Abstract
This study shows how relative fatty acid content and unsaturation index of Dunaliella salina strains can be estimated rapidly by analysis with flow cytometry and Nile Red staining. This technique is valid for analysing intraspecific variability and shows no significant distortion phenomena, such as variability in the staining of strains not associated with different fatty acid composition. These phenomena have been related to variations in the permeability of the cell membrane and cell wall to Nile Red in a number of microalgae strains and species. The saturated fatty acids/monounsaturated fatty acids/polyunsaturated fatty acids ratio was estimated indirectly from the relative polar and neutral lipid composition. High intraspecific variability was noted in the fatty acid composition of D. salina, which makes it an ideal species for conducting screening tests of new strains for oil production, using the strategic advantages associated with this species such as the possibility of mass culturing in inexpensive, open systems and high biomass processability due to its lack of cell wall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2003, we began a series of studies designed to assess the utility of the techniques of flow cytometry and Nile Red (NR) staining to develop methods for quick estimation of lipid and fatty acid content in microalgae and their potential application in culture control and mass screening of new strains (de la Jara et al. 2003; Mendoza et al. 2008a). These studies showed the utility of flow cytometry and NR staining both for ascertaining lipid content in microalgae (de la Jara et al. 2003) and in estimating variations in these parameters associated with culture conditions (Mendoza et al. 2009) and interspecific diversity (Mendoza Guzmán et al. 2011) as well as the potential utility of these techniques for developing effective systems for culture control and optimisation (Mendoza et al. 2008b).
The basic requirements of any technique valid for undertaking screening programmes are, firstly, that the variability detected, in this case in the NR fluorescence signal in stained cells, must specifically obey the parameter under study (fatty acid composition) and, secondly, that interference from other variability factors must be minimal. Because of this, it is very important to assess the efficiency of the technique (specific nature of the signal in relation to the study variable) at different levels of biological, interspecific and intraspecific diversity and the variation associated with the culture conditions. This study assesses NR and flow cytometry in quick estimation of relative fatty acid composition in relation to intraspecific variability in Dunaliella salina.
The possibility of prospecting for intraspecific diversity using quick, simple and relatively inexpensive analysis techniques such as fluorometry and flow cytometry would make it possible to exploit one of the greatest technological potentials of microalgae: their enormous diversity. This is particularly important in species that have already demonstrated their high aptitude for large-scale mass culture, such as D. salina. Having strains of hyperlipogenic D. salina readily available would make it possible to overcome one of the dilemmas in large-scale production of oils using microalgae; i.e. the combination of high potentials for biomass production in simple, inexpensive culture systems and high levels of lipid production (Griffiths and Harrison 2009; Beal et al. 2011). In this respect, D. salina is an ideal species, as its high halotolerance allows culturing without major risk of contamination and with adequate yield in both intensive and semi-extensive open systems. Moreover, its lack of cell walls simplifies biomass processing and it is one of the species that has shown highest potential for genetic engineering, making it an ideal candidate for developing screening programmes for new strains, whether wild or modified (Barzegari et al. 2010).
Dunaliella salina is also an ideal species for evaluating the analytical capacity of new techniques at intraspecific level. Large collections of strains of this species are now held, and its extremophile nature, closely associated with isolated or semi-isolated environments such as saltworks and hypersaline lakes, means high intraspecific diversity is readily available and new strains with high variability can be easily obtained (Guevara et al. 2005; Mendoza et al. 2008a).
Material and methods
The study included 11 strains of the phototrophic halophyte organism Dunaliella salina (Table 1). The ITC 5.003 strain was obtained from the Spanish Bank of Algae. The others were isolated from solar saltworks in mainland Spain, the Canary Islands and France using the agar plating technique (Mendoza et al. 2008a) and deposited at the Canary Islands Technological Institute culture collection. All strains were identified by PCR-amplified and Internal Transcribed Spacer region analysis based on ITS and rbcl sequences, following the method described by Assunção et al. (2011). Cultures were grown in a 250-mL borosilicate flasks containing Semenenko-Abdullaev medium (Semenenko and Abdullaev 1980) and supplemented by 3% CO2-enriched air bubbling at room temperature (18–23°C) under continuous light (white light, 220 μmol photons m−2 s−1). All stocks were maintained in axenic conditions by weekly subculturing. In all cases the stock culture was used as inocula, and the experimental condition was similar to the stock culture in all strains.
