40 °C), and room temperature anaerobic digestion (temperature changes with ambient temperature) according to the reaction temperature [15]. Currently, biogas production using TAD has attracted massive interest since it has more advantages, such as higher methane content, lower hydrogen sulfide content, shorter retention time, smaller reactor volume, and higher pathogen destruction rate and organic matter degradation rate [16].
Microorganisms play important role during the biogas fermentation. With the popularity of high-throughput sequencing technologies, emerging studies have discovered microbial communities and microbial roles during the fermentation process. Lu et al. [17] studied the changes of the bacterial population during mesophilic and thermophilic fermentation. The results show that when the environment changes, the content of most bacteria and archaea will change, and even some bacteria will disappear. Based on 16S rRNA high-throughput sequencing results, Tian et al. [18] found that in the fermentation system using pig manures as raw materials at 15 °C, the most important phylum of bacteria and archaea were Firmicutes and Archaebacteria, respectively. Xiao et al. [19] studied the microbial community of anaerobic digestion of mushroom substrates. High-throughput sequencing of 16S rRNA gene amplification showed that Proteobacteria (56.7–62.8%) was the main phylum of different fermentation stages. Chen and Chang [20] investigated the effect of different fermentation temperature on the microbial community in the anaerobic sludge, and this article also found that Acidaminobacter at 35 °C, Proteiniphilum and Lutispor at 42 °C, and Gelria at 55 °C were the major producers of proteinases, while bacterial strains of polysaccharide fermentation belonged to the genus of Macellibacteroides and Paludibacter at 35 °C, the dominant communities at 42 °C was Salmonella, and the predominant communities at 55 °C was Tepidimicrobium. All of the above studies have studied the changes in the structure of the microbial community in the fermentation system through high-throughput sequencing technology and did not involve systematic analysis of the structure and function of the microbial community.
Since the global supply of renewable energy is in short supply, the biogas industry has developed rapidly. At present energy plants are widely used in the biogas industry. However, the currently used energy plants have the disadvantages that they cannot survive in low-temperature environments. But ADL-1, as a new energy plant that can survive at low temperatures, has not been completely studied so far. Therefore, this article reveals the relationship between the structure and function of microbial communities in ALD-1 during TAD of biogas. These results would contribute to the future research of TAD of energy plants.
Materials and Methods
Thermophilic Anaerobic Digestion and Samples
“Lvzhou No. 1”used in this study was obtained from the planting base of The National Engineering Research Center of JUNCAO Technology. For TAD, eight 2000-L biogas digesters were fed with “Lvzhou No. 1” with different growth periods (1 month to 8 months) and kept at thermostatic incubator at 55 °C. And then various physical and chemical parameters including pH of the slurry, methane concentration, and biogas production were recorded every day during the TAD process. After the digestion was finished, all the biogas slurry was taken out and centrifuged (GL-12B, Shanghai Anting Scientific Instrument Factory, Shanghai, China) at 10000 g for 5 min. Then the collected supernatant was filtered with a 0.22-μm water filter (Tianjin Jinteng Experimental Equipment Co., Ltd., China), and the filtrate was stored at − 20 °C until use. The TAD residues were washed with sterile water, dried and crushed, and then used for determining their lignin contents, the hemicellulose contents, and the cellulose contents.
Determination of Biogas Production and Methane Concentration
The biogas yields were determined by water replacement. The methane concentration in biogas was determined by a biogas analysis meter (BX568, Henan Hanwei Electronics Co., Ltd.).
Determination of Volatile Fatty Acids (VFA) Content and pH
The pH was measured by pH meter (Mettler Toledo, FiveEasy Plus, Switzerland). VFA content was determined by gas chromatography (GC7890, Agilent, USA) using the HP-INNOWax (30 m*0.32 mm ID*0.25 um) Column and FID detector. The flow rate of N2 (carrier gas) was 19.0 mL/min, the split ratio was 1:10, the H2 flow rate was 30 mL/min, and the air flow rate was 300 mL/min. Both detectors were kept at 240 °C. The temperature program was as follows: 100 °C for 0.5 min, 4 °C/min heating rate to 150 °C, and then 5 °C/min heating rate to 180 °C. And each sample was measured in triplicate.
