Abstract
Lignocellulosic biorefineries have tonnage and throughput requirements that must be met year round, and there is no single feedstock available in any given region that is capable of meeting the price and availability demands of the biorefineries. Ionic liquid (IL) pretreatment with certain ILs is receiving significant attentions as a potential process that enables fractionation of a wide range of feedstocks and produces high yields of fermentable sugars suitable for biofuel production. Building on the large-scale demonstration of a single herbaceous feedstock (switchgrass), this work extends scale-up of IL pretreatment to woody (eucalyptus) and mixed feedstock (mixtures of two) by 30-fold, relative to the bench scale (6 vs 0.2 L) at 10 % solid loading. The mixed feedstock recovered similar yields of glucan (99.7 %), xylan (62.8 %), and lignin (59.9 %) as switchgrass and eucalyptus at 6-L scale operation, and results of all three feedstocks are better than those obtained from small-scale studies. By integrating the process of IL pretreatment with efficient and scalable homogenization, washing, and product recovery system, IL contents in the recovered materials were decreased to 0.2 %, mitigating the risk to downstream enzymatic saccharification and microbial fermentation. Results indicate that mixed feedstock are viable and valuable resource to consider when assessing biomass availability and affordability for lignocellulosic biorefineries. This scale-up evaluation demonstrates that IL pretreatment technology is feedstock agnostic and can be effectively scaled to larger operations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Technology is rapidly advancing to utilize agricultural residues, perennial grasses, woody perennials, and forest products for the production of biofuels and chemicals via a renewable lignocellulosic platform [1]. Two key factors in the successful large-scale production of cellulosic biofuels are efficient and cost-effective pretreatment and a consistent and stable supply of feedstock [2, 3].
To produce the fermentable sugars required for liquid biofuel production via the biochemical conversion route, pretreatment is a necessary step to overcome the recalcitrance of lignocellulose and render the polysaccharides within the plant cell walls amenable to enzymatic saccharification [4]. Pretreatment represents one of the most significant costs from an operational perspective, and researchers are developing current as well as novel biomass pretreatments to help drive down the overall costs of the biorefinery [5, 6]. For the current common biomass pretreatments (e.g., dilute acids, auto hydrolysis, dilute alkaline, steam explosion, and lime), one major cause of high expense, and limited deployment thus far, is that they are typically only effective on a narrow range of the available lignocellulosic feedstocks [7, 8]. For instance, while dilute acid and ammonia fiber expansion may be relatively effective in pretreating grasses and corn stovers, they are not particularly effective in pretreating softwoods and hardwoods [5, 7, 9]. The challenges of “fine tuning” of pretreatment for each type of feedstock can highly limit the biorefinery’s ability to efficiently process diverse biomass materials [9].
Feedstock diversity varies significantly from region to region in the USA, and each feedstock within a given region varies from year to year based on weather conditions, handling, storage, and crop variety, all of which increases the critical challenges of maintaining a consistent and stable supply of sustainable feedstocks from a variety of sources [10]. The reliance of pretreatment on an optimal feedstock to meet the requirements of any given biorefinery should be considered at high risk in terms of feedstock availability and affordability. To maintain productivity and profitability, a biorefinery must establish a robust pretreatment technology that can handle a wide range of feedstocks, most likely, in a mixed input stream with minimal impact on performance, sugar production, and fuel titers [10, 11]. Unfortunately, the majority of the research in the biomass conversion field has typically focused on the conversion of a single feedstock in the past, and only very recently have researchers begun to focus on the conversion of mixed feedstocks into fermentable sugars and fuels [2, 3, 5, 10].
Among various leading pretreatment technologies, ionic liquid (IL) pretreatment with certain combinations of anions and cations has shown to efficiently fractionate biomass and provide sugar substrates for the production of biofuels and renewable chemicals [12–15]. Previous work illustrated several favorable properties of IL pretreatment for biomass deconstruction at the bench scale. These include biomass dissolution and disruption, reduced cellulose crystallinity, reduced lignin content, enhanced biomass saccharification, and low toxicity and environmental impact [16–20]. Particularly, one uniqueness of IL pretreatment is the capability of efficiently handling a wide range of single feedstocks such as softwood, hardwood, herbaceous materials, and agricultural residues [5, 9]. In the light of these observations, ionic liquids have been further demonstrated to process mixed feedstock input with sugar yields equivalent to that of singular feedstocks [5, 10]. However, most of the IL pretreatment data to date were obtained at low solid loading (3–10 %) and at the 10 to 200 mL level of operation [9, 10, 21], which cannot be directly translated to industrially relevant scales. Thus, liter-scale experiments are a necessary intermediate step between bench and pilot scales in order to identify operational parameters and potential problems associated with scale-up prior to pilot-scale and full-scale commercial operations.