Cultures were analysed by flow cytometry when the stationary phase of cell growth had been reached, 24 h after the first indication that no significant variations in the cell density of the cultures had occurred, with daily estimates by directly counting cells in a Thoma chamber. For the fatty acid analysis cultures were first harvested by centrifugation (5 min, 5,000×g, 5°C).
Chromatography analysis
Fatty acid composition, obtained from fresh samples, was estimated following the method described by Mendoza et al. (1999). Biomass aliquots were transmethylated with MeOH-acetyl chloride. Gas chromatography analysis was conducted in a Varian CP-3800 fitted with an FID detector and a Varian capillary column CP7419 (50 m × 0.25 mm × 0.25 μm). Fatty acid methyl esters were identified by comparing retention times with the Supelco standard for FAME Mix C4-C24, and pentadecanoic acid (15:0) was used as internal standard.
Relative fatty acid composition is expressed as the ratio between the percentages of saturated fatty acids (SAT), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA), using the formula (PUFA/SAT+MUFA) (Guimarães et al. 1991; Mendoza Guzmán et al. 2011). The unsaturation index was also estimated, using the formula developed by Thi et al. (1985), the result of multiplying the number of double bonds in each fatty acid by its concentration (expressed as the percentage of total fatty acids).
Flow cytometry analysis
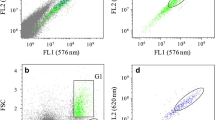
Cells from the different culture were stained with NR (Sigma Inc.) following the protocol developed by de la Jara et al. (2003) and modified to analyse of D. salina strains by Mendoza et al. (2008a). An aliquot of cell sample (1 mL at 105–106 cells mL−1) was treated with 12.5 μL of a working solution of NR (Sigma Inc.) and acetone (0.1 mg mL-1). This mix was gently vortexed and incubated for 10 min at 37°C in darkness. Flow cytometry determined yellow and red fluorescence of NR-stained cells, as well as autofluorescence (chlorophyll, size and complexity), expressed as arbitrary units, using an EPICS XL flow cytometer (Beckman Coulter Instruments) equipped with a 488-nm argon laser. The optical system used in the EPICS XL flow cytometer collects yellow light (575 band pass filter) in the FL2 channel, corresponding to the NR fluorescence in a neutral lipid matrix, and red light (620 band pass filter) in the FL3 channel, corresponding to the NR fluorescence in polar lipids (de la Jara et al. 2003; Mendoza Guzmán et al. 2011). Cells were gated according to their chlorophyll fluorescence characteristics to remove non-algal particles. Approximately 3,000 cells were analysed using a log amplification of the fluorescence signal. Equipment was calibrated daily with Flow-Check™ fluorospheres (Beckman Coulter). Unstained cells were used as autofluorescence control. Data were expressed as fluorescence, FL2 and FL3 (arbitrary units). The reference data used was the arithmetic mean of all cytometric events (3,000 cells).
Data analysis
In all cases data represent the mean of the three replicates (three experimental cultures per strain). Data were analysed for statistical significance using linear regression analysis, with a statistical significance value of p < 0.01. The variability in fatty acid content in the various strains was estimated using the coefficient of variation of each fatty acid considered within all the strains studied.
Results
The PUFA/SAT+MUFA ratio and the unsaturation index showed a significant correlation (p < 0.01) to the FL3/FL2 values (Fig. 1). As seen in earlier studies, this corresponds to the relative polar and neutral lipid content in microalgae (de la Jara et al. 2003; Mendoza Guzmán et al. 2011).
Although the group of strains presents a similar overall fatty acid profile, where the predominant fatty acids are 16:0, 18:3ω3, 18:0, 18:2ω6 and 18:1ω9 (with relative concentrations greater than 3% of all fatty acids for each strain; Table 2), considerable variability was detected in the less common fatty acids in the composition, that is 10:1, 12:0, 13:0, 14:1 and 17:1 (Fig. 2), which were not detected in all strains (Table 2). 18:3ω6 was present in all strains except ITC5.128 and ITC5.114, and the highest concentrations of this fatty acid were observed in ITC5.107 and ITC5.107. Among the predominant fatty acids, the highest variability corresponded to 18:1ω9 in its two isomeric forms (Fig. 2), with strains ITC5.114 and ITC5.105 attaining the highest concentration overall. Fatty acids 16:0 and 18:3ω3 showed lower variability and strain ITC5.105 showed the highest concentrations of 16:0. This strain also had the lowest 18:3ω3 content, along with ITC5.107.