Determination of Lignocellulose Content
The NREL laboratory analytical protocol [21] was used to quantify cellulose, hemicellulose, and lignin in the feedstock and TAD residues. The degradation rates of cellulose, hemicellulose, and lignin were given by:
XC = (MC0 – MC)/MC0
XH = (MH0 – MH)/MH0
XL = (ML0 – ML)/ML0
Here XC stands for cellulose degradation rate; MC0 refers to the original weight of cellulose in raw materials (before degradation); MC refers to the weight of cellulose in the TAD residue (after degradation); XH stands for hemicellulose degradation rate; MH0 refers to the original weight of hemicellulose in raw materials (before degradation); MH refers to the weight of hemicellulose in the TAD residue (after degradation); XL stands for lignin degradation rate; ML0 refers to the original weight of lignin in raw materials (before degradation); ML refers to the weight of lignin in the TAD residue (after degradation).
DNA Extraction
On day 1, 3, and 7, 10 mL of mixed thoroughly slurry and solid residue samples were taken out. Then the samples were centrifuged at 12,000 rpm for 10 min at 4 °C, and the pellets were collected. Total community DNA was extracted from the pellets using the CTAB method [22].
The detection of composition of microbial communities was conducted according to the protocols described previously [23]. The V4 region of the 16S rRNA gene in bacteria was amplified by PCR with the primer set of 515F (5’-GTG CCA GCM GCC GCG GTA A-3’) and 806R (5’-GGA CTA CHV GGG TWT CTA AT-3’) [23]. The primers for V4 region of archaeal 16S rRNA gene were 5’-CAG YMG CCR CGG KAA HAC C-3’ and 5’-GGA CTA CNS GGG TMT CTA AT-3’. The PCR products were detected by 2% agarose gel electrophoresis, and the aimed strips were recovered by GeneJET Glue Recovery Kit (Thermo Fisher Scientific company). The library was constructed using Thermo Fisher’s Ion Plus Fragment Library Kit 48 rxns library, and the sequencing was carried out by the Life Ion S5™ platform (Beijing Novogene Technology Co., Ltd. Beijing).
Merging of pairs of reads from the original sequenced DNA fragments was conducted by Cutadapt [24]. The Cutadapt software package was used to filter the raw tags sequences and get clean tags. The UCHIME was used to remove chimera sequences and get effective tags [25, 26]. The operational taxonomic units (OTUs) were determined from effective tags by UPARSE at the threshold of 97% [27]. Mothur method [28] and SSUrRNA database [29] were used to annotate the OTUs representative sequences (setting threshold of 0.8~1), and the community composition of each sample was statistically analyzed at various taxonomic levels. Homogenization of the sequence was performed based on the minimum amount of data in the sample. The alpha diversity analysis, including rarefaction curve, Chao1, and Shannon indices, was then conducted by QIIME [23]. All sequence data have been deposited in the NCBI Sequence Read Archive database; the number is PRJNA589176.
Biological Information Analysis
The canonical correspondence analysis (CCA) of bacteria and archaea communities at the genus level with acetic acid, propionic acid, butyric acid, gas production, methane production, cellulose degradation rate, hemicellulose degradation rate, lignin degradation rate, as well as pH was conducted by using software Canoco 4.5 [30,31,32].
Results
Determination of the Best Growth Stage of Lvzhou No.1
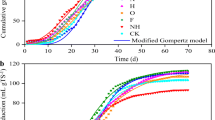
Composition of ADL-1 at different growth stage is shown in Table 1. The monthly yields of biogas of TAD using ADL-1 with different growth stages (from 1 month to 8 months) were compared. As shown in Fig. 1, results showed that the average monthly biogas and methane accumulation of dry matter first increased then decreased over time. And the average monthly biogas accumulation reached the highest value of 49,222.1 mL per plant using ALD-1 of 3-month growth period, which was chosen as raw material in the further experiment.
Changes in Various Components During Anaerobic Digestion
All anaerobic digestion reactions were carried out at 55 °C, and the changes in all components during the fermentation were summarized in Fig. 2. Among VFA detected, acetic acid was found to have the highest content, which gradually decreased as the fermentation progressed (Fig. 2a). Both acetic acid and butyric acid were present throughout the fermentation process, but propionic acid was not detected in the later stages of fermentation. The TAD process lasted for 10 days with the cumulative biogas and methane yields of 312.7 mL/g VS and 231 mL/g VS, respectively. The daily production of biogas had the same trend as methane (Fig. 2b). During the first 3 days, daily production of biogas and methane showed the gradual upward trend and reached the maximum on the third day, which were 47.0 mL/g TS and 32.9 mL/g TS, respectively. They gradually decreased from the third day to the ninth day but suddenly fell at the last day. The main components in the TAD residues were cellulose, hemicellulose, and lignin (Fig. 2d). Cellulose and lignin accounted for the majority, and hemicellulose was less. And hemicellulose had the highest degradation rate, while lignin had the lowest degradation rate (Fig. 2c). The degradation rates of cellulose, hemicellulose, and lignin were 27.99%, 41.78%, and 14.46%, respectively.