Recently, our group at the Advanced Biofuels Process Demonstration Unit, a facility funded by the DOE-Office of Energy Efficiency and Renewable Energy, in conjunction with DOE-Office of Science funded Joint BioEnergy Institute (JBEI), have demonstrated for the first time in the research community that IL pretreatment of switchgrass can be effectively scaled by 600-fold (from 0.01 to 6 L) with no fundamental issues in performance, indicating that there is a path forward to volumes relevant to biorefineries [11]. The results also provide clear evidence of enzymatic inhibition, which is magnified by the fact that pretreated materials were not washed completely prior to hydrolysis, and thus, the inhibitors generated during pretreatment, namely the ionic liquid 1-ethyl-3-methylimidaolzium acetate, will persist into hydrolysis and the downstream steps without further purification, recovery, and recycle of the IL.
As a continuation of the collaborative work on the scale-up demonstration of the IL conversion technology, the current study aims to (1) evaluate the response and scaling effects of mixed feedstock and compare with two single feedstocks, i.e., switchgrass and eucalyptus, at liter scales; (2) optimize the process by integrating with efficient and scalable washing and recovery system; (3) track the material balance for the product/solvent recovery; and (4) facilitate the further process integration of downstream unit operations.
Materials and Methods
Raw Materials
The two ground feedstocks used in this study, switchgrass and eucalyptus, were kindly provided by Idaho National Laboratory (INL; ID, USA). The switchgrass was grown near Guymon (OK, USA), harvested in October 2010, delivered to INL in the form of 3 × 4 × 8-ft bales, and have been stored since delivery under tarps. The switchgrass bales were ground using a Vermeer BG480 grinder (Vermeer, IA, USA) designed for processing up to 4 × 4-ft bales. A 1-in. screen was used for these grinds. The eucalyptus was harvested in Davis (CA, USA) with tree sizes ranging up to 18 in. in diameter. The trees were delimbed for shipment, but not debarked. Then, these trees were shredded using a tub grinder as whole trees by Wilcox Logging Inc. (ID, USA) and stored at the INL in piles on an asphalt pad. The shredded eucalyptus was further ground using a Vermeer HG200 (Vermeer, IA, USA) modified to use a chipping style drum with a 0.75-in. hexagon-shaped screen to prevent oversized material from continuing further in the process. The eucalyptus chips were then conveyed to a Baker-Rullman SD75-22 Dryer System (Baker-Rullman, WI, USA) with a 10 mmBtu/h CNFGD Kinedizer LE Burner (Maxon Corporation, IN, USA) equipped with a prepiped and prewired propane gas train and stainless steel burner internals. Residence time in the drier was controlled by the outlet temperature and the airflow rate in the drier. The dried eucalyptus samples were then ground in a model E-4424-TF hammer mill made by Bliss Industries (OK, USA). A 3/16-in. screen was used for these grinds. The ground feedstocks were shipped to ABPDU and stored at 4 °C cold room. Prior to the experiments, equal quantities of two ground feedstocks (dry weight basis) were mixed at ABPDU to formulate the mixed feedstock. 1-Ethyl-3-methylimidazolium acetate ([C2C1Im][OAc], BASF, purity ≥90 %) was used as the ionic liquid for the pretreatment experiments. Trifluoroacetic acid (TFA) and the monosaccharides, including arabinose, galactose, xylose, glucose, and cellobiose, were purchased from Sigma-Aldrich (St. Louis, MO). Cellulase (Cellic® CTec2) and hemicellulase (Cellic® HTec2) were generously provided by Novozymes (Davis, CA).
IL pretreatment Reactor Setup and Operation
A 10 % (w/w) biomass/IL solution was prepared by combining 600 g (dry weight basis) of biomass with 5.4 kg [C2C1Im][OAc] in the 10-L Parr Floor Stand Reactor (Model 4558, Parr Instrument Company, Moline, IL, USA) in triplicate. For each run, the reactor was sealed and the reactants were heated at 140 °C for 1 h with a stirring speed of 50 rpm from an anchor impellor. Temperature ramping (to 140 °C) and cooling (to 60 °C) times were approximately 25 and 20 min, respectively. After 1-h incubation, the reactor was cooled to 60 °C with chilled water through the cooling coils inside the reactor, and then, 6 L of preheated hot water (60 °C) was slowly pumped into the reactor, causing precipitation of the biomass. Both solids and liquids were transferred into a 50-L bucket and added with 12 L water for overnight soaking. This was followed by a 2-min homogenization step to obtain the uniform dispersion of small particles in the solutions with a laboratory blender (LBC 15, Waring Laboratory, Torrington, CT).
