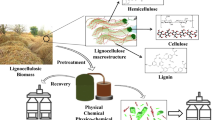
Abstract
Pretreatments have been identified as the core of lignocellulosic biorefinery design due to biomass fractionation and the influence on subsequent reaction and downstream processes. However, most pretreatments are described as single-step, maximizing the valorization of a side stream. Therefore, sequential pretreatments could better describe the integral valorization of lignocellulosic biomass to obtain platform products that can be further used for value-added products. This work experimentally analyzed the sequential pretreatments for the fractionation of rice husks to obtain individual lignocellulosic fractions. It was demonstrated that the dilute acid-wet air oxidation (DA-WAO) sequence is suitable for biorefinery designs since it is possible to solubilize up to 80% of hemicellulose during the first stage and subsequently fractionate almost 90% of lignin after the second stage, obtaining a pretreated solid with high cellulose content. The isolated lignocellulosic fractions were used as platform products to obtain furfural, levulinic acid, and phenolic compounds. As a main result, yields and conversions were improved when valorizing the cellulose platform based on sequential pretreatment. In contrast, valorizing the black liquor after a combination scheme decreased aldehyde yields such as vanillin and syringaldehyde by 4.8–11.9%. The findings indicate that from the biorefinery approach, sequential pretreatments improve the yield of platform products. Despite the decrease of phenolic compounds, levulinic acid and furfural production is significantly enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, different strategies have been proposed to limit the dependence on fossil fuels due to the different environmental and cultural issues related to the use of oil. Biotechnology has received a worldwide boost given the movement towards more sustainable economies to transform biomass (mainly waste biomass) into high value-added compounds [1]. Agricultural by-products are usually disposed of on-field as a source of nutrients or mostly incinerated. Therefore, not only is there no valorization of the different biomass constituents, but it also promotes environmental pollution by releasing pollutant gases [2]. Using biomass as feedstock in biotechnological schemes such as biorefineries could provide alternative, adequate, and sustainable routes for producing a spectrum of chemical products typically derived from petrochemical sources. Lignocellulosic biomass is a potential source for designing biorefineries due to its low cost and being abundant worldwide [3]. Among the different lignocellulosic materials, rice husk is a potential raw material due to its high production volumes (20.1 kg 100 kg−1 after rice threshing processing) and poor disposal management [4]. The problems associated with rice husk derive from its poor disposal, either by burning or burial. However, soil contamination may exist when it is directly buried due to low degradation, attributed to the hardness of the material as a result of the silica and lignin content [5]. Likewise, atmospheric pollution arises in the burning of rice husks due to the emissions of greenhouse gases, particulate matter, and soot [6, 7]. Consequently, using rice husks as a feedstock in large-scale biorefineries could be a promising alternative due to its high production rate and rich lignocellulosic content.
Prior to lignocellulosic valorization in biorefinery schemes, some challenges must be addressed concerning the complex structural arrangement of the biomass. Therefore, pretreatments are crucial to increase the accessibility to chemical or biochemical attacks. Pretreatment selection must be effective to (i) alter the heterogeneous structure to increase surface area and porosity as well as decrease crystallinity for bioconversion processes, (ii) hydrolyze hemicellulose and lignin bonds for further reactions, and (iii) isolate platform products that will be used as intermediates in biomass valorization. Other operational aspects should be considered for evaluating pretreatment efficacy, such as inhibitor formation rate and process severity, as well as cost-benefit and environmental impact [8]. Different pretreatments have been previously studied for lignocellulosic materials through single-step schemes reporting the operational improvement of conventional pretreatments and discussing new trends with emerging technologies that also enhance the accessibility to biomass constituents [9]. Most of these pretreatments focus on single-step schemes as they decrease technological complexity and operating costs and avoid accumulating unwanted products. However, implementing single-step schemes for biomass treatment with structural complexity can be challenging for isolating a lignocellulosic fraction. Therefore, these technologies cannot fully solubilize the biomass, leaving aside non-recoverable waste side streams. Pretreatments in sequential combination could overcome the problems of integral valorization and increase the economic margin in lignocellulosic biorefineries. For example, improved biofuel production has been demonstrated following a two-step pretreatment strategy where hemicellulose is first solubilized after acid treatment and then delignified with alkali [10]. The liquid hot water (LHW)-organosolv sequence has demonstrated a pretreatment opportunity for producing lignin, hemicellulose-based sugars, and pulp for papermaking [11]. Other processes have proposed the recycled aqueous ammonia expansion as a second stage for lignin solubilization [12]. The sequential fractionation through acid hydrotropes even promotes the non-destructive extraction of lignin and the solid residue maintains the fiber shape and crystalline structure of the cellulose [13]. Some authors have shown increased production and purification of hexoses as a platform product following dilute acid (DA)-LHW [14] or organosolv-alkali [15] sequences. Therefore, sequential pretreatments could be an emerging approach that improves pretreatment efficacy performance.
Although different studies demonstrate the importance of pretreatments in biorefinery design [16], there is no clear systematization of sequential combinations that maximize the recovery of lignocellulosic fractions for future valorization. Sequential pretreatments could then improve biorefinery designs as they would increase net revenues due to increased production of platform products and possibly conversions. Moreover, selectively isolating lignocellulosic fractions decreases the presence of undesirable compounds that could affect future conversions by physicochemical interactions. However, these pretreatment schemes depend on the biorefinery context since a sequential combination increases the number of processing units and the technological complexity of the process, thus increasing future capital or operating costs. Therefore, sequential pretreatments should be implemented in producing fine chemicals where the selling price offsets the overall costs. Review articles on efficient pretreatment of biorefineries [17] or recent trends in biomass pretreatments [18] do not analyze the possibility of implementing sequential technologies despite their improved selective isolation compared to conventional or single-step pretreatment. Other studies have focused on evaluating the performance of sequential pretreatments [14], but there are no reports validating pretreatments for obtaining platforms as they focus on the valorization of the hexose-rich water-insoluble solid [19]. Therefore, the possibility of maximizing the integral valorization of biomass in biorefinery schemes is not analyzed. Based on previous results of the best sequential pretreatments in biorefinery schemes [20], this work aims to experimentally evaluate the proposed combinations of DA-alkali and DA-wet air oxidation (WAO) for isolating cellulose, LHW-DA and steam explosion (SE)-DA for hemicellulose solubilization, and DA-Kraft and SE-Kraft for lignin removal using rice husk as lignocellulosic material. The performance of the pretreatments was analyzed through different operational indicators as well as for the valorization towards chemical products. The isolated or removed cellulose, hemicellulose, and lignin platforms were used to produce levulinic acid, furfural, and phenolic compounds, respectively.