Diversity of the Microbial Communities
Microbial communities of all the samples were analyzed by the high-throughput sequencing of the 16S rRNA genes. Raw reads of the bacteria and archaea in each sample were approximately 80,000 (Table 2). The rationality of the amount of sequencing data and the richness of the species in the sample could be indicated by the rarefaction curve. When the curve tends to be flat, the abundance of sequencing data was gradually reasonable (Fig. 3). Good’s coverage ranged from 99 to 100% (Tables 3 and 4). Moreover, Shannon diversity of bacteria ranged from 5.8 to 6.7 (Table 3), while Shannon diversity of archaea ranged from 2.3 to 3.5 (Table 4).
Structure of the Microbial Communities
During TAD, the bacteria at the phylum level did not change much (Fig. 4a). Firmicutes were dominant (60–65%) throughout the fermentation process. However, it decreased slightly on the third day of fermentation and increased in the later stage. Proteobacteria was the second dominant bacteria (5–12%) with slight decrease in the late fermentation. However, at the genus level, the distribution of each genus was different (Fig. 4b). Sedimentibacter had the highest abundance (10%) on the third day of fermentation. Tepidiphilus had a relatively rich abundance at the initial stages and peak period of gas production but decreased in the later stages. In contrast, the population of Petrimonas, Hydrogenispora, and Gelria increased at the later stage of fermentation. It is worth noting that Clostridium sensu stricto 1, Ruminococcus 1, and Clostridium sensu stricto 8 were found to have the highest content in the early stage of fermentation, but there were only little amount at the peak of gas production and disappeared in the late stage of fermentation.
Taxonomic classification of the bacterial communities at phylum and genus level. a Bacterial phylum. b Bacterial genus. Note: BacD1 indicates bacteria on the first day of fermentation, BacD3 indicates bacteria on the third day of fermentation, BacD7 indicates bacteria on the seventh day of fermentation, and abc indicates three groups of parallel
As shown in Fig. 5, archaea present in thermophilic anaerobic digestion were mainly divided into two orders (Methanomicrobiales and Methanosarcinales), which have been regarded to involve in hydrogenotrophic and aceticlastic methanogenesis. Methanomicrobiales was the dominant order in methanogenesis, but it had a slight decline during peak gas production. While Methanosarcinales was the most abundant during the peak period of gas production, the content was decreased in the later period. At the genus level, Methanoculleus was the dominant genus, accounting for almost 90% at the initial stages, but its content decreased during the peak period of gas production. Methanosarcina had the highest content at the peak of gas production, and it was just the opposite of the trend of Methanoculleus.
Taxonomic classification of the archaea communities at order and genus level. (a) Archaea order; (b) Archaea genus. Note: ArcD1 indicates archaea on the first day of fermentation, ArcD3 indicates archaea on the third day of fermentation, ArcD7 indicates archaea on the seventh day of fermentation, and abc indicates three groups of parallel
Biological Information Analysis
The CCA was conducted to investigate the relationship of bacterial communities and VFA and gas production. Results showed that methane production had a strong correlation with Gelria, butyric acid was related to Clostridium sensu stricto 1, and propionic acid was associated with Petrimonas (Fig. 6a). In addition, CCA results of archaea communities and VFA and gas production suggested that gas production was highly correlated with Methanoculleus, and butyric acid was related to Candidatus_Methanoperedens (Fig. 6b).