Coefficient of variation estimated from the relative fatty acid composition of all strains (standard deviation/mean of the% of each fatty acid, considering all strains studied). The bars of the predominant fatty acids in the composition of all strains (with an average greater than 3% in the entire group of strains) are shown in bold
The differences observed in fatty acid content correspond to those seen in both the relative fatty acid content, expressed as PUFA/SAT+MUFA, and the unsaturation index. The highest relative PUFA content and unsaturation index correspond to strain ITC5.107, whereas ITC5.105 had the lowest values in both parameters (Fig. 3).
Discussion
This study confirms earlier findings: NR staining and flow cytometry analysis enable rapid estimation of relative fatty acid composition in microalgae (de la Jara et al. 2003; Mendoza et al. 2009; Mendoza Guzmán et al. 2011). The correlation between NR staining and lipid fraction in various strains of D. salina was demonstrated in an earlier study (Mendoza et al. 2008a).
The utility of NR in rapid analysis of lipid content in microalgae is still questioned, particularly its use in screening tests. Cell walls inhibit permeation of NR, and between-species variability has been detected in cellular penetration of staining with this dye (Chen et al. 2009; Mutanda et al. 2011). Similarly, overlapping phenomena are frequently detected between the NR signal associated with polar lipids and chlorophyll autofluorescence, which limits the utility of NR in quantitative lipid analysis (Chen et al. 2009). Alterations in the intensity of NR fluorescence associated with the presence of certain proteins have also been detected (Brown et al. 1995). However, the results of the present study demonstrate how these techniques can be used to analyse intraspecific variability of relative fatty acid content, at least in D. salina, by indirect estimation of the PUFA/SAT+MUFA ratio through analysis of polar and neutral lipid content. Strains with the highest ratio of polar lipids have the highest PUFA content. These fatty acids are associated in microalgae primarily with membrane structures rich in polar lipids (Roessler 1990). No alterations in NR staining of the cells were detected associated with other features of intraspecific variability, such as cell permeability different to NR or interference with other cell components. A satisfactory correlation was observed between the relative fatty acid of the various strains and the NR signal.
Flow cytometry and NR have recently been successfully applied to intraspecific analysis of microalgae lipid content and isolation of stable morphotypes using uniclonal microalgae cultures (Montero et al. 2010; Doan and Obbard 2011).
The results also show the high intraspecific variability in fatty acid composition in D. salina, confirming the findings of earlier studies (Petkov et al. 1990). D. salina has a high PUFA content, specifically 18:3ω3, which poses a problem for its use in biodiesel production. Oils with more than 12% content of this fatty acid are little suited for biodiesel production (Mutanda et al. 2011). Various species of Dunaliella have been discounted for large-scale biodiesel production due to their low oil yields (Araujo et al. 2011). However, the high diversity of this genus and of D. salina in particular, associated with its high variability in fatty acid content, and above all the possibility of culturing it in inexpensive, open systems with little risk of culture collapse or contamination, continue to make this species an ideal microalga both for screening tests and biodiesel production.
Use of fatty acid content alone is a poor criterion for selecting new strains for biodiesel production. Other selection variables more closely associated with the technological aptitude of algae, such as culture yields, the simplicity of production systems and biomass processability are key aspects to consider in biodiesel production from microalgae (Beal et al. 2011).
The most suitable fatty acid profile for biodiesel production seen in this study corresponds to strain ITC5.105, which has one of the lowest 18:3ω3 contents and less than 40% PUFA. It is also one of the strains with the highest 18:1 content (Table 2). This fatty acid is associated with higher stability and resistance to oil oxidation (Knothe 2005), and with other optimum properties for biodiesel production such as the decrease in the cold filter plugging point for use in cold regions (Stournas et al. 1995). Moreover, 18:1 has been associated with the processes of β-carotene accumulation in D. salina (Mendoza et al. 1999), which would correspond to the high levels of carotenogenesis observed in this strain (data not shown), whose cultures stay a brownish colour even in low irradiation conditions. All these data make ITC5.105 a very interesting strain for future studies and show that it has highly differentiated features in relation to other strains.
Compared with other analytical techniques, flow cytometry not only offers the advantages of speed and simplicity in analysis, but also makes it possible to obtain a large variety of parameters at the cellular level, such as cell size, complexity and integrity, which are key elements for effectively conducting microalgae mass screening programmes for a variety of uses. Earlier studies showed the utility of combining several parameters when screening D. salina and revealed the high intraspecific diversity associated with these parameters in this species (Mendoza et al. 2008a). The strategic advantages of flow cytometry, coupled with the use of specific cell stains such as NR and automated isolation techniques, will provide a step forward in the development of microalgae biotechnology, which to date has primarily focussed on process engineering and cultures and to a lesser degree on effective prospecting of its wide biodiversity (Mutanda et al. 2011).