Biomass Washing
The homogenized biomass was transferred into a polypropylene filter bag (25–30-μm average pore opening) and put into a basket centrifuge (STM-2000-146, Western States Machine Company, Hamilton, OH) to remove the water at 1200 rpm for 10 min. After that, another 9 L of deionized water was pumped into basket centrifuge using a peristaltic pump (Pumpdrive 5201, Heidolph Instruments LLC, Cinnaminson, NJ), recirculating for 30 min with a centrifuge speed of 250 rpm. The speed was increased to 1200 for 10 min in order to remove the second wash liquid. This was followed by another three water washes (9 L). The liquids were sampled at each step for IL content measurements. The washed solids were collected to determine the residual IL content, moisture content, and weight to calculate the product recovery from IL pretreatment. Approximately10 g (dry basis) of the pretreated biomass was dried in the vacuum oven at 45 °C to constant weight for composition analysis, and the rest was stored in sealed containers at 4 °C for future use.
Chemical Characterization of Biomass
Acid-insoluble and acid-soluble lignins and structural carbohydrates, i.e., glucan, xylan, arabinan, and galactan, of switchgrass, eucalyptus, and mixed feedstock before and after pretreatment were determined according to analytical procedure of the National Renewable Energy Laboratory (NREL) by a two-step sulfuric acid hydrolysis [22, 23]. Carbohydrates were analyzed by high-performance anion-exchange chromatography (HPAEC) on an ICS-3000 system (Dionex, Sunnyvale, CA), equipped with an electrochemical detector and a 4 × 250-mm CarboPac PA20 analytical column. Elution was initiated with 97.2 % (v/v) water and 2.8 % (v/v) 1 M NaOH for the first 15 min, with 20-μL injection volume. Elute concentration was then switched to 55.0 % (v/v) water and 45.0 % (v/v) 1 M NaOH for the next 20 min and returned to 97.2 % (v/v) water and 2.8 % (v/v) 1 M NaOH for the last 10 min to equilibrate the column. The flow rate was 0.5 mL/min. The monosaccharides, including arabinose, galactose, xylose, and glucose, were used as the external standards for HPAEC and prepared at levels of 0 to 100 mM before use.
To obtain the sugar balance from dissolution of hemicellulose during IL pretreatment, a TFA hydrolysis was performed. The supernatants from the water wash steps were collected and concentrated; 30 μL of solution was diluted 10-fold with water and treated with 150 μL of TFA at 120 °C for 1 h. The hydrolyzed solution was centrifuged at 10,000 g for 10 min, and the supernatant was analyzed using HPAEC for the monosaccharide analysis after evaporation of the TFA residues in a Centri-Vap Vacuum Concentrator (Labconco Corp, MO) at 30 °C overnight.
IL measurement
The [C2C1Im]+ content was determined using a Dionex UltiMate 3000 UHPLC (Dionex, Sunnyvale, CA) with UV–vis detector at 240-nm wavelength. A Dionex acclaim 120 C18 column achieved the separation by isocratic elution with a mobile phase consisting of 20 mM ammonium acetate and 1 % acetic acid at 1.0 mL/min and 20 °C. The IL supernatant taken from various washing steps were directly measured for IL content after proper dilutions. To measure the residual [C2C1Im][OAc] remaining in the biomass after final wash step, the recovered solids were saccharified for 120 h using an extreme excess of enzymes (540 mg/g glucan of CTec2, 300 mg/g glucan of HTec2) at 50 °C and pH 5.5 to completely solubilize the solids and ensure that the [C2C1Im][OAc] attached to the solids went into the liquid fraction. Then, the liquid samples were diluted and measured on HPLC for [C2C1Im][OAc] content [11].
Results and Discussion
Integrated Scale-Up Process
Certain ILs, such as [C2C1Im][OAc], can dissolve a wide range of feedstocks at small scales [9, 19]. The chemical and biological processing of cellulosic feedstocks at larger scale involves the integration of the process and equipment of feedstock handling, processing, and recovery with pretreatment and enzymatic hydrolysis technologies. As the first attempt, our previously published study demonstrated a successful scale-up of switchgrass IL pretreatment by 600-fold in a 10-L reactor with the process integration of water precipitation, water and ethanol washing, and squeeze filtration through cheese cloth [11]. The previous study revealed that the pretreatment configuration requires extensive washing of the biomass post-pretreatment to remove the residual [C2C1Im][OAc], which is known to inhibit downstream saccharification and microbial fermentation [12]. The excessive use of water and waste disposal associated with washing pose challenges for the scale-up of IL pretreatment technology. As a follow-up study, here, we present an improved process which integrates the use of a basket centrifuge to facilitate wash solvent (i.e., water) recycling and efficient solid/liquid separation. The goal was to reduce both downstream IL inhibition and wash solvent volumes.