CCA analysis relating performance parameters (acetic acid, propionic acid, butyric acid, gas production, methane production, cellulose degradation rate, hemicellulose degradation rate, lignin degradation rate, pH) with changes in OTUs of bacterial (a) and archaeal (b) phylotypes at genus level in AD. Note: Bacterial 1, Sedimentibacter; 2, Tepidiphilus; 3, Gelria; 4, Clostridium sensu stricto 1; 5, Petrimonas; 6, Ruminococcus 1; 7, Hydrogenispora; 8, Clostridium sensu stricto 8; 9, Pseudomonas Archaeal; 1, Methanoculleus; 2, Methanosarcina; 3, Methanobacterium; 4, Methanocella; 5, Candidatus Methanoperedens; 6, Methanosaeta; 7, Methanothermobacter; A, acetic acid; B, propionic acid; C, butyric acid; D, gas production; E, methane production; F, cellulose degradation rate; G, hemicellulose degradation rate; H, Lignin degradation rate; I, pH
Discussion
Our results showed that the VFA content was highest on the first day of anaerobic digestion, possibly because bacteria in the microbial communities are more active. In the early stage of fermentation, the microbial communities first consumed complex organic substances such as carbohydrates, crude protein, etc., originally present in the raw materials. Microbial communities could convert these organic compounds into VFA. With the progress of anaerobic digestion, the VFA content gradually decreased, and the amount of biogas gradually increased, indicating that VFA was used by microorganisms to produce methane [33,34,35]. Our results indicated that the biogas anaerobic digestion process mainly consisted of two stages, namely, the acid-producing stage and the methanogenic stage. The acid generation stage was mainly microorganism first converted complex organic substances such as proteins and carbohydrates into simple monosaccharides, long-chain fatty acids, and amino acids. Monosaccharides, long-chain fatty acids, and amino acids are then further decomposed into acetic acid, H2, and CO2. The methanogenic stage was mainly methanogen using acetic acid and H2 to produce methane. This phenomenon was consistent with the result of the study conducted by Lauwers [36]. When these complex compounds (carbohydrates, crude protein, etc.) were decomposed by fermentation, the lignocellulose content in the TAD residue went up [37, 38]. As the fermentation progressed, cellulose, hemicellulose, and lignin are continuously consumed by the microbial communities. As polysaccharides in nature, cellulose and hemicellulose are both components of the plant cell wall. The difference is that the polymerization unit of cellulose is only glucose, while the polymerization unit of hemicellulose is not only one type, including xylose, rhamnose, mannose, etc. This leads to a more branched chemical structure of hemicellulose, which could be more easily hydrolyzed than cellulose under acidic conditions. Although cellulose could not be easily degraded, in the middle stage of fermentation, there were a large number of acetogenic bacteria; they used cellulose as substrates to decompose and produce acetic acid by fermentation, and then acetic acid would be converted to methane [39, 40]. As a biopolymer with a three-dimensional network structure and a relatively high molecular weight, lignin had different degradation degrees during TAD. Lignin had many active functional groups which may also lead to slight sulfonation and lead to its degradation in this condition. At the same time, the acetogens could also play role in partial degradation of lignin. But cellulose, hemicellulose, and lignin fractions were almost constant after day 6 (Fig. 2d). This slight but not obvious reduction in the lignocellulose content after sixth day possibly resulted from the decreasing activities of the microbial communities (including the ability to degrade the substrate and the ability to produce the biogas) which could be seen from Fig 2 c and b. This result was similar to the results reported by Neshat SA [41].
As for the analysis of microbial community diversity, high Shannon index and Chao1 indicated that bacterial and archaea taxa were very rich in these habitats [42].
As a genus of Firmicutes, Gelria had a higher abundance during the late fermentation period (Fig. 4b). It was worth noting that Firmicutes were especially common in TAD systems [43, 44], which suggested that Gelria would play an important role in TAD systems. As a genus of Clostridium, Clostridium sensu stricto 1 was most abundant in the early stages of gas production and during the peak production. At this stage, VFA also existed in large quantities. The main function of Clostridium was to further degrade the hydrolyzed products into small molecules such as alcohols, organic acids, carbon dioxide, hydrogen, and ammonia. When in the late stage of digestion, the content of small molecules was reduced. This could help explain the fact that the number of these bacteria was reduced at the later stages of fermentation.
Petrimonas was a hydrogen-producing acetogen, and CCA results showed that this genus had a strong correlation with propionic acid. Also it was found to involve in methanogenesis as a methanogen. Our results showed that Petrimonas population increased throughout the TAD. Due to its capability of biogas (hydrogen and methane) production under the anaerobic condition, Petrimonas has aroused much attention as microbial fuel cells [8, 45].
Methanomicrobiales were very common in bioreactors, landfills, and wastewater. Methanomicrobiales belong to hydrogen trophic methanogens. They were the most important methanogens in anaerobic sludge [46]. Although they had declined during the peak period of gas production, they still were dominant population. As another common methanogens, the number of Methanosarcinales increased during peak period of gas production, which indicated that Methanosarcinales were related to gas production. High abundance of Methanosarcinales would be due to their substrate versatility, acid tolerance, and higher specific growth rates [47, 48]. Previous studies have shown that when acetic acid content dropped, Methanosarcinales content would also be reduced [47,48,49]. However, different results were found in our study, which showed that the reduction in acetic acid content did not lead to the decrease of Methanosarcinales content. On the contrary, Methanosarcinales increased in the case of a decline in acetic acid content during the peak period of gas production.