2 Material and methods
2.1 Feedstock and chemicals
The rice husks were received from a rice thresher located in El Espinal, Tolima, Colombia (4°09′34.7″ N 74°54′44.3″ W). The rice husk was milled and screened with a 40 mesh (425 μm) sieve. The screened feedstock was stored in airtight plastic bags for further use. Analytical reagents for raw material characterization and pretreatments were purchased from PanReac AppliChem (Darmstadt, Germany), chromatographic solvents from Scharlau (Barcelona, Spain), and chromatographic standards from Sigma-Aldrich (MO, USA), and used as received.
The characterization of the raw material was carried out in triplicate following different procedures. The analysis included three types of characterization: (i) chemical analysis to calculate the mass balances involved in the pretreatment and reaction stages as well as the yields and conversions, (ii) proximate analysis to evaluate the performance in thermal processes, and (iii) solid content. Initially, the moisture content was reduced to 10% through a forced air oven at 45 °C. For the chemical analysis, the extractive content was determined after sequential Soxhlet reflux of water and ethanol for 8 h (NREL/TP-510-42619) [21], while the fat was by hexane reflux during the same period time [22]. Holocellulose content was estimated through the chlorination method (ASTM D1104) [23]. Meanwhile, cellulose after several sodium hydroxide dosages using the remaining holocellulose solid (ASTM D1103) [23]. The difference between holocellulose and cellulose gives hemicellulose. Insoluble lignin was determined as Klason type (NREL/TP-510-42618) [24]. Total pectin was estimated after extraction with concentrated sulfuric acid by photometric measurement of galacturonic acid with carbazole at 240 nm [25]. Protein was quantified by the Kjeldahl method with a conversion factor of 6.25 (NREL/TP-510-42625) [26]. On the other hand, for the proximate analysis, the content of volatile material was determined after sample calcination at 950 °C (ASTM E872-82) [27], ash by slow heating up to 575 °C (NREL/TP-510-42622) [28], and fixed carbon by difference. The heat capacity of the sample was determined using a calorimetric pump (SDACM 3100, Sundy, China) and following the procedure of the ASTM E711-87 [29]. Finally, the analysis of the solid content involved the determination of total and volatile solids after heating to 105 °C and 550 °C, respectively [30].
2.2 Pretreatments of rice husk
Rice husk pretreatments were performed based on the heuristic analysis of pretreatment sequences for isolating lignocellulosic fractions [20]. The following schemes were considered: DA-alkali and DA-WAO for cellulose isolation, LHW-DA and SE-DA for hemicellulose fractionation, and DA-Kraft and SE-Kraft for lignin removal. The sequential pretreatment analysis also involved single-step pretreatments to calculate the experimental enhancement of the proposed combinations.
All pretreatments, except for SE, were performed in a 300-mL high-pressure reactor with a working capacity of 50% (HP AutoLAB reactor E1823, HEL Group, UK). The reactor has a temperature controller for an electrical resistance jacket, a thermocouple in the 316 stainless steel vessel, an axial turbine stirrer, and a tap water cooling system. Additionally, the reactor has three butterfly inlet valves for gases as well as a relief and a purge valves. On the other hand, the SE was performed in a reaction system of two stainless steel vessels of 250 mL fed with feedstock and 750 mL of water storage (500 mL of working volume). Both vessels are connected through a completely sealed ball valve. The water vessel heats the fluid through an electrical resistance until saturated steam is obtained at the desired pressure conditions measured through a manometer. After heating, the ball valve is opened (time zero) and the steam comes into contact with the sample. After the reaction time, the system is cooled by convection in a cold room at 5 °C.
In general, the rice husk was fed on a dry basis and an inert atmosphere was ensured by purging with nitrogen for 5 min prior to pretreatment. Those pretreatments performed in the high-pressure reactor were carried out at 150 rpm. Table 1 summarizes the operating conditions for each pretreatment scheme. All pretreatments were performed in duplicate and the average of both runs was reported. After the residence time, the pretreated rice husk was separated by vacuum filtration. The hydrolysate was stored at 5 °C to quantify soluble sugars and degradation products by high-performance liquid chromatography (HPLC). The water-insoluble solid (WIS) was washed with distilled water at 70 mL g−1 of the pretreated sample, ensuring a washing water pH close to neutral. The WIS was then dried at 60 °C and recharacterized in terms of lignocellulosic composition based on the previously mentioned standards.
2.3 Valorization of lignocellulosic fractions
The sequential pretreatment that best had effective selectivity was selected for further valorization to value-added compounds. For the cellulose-based schemes, the WIS was valorized to produce levulinic acid using sulfuric acid as a catalyst. On the other hand, the hydrolysates obtained from the hemicellulose fractionation pretreatments were used to produce furfural by dehydration reaction with hydrochloric acid. Lignin-rich liquors were also valorized for producing low-molecular-weight phenolic compounds through oxidation reactions. Figure 1 illustrates a schematic flow chart of the process. All valorizations were performed in duplicate in the high-pressure reactor with a working volume of 150 mL, reporting the average of the results. Nitrogen was bubbled for 5 min before each reaction to ensure an inert atmosphere.
For the production of levulinic acid, a sufficient amount of pretreated rice husk (7.5 g oven-dried) was fed in the reactor vessel with a 2.5 M [H+] solution of sulfuric acid in a solid-to-liquid ratio of 1:20 (mass:volume). The reaction conditions were 200 °C for 2 h at 200 rpm [31]. The remaining solid and the levulinic acid–rich hydrolysate were separated by vacuum filtration. For furfural production, the hydrolysates were previously neutralized with slow addition of calcium hydroxide powder and continuous stirring at 40 °C until pH 5–6, leaving a final settling time of 30 min without stirring. After neutralization, the reaction volume was prepared by adding hydrochloric acid to the neutralized supernatant up to a concentration of 0.44 M and sodium chloride at a ratio of 2 g 10 g−1 reaction volume. The acid hydrolysate was fed into the reactor vessel and nitrogen was supplied up to a pressure of 10 bar. The dehydration reaction was carried out at 164 °C for 2 h and 600 rpm [32]. Concerning the production of phenolic compounds, the black liquors were characterized as total non-volatile solids [33]. The black liquor was prepared to 60 g L−1 (based on total non-volatile solids), and sufficient NaOH was added until a reaction liquor concentration of 80 g L−1. Subsequently, the liquor was fed to the reactor vessel and nitrogen was supplied up to a pressure of 6.5 bar. The oxidation reaction occurred at 120 °C for 20 min and 1100 rpm [34]. Upon reaching the temperature, oxygen was supplied until a total system pressure of 10 bar was obtained, this being the initial time of the reaction. Finally, all hydrolysates after valorizations were stored at 5 °C for subsequent chromatographic analysis.