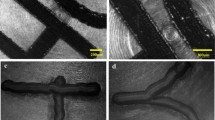
For the current study, 0.6 kg of milled switchgrass, eucalyptus, or mixed feedstock was added into [C2C1Im][OAc] and processed at 6-L scale at a solid loading of 10 % (w/w) in a 10-L Parr reactor. Figure 1 presents the images taken at different stages of the IL pretreatment process. At this solid loading, the single or the mixed feedstock was observed to be significantly solubilized in IL after 1-h reaction at 140 °C (Fig. 1b) without the presence of biomass fibrous structure, which is similar to that observed at lower or same solid loading at severe reaction conditions (160 °C and 3 h) in 10-mL small-scale reaction tube or 200-mL scale glass reactor [9, 10]. We attribute this finding to the effective and uniform mixing and heating provided by the Parr reactor with the anchor impeller and internal temperature control. Unlike other pretreatment methods that preserve the fibrous structure of the biomass in the slurry [24, 25], the three biomass/IL mixtures have morphologies that are highly viscous with no visible signs of fibrous material remaining. Further rheological measurements also show that the three feedstocks studied have different viscoelastic properties [26]. Figure 1c depicts the formation of a gel phase when water is added into the mixed feedstock/IL slurry. The same phenomenon has been observed for switchgrass at various solid loadings (3–15 %) at both small- and large-scale studies [11, 13], requiring a further homogenization step to mechanically break down the gel and facilitate complete biomass dispersion in water (Fig. 1d). Surprisingly, eucalyptus/IL slurry did not present gel morphology upon water precipitation and suggested the feasible minimization of unit operations by skipping the homogenization step to reduce the process economics for this mixture.
Photographs of process integration of [C2C1Im][OAc] pretreatment, water washing, and solid/liquid separation: a biomass after size reduction (3/16-in. screen), b expansion and solubilization of biomass in [C2C1Im][OAc] after 1-h reaction at 140 °C in Parr reactor, c water precipitation, d homogenization for biomass dispersion, e basket centrifuge for biomass continuous washing, and f recovery of pretreated biomass
The efficient recovery of pretreated biomass and removal of residual solutes is a key step in the scaled-up IL process. In the current study, basket centrifuge was used and developed for the continuous washing and solid/liquid separation (Fig. 1e). Biomass went through a total four water washing steps. During each step, water was recycled by pumping into centrifuge and kept recirculation for 30 min at centrifuge speed of 250 rpm to obtain IL-rich liquid washes and recover pretreated solids. In the end, centrifugation speed was increased to 1200 rpm for 19 min to recover the clean pretreated biomass (Fig. 1f) that can be readily saccharified to fermentable sugars. In comparison to our previous study that using eight steps of water (60 L) and ethanol (18 L) washes for switchgrass IL pretreatment, the current work used only water in four washing steps (45 L), significantly reduces the solvent usage and simplifies the unit operation process [11]. For all three feedstocks, the unit operation for material drying was not employed in the current process and all pretreated materials were kept wet and stored at 4 °C for further usage, i.e., enzymatic saccharification in the subsequent studies.
Overall, the successful integration of these unit operations, i.e., pretreatment, material handling/washing, and liquid/solid separation, is the essential next step to facilitate the further development for a commercially scalable and cost competitive process.
Efficient IL Removal as a Function of Water Washing Process
To track the efficiency of washing steps, the [C2C1Im][OAc] content in the wash liquid streams taken at four steps and final solid fractions was measured for all three feedstocks. Table 1 shows that, with the efficient water washing in basket centrifuge, the amount of [C2C1Im][OAc] in the liquid stream was significantly reduced from 23.9 % (w/w, switchgrass), 24.7 % (eucalyptus), and 24.0 % (mixed feedstock) in the first wash to below 0.03 % (w/w, negligible) in the fourth wash. This dramatic decrease of IL content is similar to the results reported from previous study with eight water and ethanol washing steps for switchgrass pretreatment [11]. A certain amount of IL residue is hypothesized to stay in the solid fraction and may not be easily removed. Further IL measurement was conducted on the three recovered solid fractions from four washing steps, using excess concentration of Cellic® HTec2 and CTec2 for 5 days to liberate the [C2C1Im][OAc] [11]. As shown in Table 1, the residual IL concentrations in the recovered solids were 0.2 % (w/w) for all three feedstocks, much less than IL concentration of 3.5 % from the previous study with eight washing steps, suggesting a successfully improved IL removal process with reduced solvent usage through this developed centrifuge washing system [11].