Methanoculleus was found to be the most dominant archaea in TAD reactors. In taxonomy, Methanoculleus was a genus of microbes within the family Methanomicrobiaceae. The species of the genus Methanoculleus usually live in bioreactors, landfills, and wastewater. Methanoculleus could convert hydrolyzed products into small molecules, volatile fatty acids, carbon dioxide, hydrogen, etc. It was mentioned above that Methanoculleus could use ethanol and some secondary alcohols as electron donors to produce methane [50]. Kougias et al. detected samples from 22 bioreactors and found that Methanoculleus was one of the archaea with high abundance [51]. Methanoculleus was the most widely represented in the methanogenic phase of the four stages of the anaerobic digestion system [52,53,54,55]. Results of the CCA showed that Methanoculleus had an irreplaceable role in the methanogenesis phase of biogas fermentation.
Methanosarcina belonging to euryarchaeote archaea genus live on the surface of the Earth, groundwater, deep sea vents, and animal digestive tracts. These single-celled organisms and anaerobic methanogens produce methane using all three metabolic pathways for methanogenesis. They were capable of using no less than nine methanogenic substrates to produce methane, including acetate [56, 57]. Since Methanosarcina reactors operate at temperatures ranging from 35 to 55 °C and pH ranges of 6.5–7.5, this genus would play a very important role in TAD reactors.
Conclusion
In conclusion, the present study focused on dynamic changes in microbial community structure and function during on TAD. The use of ADL-1 energy crops as new raw materials not only can be widely planted but also can increase biogas production and promote the rapid development of biogas industry. Furthermore, the results of the correlation between microbial communities and VFA and gas production during TAD found that bacteria could promote the production of VFA, archaea were positively related to produce the methane, and the two complement each other. Therefore, the results of this study revealed the dynamic changes in the structure and function of microbial communities during TAD, providing a theoretical basis for the development of biogas technology.
References
Deviram G, Mathimani T, Anto S, Ahamed TS, Ananth DA, Pugazhendhi A (2019) Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J Clean Prod 235:119770. https://doi.org/10.1016/j.jclepro.2019.119770
Saravanan AP, Mathimani T, Deviram G, Rajendran K, Pugazhendhi A (2018) Biofuel policy in India: a review of policy barriers in sustainable marketing of biofuel. J Clean Prod 193. https://doi.org/10.1016/j.jclepro.2018.05.033
Dharmaraja J, Shobana S, Arvindnarayan S, Vadivel M, Atabani AE, Pugazhendhi A, Kumar G (2020) Biobutanol from lignocellulosic biomass: bioprocess strategies. Lignocellulosic Biomass to Liquid Biofuels:169–193. https://doi.org/10.1016/B978-0-12-815936-1.00005-8
Prabakar D, Manimudi VT, Suvetha KS, Sampath S, Mahapatra DM, Rajendran K, Pugazhendhi A (2018) Advanced biohydrogen production using pretreated industrial waste: outlook and prospects. Renew Sust Energ Rev 96:306–324. https://doi.org/10.1016/j.rser.2018.08.006
Anto S, Mukherjee SS, Muthappa R, Mathimani T, Deviram G, Kumar SS, Verma TN, Pugazhendhi A (2019) Algae as green energy reserve: technological outlook on biofuel production. Chemosphere 125079. https://doi.org/10.1016/j.chemosphere.2019.125079
Pugazhendhi A, Mathimani T, Varjani S, Rene ER, Kumar G, Kim SH, Ponnusamy VK, Yoon JJ (2019) Biobutanol as a promising liquid fuel for the future - recent updates and perspectives. Fuel 253:637–646. https://doi.org/10.1016/j.fuel.2019.04.139
Herrmann A (2012) Biogas production from maize: current state, challenges, and prospects. 1. Methane yield potential. Bioenerg Res 5:1027–1042. https://doi.org/10.1007/s12155-012-9202-6