2.4 Analytical methods
Aliquots (5 mL) of the soluble fractions of the pretreatment schemes were hydrolyzed through dilution with 4% H2SO4 at 121 °C for 1 h to quantify oligosaccharides as monosaccharides of each corresponding sugar (i.e., glucooligosaccharide as glucose or xylooligosaccharide as xylose) [35]. Soluble sugars (glucose, xylose, arabinose, mannose, and galactose) and degradation products (xylitol, formic acid, acetic acid, levulinic acid, furfural, and 5-hydroxymethylfurfural (HMF)) were determined on an HPLC (Agilent 1260 Series, Agilent Technologies, USA) equipped with an ICSep ICE-COREGEL 87H3 column (7.8 × 300) based on refractive index detector at 35 °C. A solution of 5 mM H2SO4 was used as the mobile phase at 0.6 mL min−1 for 50 min. The oven temperature was set at 65 °C. Meanwhile, low-molecular-weight phenolic compounds such as vanillin, vanillic acid, syringaldehyde, and syringic acid were quantified on an HPLC (Shimadzu LC-2010A HT Series, Shimadzu Corporation, Japan) equipped with a Kromasil C18 column (150 mm × 4.6 mm × 5 μm). Chromatographic separation was carried out by the gradient method using eluents of (A) methanol and (B) 3% aqueous acetic acid at a rate of 1 mL min−1 according to the following method: initially 100% B, 10% A and 90% B (10 min), 30% A and 70% B (40 min), and 100% A (44–47 min). The oven temperature was 25 °C, and the quantification was performed in a UV detector at 280 nm with 20 μL of sample injection. Soluble sugars, degradation products, and phenolic compounds were quantified based on the linear calibration curves of the standard compounds. Xylooligosaccharides were determined by the difference with total monosaccharides. The stock solution of sugars and degradation products was prepared in ultrapure water, while phenolic compounds were prepared in methanol. Samples were filtered on a 0.20-μm pore size filter.
2.5 X-ray diffraction
X-ray diffraction (XRD) analysis was performed for the WIS in a diffractometer (Rigaku MiniFlex II Series, Rigaku, Japan) using Cu Kα radiation at 30 kV and 15 mA. The samples were analyzed in an angular range (2Ɵ) between 3° and 60° (step size of 0.02°) at 2° min−1 at room temperature. The crystallinity of the samples was estimated through the crystallinity index (CrI) as shown in Eq. (1), where I002 is the intensity of the crystalline fraction of the biomass (cellulose at 2Ɵ of 22.5) and Iam is the intensity of the amorphous fraction (2Ɵ of 17.8).
3 Results
3.1 Rice husk characterization
Table 2 shows the results of the physicochemical characterization of the rice husk. In general, the characterization of rice husks was comparable with other reports in the literature [36, 37]. Some disagreements may be related to the species of rice cultivated, harvesting or crop season, and climatic conditions. For example, some authors report a protein content of 3.1% and a fat content of 2.7% for rice husks grown in Sukawati, Gianyar [38]. Regarding cellulose and hemicellulose, the total carbohydrate content was 44.3%, which could be optimal for bioconversion processes. Lignocellulosic biomass with total carbohydrate contents higher than 40% has been shown to be feasible for enzymatic and fermentative processes [39, 40]. Concerning the lignin content, it was higher than non-wood lignocellulosic feedstocks such as corn stover (19.5%) [41], wheat straw (19.2%) [42], and corn stalks (19.6%) [12]. High lignin contents substantially decrease the efficiency of enzymatic hydrolysis of cellulose as the enzyme adsorbs on lignin through different interactions such as hydrogen bonds or electrostatic/hydrophobic bonds, subsequently blocking the active sites [43]. Therefore, the selective isolation of lignin is fundamental during the design of integral biorefineries, whose valorization is essential to increase total revenues. Regarding the proximate analysis, volatile material and fixed carbon parameters have been used to estimate the performance of thermal processes such as gasification, providing an idea of maximum temperatures in the reactor. Values between 3 and 4 of these two parameters are suggested for gasifications. This ratio was 3.49 for rice husk, indicating higher temperatures would be achieved than wood materials [34]. On the other hand, despite the high calorific value of rice husks compared to herbaceous materials, the high ash content significantly decreases the energy density [44]. After the thermal process, large ash volumes are generated, representing a disposal problem since it does not degrade easily and is an air pollutant [45]. Due to the high silica content (SiO2 between 85 and 95%), rice husk is normally used as a pozzolanic material or mineral additive in cementitious products [46].
3.2 Effect of pretreatment on the chemical compositional of rice husk
As described in Section 2, rice husk was pretreated based on sequential combinations for the individual isolation of each lignocellulosic fraction. The DA-alkali and DA-WAO sequence was proposed for cellulose isolation, the LHW-DA and SE-DA sequence for hemicellulose removal, and the DA-Kraft and SE-Kraft sequence for lignin fractionation. Additionally, all pretreatments were performed as a single step to compare the experimental performance of the proposed sequences. Comparing the effect of the single-step pretreatments on solid recovery (defined as the mass ratio between the WIS of each pretreatment and the raw feedstock on a dry basis), the alkaline pretreatments (i.e., alkali and Kraft) and WAO showed higher biomass solubilization (see Table 3.). In contrast, DA demonstrated less ability to release soluble compounds. Subjecting rice husk to sequential pretreatment further increases the removal of lignocellulosic material, gradually decreasing the total solid content of the biomass, especially in the combinations where alkaline pretreatment was performed as the second stage. This loss of solids may be because the DA pretreatments increased the surface area of the biomass through swelling of the cellulosic fibers [14]; therefore, the alkaline agent easily penetrated the matrix to solubilize more lignocellulosic content.