Low levels of [C2C1Im][OAc] (higher than 3.5 %) can significantly inhibit the activity of common cellulases, such as those derived from the fungus Trichoderma reesei [11, 12, 14]. When using hydrolysates derived from biomass pretreated with [C2C1Im][OAc], the carryover of residual IL was found to be the primary source of inhibition on downstream microbial growth and ethanol production [12]. Furthermore, [C2C1Im]+ and acetate together can have a synergistic effect and generate greater levels of inhibition than [C2C1Im]Cl [12]. Experiments conducted at controlled amounts of [C2C1Im][OAc] in corn stover and switchgrass showed that 0.2 % of this IL has insignificant inhibitory effects on Saccharomyces cerevisiae growth and ethanol production, which suggest that the three types of recovered biomass are likely free of IL at significant levels for subsequent downstream processing.
Solid Recovery and Mass Distribution of Three Feedstocks
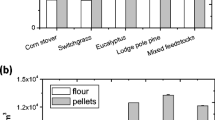
An analysis of the composition in all three feedstocks has been carried out to understand the proper mass distribution changes after IL pretreatment, as illustrated in Fig. 2. In the three untreated biomass samples, cellulose, hemicellulose, and lignin accounts for 31.1–39.2, 13.4–22.7, and 22.5–38.1 % of total biomass, respectively, with the remaining 8.8–23.8 % as structural inorganics, acetyl and proteins. The cell wall chemical compositions are different for switchgass and eucalyptus. The switchgrass has lower glucan (31.1 %) and lignin (22.5 %) but higher xylan content (18.5 %), in comparison to eucalyptus with higher glucan (41.1 %) and lignin (38.1 %), but lower xylan content (11.1 %). Despite the inherent differences between the polysaccharide linkages present in grass and woody biomass samples, arabinan and galactan accounted for only a small portion of the biomass composition and were included together with xylan. Mixed feedstock composition was not measured but calculated from the composition of switchgrass and eucalyptus following 1:1 ratio. The high structural inorganics in switchgrass (23.8 %) is likely due to the high soil contents carried over during biomass harvesting and pre-processing. The significant mass loss into washes is attributed to the solubilization of components such as xylan, lignin, and other extractives during pretreatment with [C2C1Im][OAc]. Results show that switchgrass upon IL treatment at 140 °C and 1 h lost 1.1 % of glucan (% of the total mass, same below), 7.8 % of xylan, and 10.8 % of lignin into the water washes. In comparison, mixed feedstock shows the lowest glucan (0.1 %), similar xylan (7.2 %) and lignin (12.1 %) loss, whereas eucalyptus released relatively lower glucan (0.6 %) and similar lignin (13.0 %), but much less xylan (2.6 %) into water washes.
Based on the results of all the 6-L scale runs at 140 °C and 1 h, 61.5 % of starting switchgrass, 76.7 % of starting eucalyptus and 70.3 % of mixed feedstock was recovered as solids after pretreatment. Figure 3 illustrates the scale-up, loading effects, and pretreatment severity on the changes of solid recovery and three major biomass components, in comparison to the results obtained at the small-scale data from previous studies [9, 10, 17]. Comparison results show that these amounts of solids recovered at the 6-L scale are higher than previous values of 49.3, 59.9, and 64.9 % for three similar feedstocks pretreated at 3–10 % (w/w) solid loading but severer condition of 160 °C and 3 h in the 10 or 200 mL scales [9, 10, 17], suggesting that solid recoveries can be improved effectively with scaling to higher solid loadings, larger volumes, and milder severity.
For switchgrass and eucalyptus, unlike the significant loss of glucan, xylan and lignin observed at 10-mL scale, 160 °C, and 3 % solid loading [9], 6-L scale significant preserved structural carbohydrates, i.e., glucan (96.6 and 98.6 % vs 84.4 and 77.1 %), xylan (64.4 and 87.5 % vs 18.5 and 20.2 %), and lignin (52.2 and 65.9 % vs 29.4 and 45.1 %). Mixed feedstock pretreated at 6-L scale also effectively retained the glucan (99.7 vs 89.1 %) and xylan (62.8 vs 33.9 %), with a slightly lower lignin recovery (59.9 vs 65.1 %), in comparison to the 200-mL scale study at 10 % solid loading, 160 °C and 3 h [10]. Previous studies have shown that hemicellulose and lignin depolymerization during IL pretreatment highly depends on the pretreatment severity [27]. The current study at the larger scale further demonstrates that higher solid loading, lower severity of pretreatment conditions at 140 °C and 1 h, uniform mixing from anchor impeller of the reactor, and effective biomass washing/recovery system can favorably preserve the structural carbohydrates for subsequent enzymatic saccharification and fermentation.