Nges IA, Escobar F, Fu X, Björnsson L (2012) Benefits of supplementing an industrial waste anaerobic digester with energy crops for increased biogas production. Waste Manag 32:0–59. https://doi.org/10.1016/j.wasman.2011.09.009 53
Herrmann C, Idler C, Heiermann M (2016) Biogas crops grown in energy crop rotations: linking chemical composition and methane production characteristics. Bioresour Technol 206:23–35. https://doi.org/10.1016/j.biortech.2016.01.058
Nguyen NS, Soda S, Ishigaki T, Ike M (2012) Microorganisms in landfill bioreactors for accelerated stabilization of solid wastes. J Biosci Bioeng 114:243–250. https://doi.org/10.1016/j.jbiosc.2012.04.007
Li X, Liu Y, Zhang X, Ge C, Piao R, Wang W, Cui Z, Zhao H (2017) Evaluation of biogas production performance and dynamics of the microbial community in different straws. J Microbiol Biotechnol 27:524–534. https://doi.org/10.4014/jmb.1608.08062
Sun W, Sun XX, Cupples AM (2014) Identification of Desulfosporosinus as toluene-assimilating microorganisms from a methanogenic consortium. Int Biodeterior Biodegradation 88:13–19. https://doi.org/10.1016/j.ibiod.2013.11.014
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. https://doi.org/10.1021/ac071042
Azman S, Khadem AF, Lier JBV, Zeeman G, Plugge CM (2015) Presence and role of anaerobic hydrolytic microbes in conversion of lignocellulosic biomass for biogas production. Crit Rev Env Sci Tec 45:2523–2564. https://doi.org/10.1080/10643389.2015.1053727
Hameed SA, Riffat R, Li B, Naz I, Badshah M, Ahmed S, Ali N (2019) Microbial population dynamics in temperature phased anaerobic digestion of municipal wastewater sludge. J Chem Technol Biotechnol 94:1816–1831. https://doi.org/10.1002/jctb.5955
Xiao BY, Zhang WZ, Yi H, Qin Y, Wu J, Liu JX, Li YY (2019) Biogas production by two-stage thermophilic anaerobic co-digestion of food waste and paper waste: effect of paper waste ratio. Renew Energ 132:1301–1309. https://doi.org/10.1016/j.renene.2018.09.030
Yucai L, Ning LI, Jinling G, Dachun G, Xiaofen W, Zongjun C (2016) Microbial community structure variation during the startup of culture enrichment under thermophilic condition inoculated with a mesophilic community for anaerobic digestion. Acta Sci Circumst 36:1986–1997. https://doi.org/10.13671/j.hjkxxb.2015.0648
Tian GL, Zhang WD, Dong MH, Yang B, Zhu R, Yin F, Zhao XL, Wang YX, Xiao W, Wang Q, Cui XL (2017) Metabolic pathway analysis based on high-throughput sequencing in a batch biogas production process. Energy 139:571–579. https://doi.org/10.1016/j.energy.2017.08.003
Xiao Z, Lin M, Fan J, Chen Y, Zhao C, Liu B (2018) Anaerobic digestion of spent mushroom substrate under thermophilic conditions: performance and microbial community analysis. Appl Microbiol Biotechnol 102:499–507. https://doi.org/10.1007/s00253-017-8578-9
Chen H, Chang S (2017) Impact of temperatures on microbial community structures of sewage sludge biological hydrolysis. Bioresour Technol 245:502–510
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of extractives in biomass. National Renewable Energy Laboratory Technical Report 510:42619
Siming H, Jiqiang D, Xueni L, Xiaowei D, Fu X, Feifu L (2005) Optimized CTAB protocol for extracting the total DNA of ramie. Xibei Zhiwu Xuebao 25:2193–2197
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Martin M (2011) Cut adapt removes adapter sequences from high-throughput sequencing reads. EMBnet 17:10–12
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methé B, DeSantis TZ, Human MC, Petrosino JF, Knight R, Birren BW (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504. https://doi.org/10.1101/gr.112730.110
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible in ARB. Appl Environ Microbiol 72:5069–5072
Gonzalez-Martinez A, Garcia-Ruiz MJ, Rodriguez-Sanchez A, Osorio F, Gonzalez-Lopez J (2016) Archaeal and bacterial community dynamics and bioprocess performance of a bench-scale two-stage anaerobic digester. Appl Microbiol Biotechnol 100:6013–6033. https://doi.org/10.1007/s00253-016-7393-z