The compositional analysis of the raw material and WIS after each pretreatment is summarized in Fig. 2. It is observed that for all samples, cellulose is the biopolymer less affected by the thermochemical action of the pretreatment, achieving maximum removals of 14.15% for LHW in single-step and 15.08% for the SE-DA sequence, as shown in Table 3.. This isolation hindrance is explained by the abundance of hydrogen bonds of both intramolecular and intermolecular hydroxyl groups, which are difficult to hydrolyze [47]. The removed cellulose has been mainly attributed to amorphous fractions within the molecular matrix, obtaining smaller polymers and oligomers [48]. Glucose was detected in the hydrolysates (see Table 4), which was not only due to amorphous cellulose but could also be explained by hemicellulose glucans such as xyloglucans, glucomannans, or even branched glucans [49]. Pretreatment sequences for cellulose isolation showed maximum removals of 11.2% and recoveries of ~78–85% (defined as cellulose ratio between the WIS to feedstock). These results agree with some reports for hazelnut husk pretreatment under the same sequential technology [14]. Comparing both sequences for cellulose, the DA-WAO combination was able to recover 9% more insoluble hexoses, explained by the fact that alkali-catalyzed pretreatments easily hydrolyze amorphous regions of hemicellulose [50]. Although the DA-alkali combination totally removed hemicellulose, the WIS has an accessibility of 84.5% (see Poveda-Giraldo et al. for accessibility definition [11]), being slightly lower than the DA-WAO sequence of 93.9%, as shown in Table 5. Therefore, the WIS would be affected to a small extent by the remaining undepolymerized lignin. Regarding lignin, although the single-step of alkali favors delignification (71.5%), it was observed that the DA-alkali sequence does not reach these values, except for DA-WAO. Therefore, the WIS would be affected by the remaining undepolymerized lignin, disturbing possible future bioconversion processes. To explain this, some reports have shown that after hydrothermal treatments close to the melting point of lignin, it tends to liquefy and then coalesce into small droplets during cooling, adhering to the surface matrix of the biomass [51]. Since alkaline processes break the β-O-4 bonds of lignin, the alkaline catalyst does not have sufficient lignin surface area to enhance delignification processes. However, studies have shown that the thermal treatment of biomass through LHW improves further delignification processes in soda-anthraquinone (soda-AQ) pulpings [52]. On the other hand, the total hemicellulose removal after the second stage in the DA-alkali combination showed that the formation of xylo-oligomers was favored (see Table 4), a trend that has also been demonstrated in alkali-catalyzed processes [53]. Table 3. also shows that extractives and ash content decrease considerably over the pretreatments, as has also been described for wood materials with increasing doses of alkali [54] or for acid pretreatments [55]. In general, sequential combinations for cellulose isolation improved some technical indicators compared to single-step pretreatments. For example, performing DA pretreatment allows accessibility in the pretreated solid of 74.2% and a cellulose recovery of 88.2% (see Table 5). In contrast, the DA-alkali combination increased these indicators to 84.5% and 78.3%, while DA-WAO to 93.4% and 85.4% for WIS accessibility and total cellulose recovery, respectively. The DA-WAO pretreatment is the best sequential scheme for cellulose isolation as it has the best results in technical indicators, and it is possible to fractionate more lignin in the second stage. Indeed, the technical indicators are 95.3% and 87.8% of the theoretical values for accessibility and cellulose recovery based on the results presented in previous studies [20]. DA-WAO also concluded that after DA pretreatment, a first hydrolysate rich in sugars mainly of the pentose type (10.3 g L−1 as oligo- and monosaccharides) is obtained, and after sequencing, a second hydrolysate rich in soluble lignin is favored.
The pretreatment sequences for hemicellulose removal consisted mainly of acid-catalyzed processes. Both combinations showed no significant difference as there were low removals of cellulose (12.1–15.1%) and lignin (11.2–11.6%), as well as total hemicellulose solubilizations at the end of the sequence of 97.5–98.5%, substantially improving upon the single-step of LHW and SE processes. Small differences were observed in the production of soluble compounds after the second stage of the sequence, obtaining 24.4 g L−1 of total pentoses (mono- and oligosaccharides) for LHW-DA and 28.9 g L−1 for SE-DA, as shown in Table 4. In addition, this last sequential combination resulted in a higher content of degradation products, especially furfural (0.63 g L−1). The selection of the best sequential pretreatment for hemicellulose removal was then evaluated depending on the objective of the process. If bioconversion processes for biofuel production are desired, for instance, the LHW-DA combination would be more favorable due to its lower content of inhibitory compounds (see Table 4). In contrast, the SE-DA sequence is more favorable if a furan production process is desired. Unlike the cellulose schemes, the SE-DA and LHW-DA combinations do not allow obtaining the lignocellulosic fractions individually but are more focused on pentose valorization processes. This fact can be affirmed by the accessibility of the second hydrolysate aimed at valorizing soluble lignin, as illustrated in Table 5. Sequential pretreatments enhanced the total hemicellulose solubilization performance, achieving 97.1% and 96.6% values for LHW-DA and SE-DA, respectively. Despite these promising removals, the hydrolysates have lower accessibility (63.4–75.1% for both hydrolysates) than the cellulose combinations. This idea is supported by total cellulose and lignin removal accumulation along the sequences (see Table 3.).
The pretreatment sequences for lignin fractionation in addition to obtaining the lowest biomass solid recovery yields also removed the highest content of extractives. The removal of extractives was favored by increasing the concentration of the alkali catalyst, as evidenced elsewhere [54]. Total lignin removal during the whole sequence was higher for the SE-Kraft (86.1%). Therefore, this combination would solubilize higher hemicellulose content during the first stage (removal comparison between SE and DA) and higher lignin during the second pretreatment stage. In fact, Table 4 shows a higher content of pentose sugars (11.37 g L−1 as mono-oligomers) after individual SE pretreatment. Additionally, the production of xylose monomers (16.0 g L−1 for DA-Kraft and 24.0 g L−1 for SE-Kraft) was favored following the sequential schemes. Concerning inhibitory compounds, the furan compound concentrations were small or not detected. As in the sequences for hemicellulose, there is no significant difference in choosing a better lignin pretreatment combination since both have similarities in removals. Based on total lignin removal, the SE-Kraft scheme would be optimal for biopolymer fractionation and further valorization. However, the second hydrolysate presents high xylose contents that could affect the phenolic compound production. Unlike the sequential combinations for cellulose and hemicellulose isolation, the sequential pretreatments for lignin fractionation do not improve the total heteropolymer removal compared to the Kraft process. Total lignin removal decreased by 13.4% for the DA-Kraft sequence and 4.1% for the SE-Kraft.
3.3 X-ray analysis of pretreated rice husk
Figure 3 shows the effect of single-step and sequential pretreatments on rice husk structure. By analyzing the raw feedstock, three main peak intensities could be identified: at 15.9° which represents cellulose allomorphs (i.e., tricyclic and monocyclic), 22.0° from the hydrogen-bonded sheets of cellulose I, and 34.5° which may represent cellobiose bond lengths [56]. As shown in Fig. 2A, single-step pretreatments changed the biomass structure, specifically the crystallinity of cellulose, by cleaving inter- and intramolecular hydrogen bonds. It is observed that for alkaline pretreatments, there is peak overlap at diffraction angles between 20° and 21° explained by the formation of cellulose II structures [57]. In contrast, there was an extensive reduction in the peak intensities for the DA pretreatment, implying that the sample was totally amorphous. This analysis was confirmed by calculating the CrI (Table 6), showing a clear decrease compared to the raw rice husk. In contrast, the Kraft pretreatment showed an apparent increase in CrI, explained by the partial removal of amorphous fractions such as hemicellulose and lignin, leading to an accumulation of crystalline cellulose [56]. Unlike the SE-Kraft sequence, on the other hand, the pattern of intensity decrease in the diffractograms was also observed for the sequential pretreatments at a higher rate, as observed in Fig. 2B. In fact, the CrI calculated from the sample pretreated with the sequential combinations is significantly lower than for the raw feedstock, especially for DA-alkali. This decrease in crystallinity indicates that the recovered product is highly amorphous and could have a higher accessibility to the cellulose surface, favoring bioconversion processes [58].