Hemicellulose in the Liquid Fractions
IL pretreatment can remove significant amounts of hemicellulose and small quantities of amorphous cellulose depending on the severity of reaction conditions [27], and, under some pretreatment conditions, hemicellulose can be depolymerized to oligosaccharides [17]. To understand the effect of washing on hemicellulose removal, an analysis on sugar released from three feedstocks during the first two water washing steps was conducted using HPAEC after TFA digestion (Fig. 4). These results show that the pattern of hemicellulose release, as measured by the xylose, arabinose, and galactose contents, strongly depends on the washing steps. The first wash liquor removed high amounts of xylose from switchgrass (26.5 g, 78.8 % of total xylan loss), eucalyptus (7.8 g, 82.5 % of the total xylan loss), and mixed feedstock (30.8 g, 82.0 % of total xylan loss), whereas the second wash further removes small quantities and increased the total xylose amounts up to 88.0, 92.0, and 85.5 % of the total xylan loss, respectively. The total arabinose and galactose removal in all three feedstocks during the two washing steps also reached up to 74.4–98.4 and 73.9–95.7 %, respectively. It is clear that major fractions of hemicellulose bound to the solids can be effectively washed out during two washing steps, which are much more effective than the process used in the previous study which employed five water and three ethanol washing steps [11]. Small amounts of glucose were also observed in the washes of three feedstocks, which were likely released from the amorphous cellulose initially present in the biomass. These results are consistent with the data of compositional changes and mass distribution from Figs. 2 and 3. In comparison with previous studies at small scale with lower solid loadings and more severe pretreatment conditions, the total loss of the glucan in the current study are much lower, suggesting higher solid loadings and mild pretreatment condition at large scale can help retain the structural carbohydrates in the recovered solid streams [9, 11, 25, 27, 28].
Mass Balance
Both single and mixed feedstocks have shown consistent solids and carbohydrate recovery. Using mixed feedstock as the model, an analysis of the mass balance of the ionic liquid pretreatment, the subsequent biomass recovery through precipitation and washing, and their resultant composition of the products generated is summarized in Fig. 5 to develop a clear overview of this pretreatment technology scale up. On the 600-g basis of untreated mixed feedstock, 421.7 g of pretreated solids can be recovered that retain 51.2 % of glucan, 13.2 % of xylan, and 25.8 % of lignin. On the same basis, 0.3 g of glucose oligomers, 33.0 g of xylo-oligomers, 72.8 g of lignin, plus 4.5 g arabinan and 5.8 g of galactan, respectively, can be recovered post pretreatment. Furthermore, of the 5400 g of ionic liquid used, 5373 g was detected in the liquid fraction and available for further recovery and recycling. A very small portion of IL (0.9 g) was retained with pretreated mixed feedstock, a level that will not generate inhibitory effects for downstream enzymatic hydrolysis and microbial fermentation [11]. The overall IL balance closure is 99.5 %, much higher than the 92 % from previous study [11]. The material balance indicates some mass loss during pretreatment and precipitation/washing process, yet the overall glucan recovery from solid streams remains over 99.7 %, confirming that IL pretreatment can preserve most of these sugars for subsequent enzymatic hydrolysis. The overall glucan recovery on the basis of the recovered solids after pretreatment (99.7 %) is higher than xylan (62.8 %), which is attributed to the greater chemical robustness of glucose during the IL pretreatment [10]. A fraction of the hemicellulose remained in the liquid stream after pretreatment but is not considered lost to the overall conversion process [29]. Our recent lab-scale study has demonstrated that the application of liquid-liquid extraction achieved over 90 % xylose recovery from [C2C1Im][OAc] water mixture [14, 26]. During pretreatment, a significant amount of lignin was also solubilized into the liquid stream, causing the lignin reduction in the pretreated solids. However, the residual solids after enzymatic saccharification are rich in lignin content, indicating potential opportunities for lignin valorization. For a long-term development of biorefinery, lignin supply will progressively increase as many types of lignocellulosic feedstocks are implemented in the future. Adding value to the lignin rich residue will significantly enhance the competitiveness of biomass-to-biofuel conversion [30].