Castellano-Hinojosa A, Armato C, Pozo C, Gonzalez-Martinez A, Gonzalez-Lopez J (2018) New concepts in anaerobic digestion processes: recent advances and biological aspects. Appl Microbiol Biotechnol 102:5065–5076. https://doi.org/10.1007/s00253-018-9039-9
Software canoco 4.5. http://www.canoco4.5.com/
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energ Combust 42(1):35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Zhao Y, Xu C, Ai S, Wang H, Gao Y, Yan L, Mei Z, Wang W (2019) Biological pretreatment enhances the activity of functional microorganisms and the ability of methanogenesis during anaerobic digestion. Bioresour Technol 121660. https://doi.org/10.1016/j.biortech.2019.121660
Liu T, Zhou X, Li Z, Wang X, Sun J (2019) Effects of liquid digestate pretreatment on biogas production for anaerobic digestion of wheat straw. Bioresour Technol 280:345–351. https://doi.org/10.1016/j.biortech.2019.01.147
Lauwers J, Appels L, Thompson IP, Degrève J, Van Impe JF, Dewil R (2013) Mathematical modelling of anaerobic digestion of biomass and waste: power and limitations. Prog Energ Combust 39:383–402. https://doi.org/10.1016/j.pecs.2013.03.003
Bahmani MA, Shafiei M, Karimi K (2016) Anaerobic digestion as a pretreatment to enhance ethanol yield from lignocelluloses. Process Biochem 51:1256–1263. https://doi.org/10.1016/j.procbio.2016.05.012
Kumar R, Sharma RK, Singh AP (2017) Cellulose based grafted biosorbents - journey from lignocellulose biomass to toxic metal ions sorption applications - a review. J Mol Liq 232:62–93. https://doi.org/10.1016/j.molliq.2017.02.050
Li W, Khalid H, Zhu Z, Zhang R, Liu G, Chen C, Thorin E (2018) Methane production through anaerobic digestion: participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl Energ 226:1219–1228. https://doi.org/10.1016/j.apenergy.2018.05.055
Pore SD, Shetty D, Arora P, Maheshwari S, Dhakephalkar PK (2016) Metagenome changes in the biogas producing community during anaerobic digestion of rice straw. Bioresour Technol 213:50–53. https://doi.org/10.1016/j.biortech.2016.03.045
Neshat SA, Mohammadi M, Najafpour GD, Lahijani P (2017) Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew Sust Energ Rev 79:308–322. https://doi.org/10.1016/j.rser.2017.05.137
Han S, Liu Y, Zhang S, Luo G (2016) Reactor performances and microbial communities of biogas reactors: effects of inoculum sources. Appl Microbiol Biotechnol 100:987–995. https://doi.org/10.1007/s00253-015-7062-7
Gieg LM, Davidova IA, Duncan KE, Suflita JM (2010) Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ Microbiol 12:3074–3086. https://doi.org/10.1111/j.1462-2920.2010.02282.x
Mbadinga SM, Li KP, Zhou L, Wang LY, Yang SZ, Liu JF, Gu JD, Mu BZ (2012) Analysis of alkane-dependent methanogenic community derived from production water of a high-temperature petroleum reservoir. Appl Microbiol Biotechnol 96:531–542. https://doi.org/10.1007/s00253-011-3828-8
Sun R, Zhou A, Jia J, Liang Q, Liu Q, Xing D, Ren N (2015) Characterization of methane production and microbial community shifts during waste activated sludge degradation in microbial electrolysis cells. Bioresour Technol 175:68–74. https://doi.org/10.1016/j.biortech.2014.10.052
Grabowski A, Tindall BJ, Bardin V, Blanchet D, Jeanthon C (2005) Petrimonas sulfuriphila gen. nov., sp nov., a mesophilic fermentative bacterium isolated from a biodegraded oil reservoir. Int J Syst Evol Microbiol 55:1113–1121. https://doi.org/10.1099/ijs.0.63426-0
Wang X, Zhu M, Li F, Zhang C, Zhu X (2018) Long-term effects of multi-walled carbon nanotubes on the performance and microbial community structures of an anaerobic granular sludge system. Appl Microbiol Biotechnol 102:9351–9361. https://doi.org/10.1007/s00253-018-9273-1
Leite AF, Janke L, Lv Z, Harms H, Richnow HH, Nikolausz M (2015) Improved monitoring of semi-continuous anaerobic digestion of sugarcane waste: effects of increasing organic loading rate on methanogenic community dynamics. Int J Mol Sci 16:23210–23226. https://doi.org/10.3390/ijms161023210