3.4 Valorization of pretreatment side streams for producing value-added compounds
After sequential pretreatments, the side streams of the schemes were valorized for the production of value-added compounds. The production of levulinic acid from the WIS cellulose platform, as well as furfural and phenolic compounds from the hydrolysates, was proposed. Since an integrated biorefinery seeks to maximize the profit margin, pretreatment schemes should be designed to minimize losses of lignocellulosic fractions. Based on the sequential pretreatment results, there were combinations where individual lignocellulosic fractions were not obtained. For example, hemicellulose schemes maximized pentose solubilization throughout the sequences with moderate amounts of lignin and cellulose removals of more than 20%, or lignin schemes where after the second stage, the hydrolysates contained large xylose composition that would not be valorized in the first pretreatment stage. Therefore, the sequential pretreatment in a biorefinery scheme should be such that besides removing large amounts of the platform products, it should obtain them individually with the least amount of unwanted fractions and preserve cellulose recovery. Therefore, the sequential pretreatment DA-WAO shows to be an alternative for the valorization of the side streams obtaining a first hydrolysate for the production of furfural, a second hydrolysate rich in lignin and potential for the production of phenolic compounds, and an insoluble solid that can be used for the production of levulinic acid. Figure 4 illustrates the mass balances of the DA-WAO sequential pretreatment for producing lignocellulosic platforms further valorized to value-added compounds.
The first hydrolysate from the sequential DA-WAO combination was used as feedstock for furfural production. The dehydration reaction also considered raw rice husk as substrate in order to evaluate the pretreatment performance, as shown in Table 7. After the reaction, the furfural concentration increased by more than 700% compared to the initial content of the pretreatment hydrolysate (0.41 g L−1), achieving a higher conversion of 95% of xylose, which agrees with some reported results in the literature. Studies have shown that after 1 h of reaction, all the xylose can be consumed even without adding a catalyst [59]. The yield of furfural production was also much higher than the raw feedstock and can be explained by the presence of organic acids in the hydrolysate, which acts as catalysts in the reaction, promoting the hydrolysis of even the pentose oligosaccharides. After the dehydration reactions, the presence of xylooligosaccharides was not detected, and their hydrolysis was sufficiently efficient [60]. The furfural yields were in agreement and even higher than some literature reports. Yields of 33.8% have been reported for the reaction of neutralized hydrolysates of coffee-cut steams after DA pretreatments [32]. Other studies have achieved maximum yields of 54% following reactions at 170 °C for 1 h from LHW hydrolysates [61]. Under controlled two-phase reactor systems, xylose conversions can be in the range of 24.6–100% and yields above 90% [60]. Differences were found possibly to the dehydration of mono- and xylooligomers that favored the reaction. The dehydration performance is also affected by the presence of soluble lignin, which can react with the generated furfural and form undesired products [61]. On the other hand, furfural is also a degradation product of the DA process, so its composition is added to the final yield of the process, favoring hydrolysates with pre-existing furan compound formation. According to these results, it is possible to conclude that the DA pretreatment enhanced the furfural production compared to the raw feedstock with yields comparable to the literature. Additionally, less than 5% of xylose is destined as residue since the conversions could be favored by the content of organic acids in the hydrolysate (see Table 4).
The black liquor or lignin-rich hydrolysate obtained after the second stage of sequential pretreatment was used to produce phenolic compounds through oxidation reactions. The analysis of results also involved the pretreatment of hydrolysates from the WAO process and raw rice husk to evaluate the oxidation reaction. The loading of the black liquor into the reactor was performed based on the content of total non-volatile solids as described elsewhere [62]. Table 8 shows the yields and composition of the liquors after the oxidations. As a main result, the pretreatment schemes significantly favored the formation of phenolic compounds, possibly explained by the ability of the alkali catalyst to hydrolyze short-chain soluble lignin bonds, which are more free and accessible compared to the complex lignocellulosic matrix of the biomass. As can be seen in Table 8, the phenolic compounds most frequently identified were vanillin, syringaldehyde, and vanillic acid, which are produced after breaking the Cα-Cβ bonds of coniferyl and sinapyl alcohols or guaiacyl and syringyl moieties of lignin [63]. It is also possible to observe that the production of phenolic compounds was not favored with the sequential combination approach. This trend could be due to the accumulation of inorganic compounds (i.e., NaOH, Na2S, Na2SO4) from the chemical agents of the pretreatments, which exert ionic strength to the oxidation solution and consequently decrease the solubility of oxygen [64]. Therefore, the gas consumption and the formation of phenolic compounds are reduced. Furthermore, it is possible that soluble sugars undergo degradation through the alkali agent and oxygen, forming undesired compounds that will alter the pH of the medium or the pressure of the system through the formation of carbon dioxide, unfavorable for oxidation performances [33]. Aldehyde selectivity, on the other hand, can easily be determined through the ratio of vanillin/vanillic acid (V/VA) and syringaldehyde/syringic acid (S/SA) composition. The S/SA ratio for the DA-WAO pretreatment was 1.6 times higher than for the single-step scheme. In contrast, the trend of the V/VA ratio was the opposite, showing a slight increase for the simple DA scheme (V/VA of 1.67). The formation of acids from vanillin and syringaldehyde aldehydes should be understood as oxidation by-products rather than aldehyde degradation products, usually formed from lignin structures with carbonyl groups at the Cα position [65]. The yields of phenolic compounds are highly sensitive to operating conditions such as temperature, system pressure, alkali catalyst loading, and time, achieving vanillin variations between 6.3 and 10.8% [66]. Pinto and co-authors reported maximum aldehyde yields (on a total solid basis) of 3.2% for syringaldehyde and 1.5% for vanillin following sulfite liquor oxidation, as well as 2.0% for syringic acid and 0.8% for syringic acid from isolated solid lignin [33]. Comparing the results of the present work with previous studies from black liquors based on soda-type pretreatments, the yields decreased by 34.5% [34], respectively. Despite these results, sequential DA-WAO pretreatment demonstrates a potential for producing phenolic compounds from solubilized lignin-rich hydrolysate as it shows higher yields even for isolated lignin and high selectivity for aldehydes rather than acids. Also, the oxidation reactions were improved compared to using raw feedstock.