It should be addressed that due to their current high cost, recovery and recycle of ILs has been given more and more attention as a requirement of its commercial use in biomass pretreatment. These include using anti-solvent such as acetone, followed by distillation/evaporation for separation [13, 19], biphasic system with addition of an aqueous solution containing kosmotropic anion, such as phosphate, carbonate, or sulfate [31, 32], and sequential membrane filtration and vacuum evaporation post sugar extraction from aqueous IL hydrolysate [15, 29, 33]. Although these results show that separation and recovery of IL can be achieved by various methods, to date, all these potential alternatives have been limited to the lab-scale level of development and require more investigation before scale-up can occur. The process employed in the current report does not involve IL recovery, optimization for washing method, separation method, and removal of the remaining water; however, as a continuation of the present study, JBEI and ABPDU are working together on the scale up demonstration of newly developed wash-free IL processes [15, 29] and liquid-liquid extraction and filtration based IL recycle technologies. The present work provides an essential step to understand and evaluate the scale-up effect and important parameters and requires to further development toward a commercially scalable and cost competitive process.
Conclusions
The IL fractionations of switchgrass, eucalyptus, and their mixtures were successfully scaled up by 30-fold at 6 L with a solid loading of 10 %. In comparison with single feedstocks, mixed feedstocks appear to generate similar yields of sugar and lignin recovery at the 6-L scale, indicating that mixed feedstocks are a viable and valuable resource to consider when assessing biomass availability and affordability demands of the biorefineries scheduled for deployment. The results generated are consistent with those from the small-scale experiments that have been conducted at JBEI and elsewhere and indicate that there are no fundamental issues in terms of performance associated with the scale-up of an IL-based conversion technology. Furthermore, an integrated scale-up process including pretreatment, homogenization, continuous washing/separation, and product recovery was effectively developed to simplify feedstock handling, reduce IL inhibition, and reduce water consumption, all of which can be further integrated with downstream enzyme hydrolysis and microbial fermentation for lignocellulosic biorefinery.
References
Johnson JM, Coleman MD, Gresch R, Jaradat A, Mitchell R, Reicosky D, Wilhelm WW (2007) Biomass-bioenergy crops in the United States: a changing paradigm. Am J Plant Sci Biotechnol 1(1):1–28
Eranki PL, Dale BE (2011) Comparative life cycle assessment of centralized and distributed biomass processing systems combined with mixed feedstock landscapes. GCB Bioenergy 3(6):427–438
Corton J, Buhle L, Wachendorf M, Donnison IS, Fraser MD (2013) Bioenergy as a biodiversity management tool and the potential of a mixed species feedstock for bioenergy production in Wales. Bioresour Technol 129:142–149
Banerjee G, Car S, Liu TJ, Williams DL, Meza SL, Walton JD, Hodge DB (2012) Scale-up and integration of alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis, and ethanolic fermentation. Biotechnol Bioeng 109(4):922–931
Singh S, Arora R, Li C, Mathews IP, Simmons BA (2013) Mixed feedstocks processing using an ionic liquid. US 20130183739 A1
George A, Brandt A, Zahari S, Klein-Marcuschamer D, Parthasarathi R, Sun N, Sathitsuksanoh N, Shi J, Stavila V, Tran K, Singh S, Holmes BM, Welton T, Simmons B, Hallett J (2014) Design of low-cost ionic liquids for biomass pretreatment. Green Chem. doi:10.1039/C4GC01208A
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY, Mitchinson C, Saddler JN (2009) Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol Prog 25(2):333–339
Wyman C, Dale B, Elander R, Holtzapple M, Ladisch M, Lee Y (2005) Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour Technol 96:2026–2032
Li C, Sun L, Simmons BA, Singh S (2012) Comparing the recalcitrance of eucalyptus, pine, and switchgrass using ionic liquid and dilute acid pretreatments. Bioenerg Res 6(1):14–23
Shi J, Thompson VS, Yancey NA, Stavila V, Simmons BA, Singh S (2013) Impact of mixed feedstocks and feedstock densification on ionic liquid pretreatment efficiency. Biofuels 4(1):63–72
Li C, Tanjore D, He W, Wong J, Gardner JL, Sale KL, Simmons BA, Singh S (2013) Scale-up and evaluation of high solid ionic liquid pretreatment and enzymatic hydrolysis of switchgrass. Biotechnol Biofuels 6(1):154