Zealand AM, Mei R, Papachristodoulou P, Roskilly AP, Liu WT, Graham DW (2018) Microbial community composition and diversity in rice straw digestion bioreactors with and without dairy manure. Appl Microbiol Biotechnol 102:8599–8612. https://doi.org/10.1007/s00253-018-9243-7
Maus I, Wibberg D, Winkler A, Pühler A, Schnürer A, Schlüter A (2016) Complete genome sequence of the methanogen Methanoculleus bourgensis BA1 isolated from a biogas reactor. Genome Announc 4:e00568–e00516. https://doi.org/10.1128/genomeA.00568-16
Kougias PG, Campanaro S, Treu L, Zhu X, Angelidaki I (2017) A novel archaeal species belonging to Methanoculleus genus identified via de-novo assembly and metagenomic binning process in biogas reactors. Anaerobe 46:23–32. https://doi.org/10.1016/j.anaerobe.2017.02.009
Schlüter A, Bekel T, Diaz NN, Dondrup M, Eichenlaub R, Gartemann KH, Krahn I, Krause L, Krömeke H, Kruse O, Mussgnug JH, Neuweger H, Niehaus K, Pühler A, Runte KJ, Szczepanowski R, Tauch A, Tilker A, Viehöver P, Goesmann A (2008) The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J Biotechnol 136:77–90. https://doi.org/10.1016/j.jbiotec.2008.05.008
Jaenicke S, Ander C, Bekel T, Bisdorf R, Droge M, Gartemann KH, Junemann S, Kaiser O, Krause L, Tille F, Zakrzewski M, Puhler A, Schluter A, Goesmann A (2011) Comparative and joint analysis of two metagenomic datasets from a biogas fermenter obtained by 454-pyrosequencing. PLoS One 6:e14519. https://doi.org/10.1371/journal.pone.0014519
Rademacher A, Zakrzewski M, Schlüter A, Schönberg M, Szczepanowski R, Goesmann A, Pühler A, Klocke M (2012) Characterization of microbial biofilms in a thermophilic biogas system by high-throughput metagenome sequencing. FEMS Microbiol Ecol 79:785–799. https://doi.org/10.1111/j.1574-6941.2011.01265.x
Zakrzewski M, Goesmann A, Jaenicke S, Junemann S, Eikmeyer F, Szczepanowski R, Al-Soud WA, Sorensen S, Puhler A, Schluter A (2012) Profiling of the metabolically active community from a production-scale biogas plant by means of high-throughput metatranscriptome sequencing. J Biotechnol 158:248–258. https://doi.org/10.1016/j.jbiotec.2012.01.020
Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, Calvo S, Engels R, Smirnov S, Atnoor D, Brown A, Allen N, Naylor J, Stange-Thomann N, DeArellano K, Johnson R, Linton L, McEwan P, McKernan K, Talamas J, Tirrell A, Ye W, Zimmer A, Barber RD, Cann I, Graham DE, Grahame DA, Guss AM, Hedderich R, Ingram-Smith C, Kuettner HC, Krzycki JA, Leigh JA, Li W, Liu J, Mukhopadhyay B, Reeve JN, Smith K, Springer TA, Umayam LA, White O, White RH, Conway De Macario E, Ferry JG, Jarrell KF, Jing H, Macario AJ, Paulsen I, Pritchett M, Sowers KR, Swanson RV, Zinder SH, Lander E, Metcalf WW, Birren B (2002) The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res 12:532–542. https://doi.org/10.1101/gr.223902
Fontana A, Patrone V, Puglisi E, Morelli L, Bassi D, Garuti M, Rossi L, Cappa F (2016) Effects of geographic area, feedstock, temperature, and operating time on microbial communities of six full-scale biogas plants. Bioresour Technol 218:980–990. https://doi.org/10.1016/j.biortech.2016.07.058
Funding
This work was financially supported by grants from the Natural Science Foundation of China (31370146), Fujian Agriculture and Forestry University International Cooperation and Exchange Project (No. KXG15001A), Sub Project of National Science and Technology Support Program (2014BAD15B01-6), and Key Research and Development Plan of Jiangxi Province (20171ACF60005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, Y., Xie, C., Wang, X. et al. Thermophilic Anaerobic Digestion of Arundo donax cv. Lvzhou No. 1 for Biogas Production: Structure and Functional Analysis of Microbial Communities. Bioenerg. Res. 13, 866–877 (2020). https://doi.org/10.1007/s12155-020-10105-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10105-y