The production of levulinic acid involved the pretreated rice husk after the sequential combination of DA-WAO. Additionally, the reaction was performed considering the first stage of the sequence (DA pretreatment) as a single-step pretreatment and the raw feedstock. Table 9 shows the main results of levulinic acid production using different feedstock solids. In general, the pretreatments improved the process yield by 30.1–50.1% compared to raw feedstock, explained by the increase in cellulose surface area after removing hemicellulose and lignin, increasing the catalytic action of the acid. Comparing both pretreatment schemes, the slight decrease in the yield of the single-step pretreatment could be explained by the presence of furfural and unsolubilized lignin, which promote humin formation, negatively affecting the reaction [67]. Additionally, some authors report decreased yields due to the physicochemical interaction between lignin and levulinic acid [68]. Regarding the cellulose conversion, the non-pretreated raw material showed the highest values due to cellulose loss along the sequences. Despite these differences, the yields agree with the literature. Raspolli et al. obtained yields of 26.4% and 21.8% for material fed after the thermal treatment of poplar sawdust and paper sludge, respectively [69]. In contrast to this work, other authors have reported that there is no apparent improvement in the production of levulinic acid after delignification, obtaining yields of 54.5% based on cellulose content using pretreated rice husks, a lower value than the present work (67.15% for DA and 59.82 for DA-WAO) [70]. There are several factors by which the performance of the process is enhanced, including temperature, acid strength of the catalyst, and biomass type, among others. For example, increasing the temperature to 200 °C and using 8% H2SO4 can achieve yields of 32.6% after using sorghum flour [71]. The results of this work show that levulinic acid production performance was improved after sequential pretreatments. However, cellulose conversion also decreases due to the loss of solid biomass throughout the process. Therefore, it is advisable to perform an economic analysis to determine the cost-benefit of the capital costs of sequential pretreatments to obtain higher yields of levulinic acid.
4 Conclusions
Since pretreatments have an important role in the design of biorefineries, the effect of different sequential combinations for the production of platform products was evaluated. This work showed that the sequences for cellulose recovery (DA-alkali and DA-WAO) and lignin removal (DA-Kraft and SE-Kraft) do not present significant differences for obtaining platform products. Likewise, hemicellulose hydrolysis would be prioritized in the first stage and then lignin removal. In contrast, sequential pretreatments for hemicellulose solubilization are focused on maximizing the removal of mainly pentose sugars, generating a pretreated solid with rich lignin content. The results of the pretreatment schemes illustrated that the DA-WAO sequence had the best fractionation results of the lignocellulosic material based on a biorefinery scheme where the integral valorization of the biomass is maximized. This sequential combination was able to solubilize almost 80% of hemicellulose during the first stage (DA pretreatment) and 90% of lignin in the second hydrolysate after the sequence, obtaining a cellulose recovery in the pretreated solid of 96.8% with accessibility of 84.5%. Both the hydrolysates and the pretreated solid were used to obtain value-added products. The valorization of the hemicellulose hydrolysate achieved furfural yields of 60.8%, with xylose conversions above 95%. Regarding the lignin-rich black liquor, no improvement of the sequential approach (DA-WAO) was observed when compared to the WAO pretreatment. The results showed a slight reduction in the production of aldehyde (vanillin and syringaldehyde) and acid (syringic acid) phenolic compounds. However, it is an open proposal given the increase in production yields compared to the literature. Finally, the valorization of the pretreated solid after the DA-WAO sequence considerably improved levulinic acid production compared to the DA pretreatment and the raw feedstock. This work demonstrated that the two-stage pretreatment strategy considerably improves both the isolation of lignocellulosic fractions and the valorization yields of the platform products.
References
Saratale RG et al A critical review on biomass-based sustainable biorefineries using nanobiocatalysts: opportunities, challenges, and future perspectives. Bioresour Technol 363. Elsevier Ltd, Nov. 01, 2022. https://doi.org/10.1016/j.biortech.2022.127926
Adejumo IO, Adebiyi OA (2021) Agricultural solid wastes: causes, effects, and effective management. Strateg Sustain Solid Waste Manag. IntechOpen, IntechOpen. https://doi.org/10.5772/intechopen.93601
Rodionova MV et al (2022) A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int J Hydrogen Energy 47(3):1481–1498. https://doi.org/10.1016/j.ijhydene.2021.10.122 Elsevier Ltd
Baiyeri KP, Chukwudi UP, Chizaram CA, Aneke N (2019) Maximizing rice husk waste for Daucus carota production. Int J Recycling Org Waste Agric 8(Suppl 1):399–406. https://doi.org/10.1007/s40093-019-00312-9
Yan S, Yin D, He F, Cai J, Schliermann T, Behrendt F (2022) Characteristics of smoldering on moist rice husk for silica production. Sustainability (Switzerland) 14(1). https://doi.org/10.3390/su14010317
Luu LQ, Halog A (2016) Rice husk based bioelectricity vs. coal-fired electricity: life cycle sustainability assessment case study in Vietnam. In: Procedia CIRP. Elsevier B.V, pp 73–78. https://doi.org/10.1016/j.procir.2016.01.058
Efremova SV (2012) Rice hull as a renewable raw material and its processing routes. Russ J Gen Chem 82(5):999–1005. https://doi.org/10.1134/S1070363212050349
Henrique M et al (2015) Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 8:3366–3390. https://doi.org/10.1002/cssc.201500282
Kumar R et al (2022) Emerging approaches in lignocellulosic biomass pretreatment and anaerobic bioprocesses for sustainable biofuels production. J Clean Prod 333. Elsevier Ltd. https://doi.org/10.1016/j.jclepro.2021.130180
Romero I, López-Linares JC, Delgado Y, Cara C, Castro E (2015) Ethanol production from rape straw by a two-stage pretreatment under mild conditions. Bioprocess Biosyst Eng 38(8):1469–1478. https://doi.org/10.1007/s00449-015-1389-4
Serna-Loaiza S, Adamcyk J, Beisl S, Miltner M, Friedl A (2022) Sequential pretreatment of wheat straw: liquid hot water followed by organosolv for the production of hemicellulosic sugars, lignin, and a cellulose-enriched pulp. Waste Biomass Valorization 13(12):4771–4784. https://doi.org/10.1007/s12649-022-01824-8
Zhang C, Pang F, Li B, Xue S, Kang Y (2013) Recycled aqueous ammonia expansion (RAAE) pretreatment to improve enzymatic digestibility of corn stalks. Bioresour Technol 138:314–320. https://doi.org/10.1016/j.biortech.2013.03.091
Wu X, Zhang T, Liu N, Zhao Y, Tian G, Wang Z (2020) Sequential extraction of hemicelluloses and lignin for wood fractionation using acid hydrotrope at mild conditions. Ind Crops Prod 145. https://doi.org/10.1016/j.indcrop.2020.112086
Hoşgün EZ, Biran Ay S, Bozan B (2020) Effect of sequential pretreatment combinations on the composition and enzymatic hydrolysis of hazelnut shells. Prep Biochem Biotechnol 0(0):1–10. https://doi.org/10.1080/10826068.2020.1836657
Aggarwal N, Pal P, Sharma N, Saravanamurugan S (2021) Consecutive organosolv and alkaline pretreatment: an efficient approach toward the production of cellulose from rice straw. ACS Omega 6(41):27247–27258. https://doi.org/10.1021/acsomega.1c04030
Cantero D et al (2019) Pretreatment processes of biomass for biorefineries: current status and prospects. Annu Rev Chem Biomol Eng 10(1):289–310. https://doi.org/10.1146/annurev-chembioeng
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol Biofuels 12(1) BioMed Central Ltd. https://doi.org/10.1186/s13068-019-1634-1
Baruah J et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6. Frontiers Media S.A. https://doi.org/10.3389/fenrg.2018.00141
Haghighi Mood S et al (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27. Elsevier Ltd:77–93. https://doi.org/10.1016/j.rser.2013.06.033
Poveda-Giraldo JA, Cardona Alzate CA (2023) Analysis of sequential pretreatments to enhance the early-stage biorefinery designs. Appl Sci 13(11). https://doi.org/10.3390/app13116758
A. Sluiter, R. O. Ruiz, C. Scarlata, J. Sluiter, and D. Templeton, Determination of extractives in biomass - NREL/TP-510-42619, Golden, Colorado, 2004. 2008.