Ouellet M, Datta S, Dibble DC, Tamrakar PR, Benke PI, Li C, Singh S, Sale KL, Adams PD, Keasling JD, Simmons BA, Holmes BM, Mukhopadhyay A (2011) Impact of ionic liquid pretreated plant biomass on Saccharomyces cerevisiae growth and biofuel production. Green Chem 13(10):2743–2749
Dibble D, Li C, Sun L, George A, Cheng A, Cetinkol O, Benke P, Holmes B, Singh S, Simmons B (2011) A facile method for the recovery of ionic liquid and lignin from biomass pretreatment. Green Chem 13:3255–3264
Datta S, Holmes B, Park JI, Chen ZW, Dibble DC, Hadi M, Blanch HW, Simmons BA, Sapra R (2010) Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem 12(2):338–345
Shi J, Gladden JM, Sathitsuksanoh N, Kambam P, Sandoval L, Mitra D, Zhang S, George A, Singer SW, Simmons BA, Singh S (2013) One-pot ionic liquid pretreatment and saccharification of switchgrass. Green Chem 15(9):2579–2589
Li C, Cheng G, Balan V, Kent MS, Ong M, Chundawat SPS, Ld S, Melnichenko YB, Dale BE, Simmons BA, Singh S (2011) Influence of physico-chemical changes on enzymatic digestibility of ionic liquid and AFEX pretreated corn stover. Bioresour Technol 102(13):6928–6936
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101(13):4900–4906
Lee SH, Doherty TV, Linhardt RJ, Dordick JS (2009) Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol Bioeng 102(5):1368–1376
Sun N, Rahman M, Qin Y, Maxim ML, Rodriguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11(5):646–655
Tan SSY, MacFarlane DR, Upfal J, Edye LA, Doherty WOS, Patti AF, Pringle JM, Scott JL (2009) Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem 11(3):339–345
Wu H, Mora-Pale M, Miao J, Doherty TV, Linhardt RJ, Dordick JS (2011) Facile pretreatment of lignocellulosic biomass at high loadings in room temperature ionic liquids. Biotechnol Bioeng 108(12):2865–2875
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2004) Determination of structural carbohydrates and lignin in biomass.LAP-002 NREL Analytical Procedure. National Renewable Energy Laboratory, Golden, CO
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2004) Determination of ash in biomass. LAP-005 NREL Analytical Procedure. National Renewable Energy Laboratory, Golden, CO
Sathitsuksanoh N, Zhu ZG, Wi S, Zhang YHP (2011) Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol Bioeng 108(3):521–529
Modenbach AA, Nokes SE (2012) The use of high-solids loadings in biomass pretreatment—a review. Biotechnol Bioeng 109(6):1430–1442
Tanjore D, Wong J, Li C, Gardner JL, Baez J (2013) Inline rheometry to identify mass transfer issues in enzymatic hydrolysis of biomass at high solids concentration. In: 2013 AICHE Annual Meeting San Francisco, California, USA
Arora R, Manisseri C, Li C, Ong M, Scheller HV, Vogel K, Simmons BA, Singh S (2010) Monitoring and analyzing process streams towards understanding ionic liquid pretreatment of switchgrass (Panicum virgatum L.). BioEnergy Research 3:134–145
Cruz A, Scullin C, Mu C, Cheng G, Stavila V, Varanasi P, Xu D, Mentel J, Chuang Y-D, Simmons B, Singh S (2013) Impact of high biomass loading on ionic liquid pretreatment. Biotechnology for Biofuels 6(1):52
Sun N, Liu H, Sathitsuksanoh N, Stavila V, Sawant M, Bonito A, Tran K, George A, Sale K, Singh S, Simmons B, Holmes B (2013) Production and extraction of sugars from switchgrass hydrolyzed in ionic liquids. Biotechnol Biofuels 6(1):39
Varanasi P, Singh P, Auer M, Adams P, Simmons B, Singh S (2013) Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnology for Biofuels 6(1):14
Gutowski K, Grant A, Willauer H, Huddleston J, Swatloski R, Holbrey J, Rogers R (2003) Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc 125:6632–6633
Shill K, Padmanabhan S, Xin Q, Prausnitz JM, Clark DS, Blanch HW (2011) Ionic liquid pretreatment of cellulosic biomass: enzymatic hydrolysis and ionic liquid recycle. Biotechnol Bioeng 108(3):511–520
Brennan TCR, Datta S, Blanch HW, Simmons BA, Holmes BM (2010) Recovery of sugars from ionic liquid biomass liquor by solvent extraction. Bioenerg Res 3(2):123–133
Acknowledgments
ABPDU would like to acknowledge the funding support from Office of Biomass Program within the US DOE’s Office of Energy Efficiency and Renewable Energy and also the funding support from the American Recovery and Reinvestment Act. JBEI would like to acknowledge the funding support from US DOE’s Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US DOE. The authors would like to thank the Idaho National Laboratory for providing the switchgrass and eucalyptus used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, C., Tanjore, D., He, W. et al. Scale-Up of Ionic Liquid-Based Fractionation of Single and Mixed Feedstocks. Bioenerg. Res. 8, 982–991 (2015). https://doi.org/10.1007/s12155-015-9587-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9587-0