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: past and present panacea. J Chromatogr A 1217(16):2383–2389. https://doi.org/10.1016/J.CHROMA.2009.11.027
Han JS, Rowell JS (1996) In: Rowell R, Young R, Rowell J (eds) Chemical composition of fibers, in Paper and Composites from agro-based resources, vol 1. CRC Press, pp 83–134
Sluiter A et al (2012) Determination of structural carbohydrates and lignin in biomass - NREL/TP-510-42618. Golden, Colorado
Abbaszadeh AH (2008) Pectin and galacturonic acid from citrus wastes, Master Thesis. University of Borås
Hames B, Scarlata C, Sluiter A (2008) Determination of protein content in biomass - NREL/TP-510-42625. Golden, Colorado
Standard ASTM (2013) Standard test method for volatile matter in the analysis of particulate wood fuels - ASTM E872-82, West Conshohocken. PA. https://doi.org/10.1520/E0872-82R13
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of ash in biomass - NREL/TP-510-42622. Golden, Colorado
Standard ASTM (2004) Standard test method for gross calorific value of refuse-derived fuel by the bomb calorimeter - ASTM E711-87, West Conshohocken. PA. https://doi.org/10.1520/E0711-87R04
Sahito AR, Mahar R, Siddiqui Z, Brohi K (2013) Estimating calorific values of lignocellulosic biomass from volatile and fixed solids. Int J Biomass Renewables 2(1):1–6
Solarte Toro JC (2022) Sustainability assessment of different biorefinery schemes to enhance the development of post-conflict areas in the Colombian context: the Montes de Maria case, Master thesis,. Universidad Nacional de Colombia sede Manizales, Manizales, Colombia
V. Aristizábal-Marulanda and C. A. Cardona A., Experimental production of ethanol, electricity, and furfural under the biorefinery concept, Chem Eng Sci,. 229. 2021. https://doi.org/10.1016/j.ces.2020.116047.
Pinto PCR, Costa CE, Rodrigues AE (2013) Oxidation of lignin from eucalyptus globulus pulping liquors to produce syringaldehyde and vanillin. Ind Eng Chem Res 52(12):4421–4428. https://doi.org/10.1021/ie303349j
Poveda-Giraldo JA, Cardona CA (2021) Biorefinery potential of Eucalyptus grandis to produce phenolic compounds and biogas. Can J For Res 51(1):89–100. https://doi.org/10.1139/cjfr-2020-0201
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2006) Determination of sugars, byproducts, and degradation products in liquid fraction process samples - NREL/TP-510-42623. Golden, Colorado
Abu Bakar MS, Titiloye JO (2013) Catalytic pyrolysis of rice husk for bio-oil production. J Anal Appl Pyrolysis, Elsevier B.V:362–368. https://doi.org/10.1016/j.jaap.2012.09.005
Kirubakaran V, Sivaramakrishnan V, Nalini R, Sekar T, Premalatha M, Subramanian P (2009) A review on gasification of biomass. Renew Sustain Energy Rev 13(1):179–186. https://doi.org/10.1016/j.rser.2007.07.001
Gaga Parta IB, Belawa Yad TG, Bidura IGNG, Sri Trisna AAA, Sayang Yup W (2019) The effect of rice hull in diets on performance, antioxidant capacity and blood chemical profile of Bali duck, Int. J Poult Sci 18(2):69–75. https://doi.org/10.3923/ijps.2019.69.75
Dharma Patria R et al (2022) Bioconversion of food and lignocellulosic wastes employing sugar platform: a review of enzymatic hydrolysis and kinetics. Bioresour Technol 352. Elsevier Ltd. https://doi.org/10.1016/j.biortech.2022.127083
H. Escalante H., G. L. Carolina, and C. M. Liliana, Anaerobic digestion of fique bagasse: an energy alternative, Dyna (Medellin), vol. 81. 74–85, 2014: https://doi.org/10.15446/dyna.v81n183.34382.
Tae HK, Lee YY (2005) Pretreatment and fractionation of corn stover by ammonia recycle percolation process. Bioresour Technol 96(18) SPEC. ISS:2007–2013. https://doi.org/10.1016/j.biortech.2005.01.015
Salapa I, Katsimpouras C, Topakas E, Sidiras D (2017) Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 100:10–16. https://doi.org/10.1016/j.biombioe.2017.03.011
H. Liu, J. Sun, S. Y. Leu, and S. Chen, Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process, Biofuels Bioprod Biorefin. \, . 5. John Wiley and Sons Ltd. 648–663, 2016. https://doi.org/10.1002/bbb.1670.
Yoon SJ, Il Son Y, Kim YK, Lee JG (2012) Gasification and power generation characteristics of rice husk and rice husk pellet using a downdraft fixed-bed gasifier. Renew Energy 42:163–167. https://doi.org/10.1016/j.renene.2011.08.028
Riza FV, Rahman IA (2015) The properties of compressed earth-based (CEB) masonry blocks. In: Eco-efficient masonry bricks and blocks: design, properties and durability. Elsevier Inc, pp 379–392. https://doi.org/10.1016/B978-1-78242-305-8.00017-6
Hossain Z, Islam KT (2022) Prospects of rice husk ash as a construction material, in Sustainable concrete made with ashes and dust from different sources: materials, properties and applications. Woodhead Publishing, pp 61–92. https://doi.org/10.1016/B978-0-12-824050-2.00009-7
Nishiyama Y (2009) Structure and properties of the cellulose microfibril. J Wood Sci 55(4) Springer Japan:241–249. https://doi.org/10.1007/s10086-009-1029-1
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729. https://doi.org/10.1021/ie801542g
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289. https://doi.org/10.1146/annurev-arplant-042809-112315
Hu F, Ragauskas A (2012) Pretreatment and lignocellulosic chemistry. Bioenergy Res 5(4):1043–1066. https://doi.org/10.1007/s12155-012-9208-0
Borrega M, Nieminen K, Sixta H (2011) Effects of hot water extraction in a batch reactor on the delignification of birch wood. Bioresources 6(2):1890–1903. https://doi.org/10.15376/biores.6.2.1890-1903
Ullah S, Pakkanen H, Lehto J, Alén R (2018) A comparable study on the hot-water treatment of wheat straw and okra stalk prior to delignification. Biomass Convers Biorefin 8(2):413–421. https://doi.org/10.1007/s13399-018-0306-x
Lehto J, Alén R (2013) Alkaline pre-treatment of hardwood chips prior to delignification. J Wood Chem Technol 33(2):77–91. https://doi.org/10.1080/02773813.2012.748077
Lehto J, Alén R (2015) Alkaline pre-treatment of softwood chips prior to delignification. J Wood Chem Technol 35(2):146–155. https://doi.org/10.1080/02773813.2014.902964
He Y, Fang Z, Zhang J, Li X, Bao J (2014) De-ashing treatment of corn stover improves the efficiencies of enzymatic hydrolysis and consequent ethanol fermentation. Bioresour Technol 169:552–558. https://doi.org/10.1016/j.biortech.2014.06.088
Cheng G et al (2011) Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis. Biomacromolecules 12(4):933–941. https://doi.org/10.1021/bm101240z
Kuo CH, Lee CK (2009) Enhancement of enzymatic saccharification of cellulose by cellulose dissolution pretreatments. Carbohydr Polym 77(1):41–46. https://doi.org/10.1016/j.carbpol.2008.12.003
Li C et al (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101(13):4900–4906. https://doi.org/10.1016/j.biortech.2009.10.066
Köchermann J, Mühlenberg J, Klemm M (2018) Kinetics of hydrothermal furfural production from organosolv hemicellulose and d -xylose. Ind Eng Chem Res 57(43):14417–14427. https://doi.org/10.1021/acs.iecr.8b03402
Xing R, Qi W, Huber GW (2011) Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries. Energy Environ Sci 4(6):2193–2205. https://doi.org/10.1039/c1ee01022k
Liu L, Chang HM, Jameel H, Park S (2018) Furfural production from biomass pretreatment hydrolysate using vapor-releasing reactor system. Bioresour Technol 252:165–171. https://doi.org/10.1016/j.biortech.2018.01.006
Gomes ED, Mota MI, Rodrigues AE (2018) Fractionation of acids, ketones and aldehydes from alkaline lignin oxidation solution with SP700 resin. Sep Purif Technol 194:256–264. https://doi.org/10.1016/j.seppur.2017.11.050
Zhang R et al (2021) Characterization of lignin compounds at the molecular level: mass spectrometry analysis and raw data processing. Molecules 26(1) MDPI. https://doi.org/10.3390/MOLECULES26010178
Millero FJ, Huang F (2003) Solubility of oxygen in aqueous solutions of KCl, K2SO4, and CaCl2 as a function of concentration and temperature. J Chem Eng Data:1050–1054. https://doi.org/10.1021/je034031w
Hirano Y, Hosoya T, Miyafuji H (2023) Depolymerization of native lignin into vanillin, vanillic acid, and other related compounds via alkaline aerobic oxidation: reaction mechanisms and process control using organic cations. From Biomass Biobased Prod , IntechOpen. https://doi.org/10.5772/intechopen.112090
Fargues C, Mathias Á, Rodrigues A (1996) Kinetics of vanillin production from Kraft lignin oxidation. Ind Eng Chem Res 35(1):28–36. https://doi.org/10.1021/ie950267k
Yoon SY, Han SH, Shin SJ (2014) The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy 77:19–24. https://doi.org/10.1016/j.energy.2014.01.104
Kang S, Yu J (2016) An intensified reaction technology for high levulinic acid concentration from lignocellulosic biomass. Biomass Bioenergy 95:214–220. https://doi.org/10.1016/j.biombioe.2016.10.009
Raspolli Galletti AM, Antonetti C, De Luise V, Licursi D, Di Nasso NN (2012) Levulinic acid from waste biomass. Bioresources 7(2):1824–1835
Bevilaqua DB, Rambo MKD, Rizzetti TM, Cardoso AL, Martins AF (2013) Cleaner production: levulinic acid from rice husks. J Clean Prod 47:96–101. https://doi.org/10.1016/j.jclepro.2013.01.035
Fang Q, Hanna MA (2002) Experimental studies for levulinic acid production from whole kernel grain sorghum. Bioresour Technol 81:187–192. https://doi.org/10.1016/s0960-8524(01)00144-4
Acknowledgements
The authors express their gratitude to the “Sistema de Información de la Investigación, Extensión y Laboratorios – HERMES” for the funding approved through the mobility 12792 of the Universidad Nacional de Colombia. Likewise, the gratitude is extended to the Laboratory of Intensification and Hybrid Processes and the Laboratory of Nanostructured and Functional Materials belonging to the Universidad Nacional de Colombia sede Manizales, as well as to the Laboratory of Instrumentation of the University of Jaén (Spain) for characterizations support in this work.
Data availability
Data supporting the results of this study are available upon request from the corresponding author.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Contributions
JAPG: conceptualization, methodology, software, validation, investigation, data curation, formal analysis, writing - original draft. CACA: conceptualization, formal analysis, resources, writing - review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poveda-Giraldo, J.A., Cardona Alzate, C.A. Valorization of lignocellulosic platform products based on biorefinery schemes through sequential pretreatments. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-023-05140-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05140-6