References
Araujo GS, Matos LJBL, Gonçalves LRB, Fernandes FAN, Farias WRL (2011) Bioprospecting for oil producing microalgal strains: evalulation of oil and biomass production for ten microalgal strains. Bioresource Technol 102:5248–5250
Assunção P, Jaén-Molina R, Caujapé-Castells J, de la Jara A, Carmona L, Freijanes K, Mendoza H (2011) Phylogenetic position of Dunaliella acidophila (Chlorophyceae) based on ITS and rbcL sequences. J Appl Phycol. doi:10.1007/s10811-011-9676-1
Barzegari A, Hejazi MA, Hosseinzadeh N, Eslami S, Agdam EM, Hejazi MS (2010) Dunaliella as an attractive candidate for molecular farming. Mol Biol Rep 37:3427–3430
Beal CM, Smith CH, Webber ME, Ruoff RS, Hebner RE (2011) A framework to report the production of renewable diesel from algae. Bioenerg Res 4:36–60
Brown MB, Miller JN, Seare NJ (1995) An investigation of the use of Nile Red as a long-wavelength fluorescent probe for the study of α1-acid glycoprotein-drug interactions. J Pharm Biomed Anal 13:1011–1017
Chen W, Chengwu Z, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Meth 77:41–47
De la Jara A, Mendoza H, Martel A, Molina C, Nordströn C, de la Rosa V, Díaz R (2003) Flow cytometric determination of lipid content in marine dinoflagellate Crypthecodinium cohnii. J Appl Phycol 15:433–438
Doan TY, Obbard JF (2011) Enhanced lipid production in Nannochloropsis sp. using fluorescence-active cell sorting. GCB Bioenergy 3:264–270
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Guevara M, Lodeiros C, Gómez O, Lemus N, Núñez P, Romero L, Vásquez A, Rosales N (2005) Carotenogenesis of five strains of the algae Dunaliella sp (Chlorophyceae) isolated from Venezuelan hypersaline lagoons. Rev Biol Trop 53:331–337
Guimarães ARP, Costa Rosa LFBP, Sitnik RH, Curi R (1991) Effect of polyunsaturates (PUFA n-6) and saturated fatty acid-rich diets on macrophage metabolism and function. Biochem Int 23:1739–1751
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Mendoza Guzmán H, de la Jara VA, Carmona Duarte L, Presmanes F (2011) Analysis of intraespecific variation in relative fatty acid composition: use of flow cytometry to estímate unsaturation index and relative polyunsaturated fatty acid content in microalgae. J Appl Phycol 23:7–15
Mendoza H, Martel A, Jiménez del Río M, García-Reina G (1999) Oleic acid is the main fatty acid related with carotenogenesis in Dunaliella salina. J Appl Phycol 11:15–19
Mendoza H, de la Jara A, Freijanes K, Carmona L, Ramos AA, de Sousa DV, Varela JCS (2008a) Characterization of Dunaliella salinastrains by flow cytometry: a new approach to select carotenoid hyperproducing strain. Electron J Biotechnol. doi:10.2225/vol11-issue4-fulltext-2
Mendoza H, Molina Cedrés C, de la Jara A, Nordström L, Freijanes K, Carmona L (2008b) Quantitative and qualititative variation of the fatty acid composition in the dinoflagellate Cryothecodinium cohnii under nitrogen starvation conditions. Grasas y Aceites 59:27–32
Mendoza H, de la Jara A, Carmona Duarte L, Freijanes Presmanes K (2009) Estímate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquac Int 18:189–199
Montero MF, Aristizábal M, García Reina G (2010) Isolation of high-lipid content strains of the marine microalga Tetraselmis suecica for biodiesel production by flow cytometry and single-cell sorting. J Appl Phycol. doi:10.1007/s10811-010-9623-6
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102:57–70
Petkov GD, Klyachko-Gurvich GL, Furnadzhieva ST, Pronina NA, Ramazanov ZM (1990) Sov Plant Physiol 3:268–272
Roessler PG (1990) Environmental control of glycerolipids metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399
Semenenko VE, Abdullaev AA (1980) Parametric control of β-carotene biosynthesis in Dunaliella salina cells under conditions of intensive cultivation. Sov Plant Physiol 27:22–30
Stournas S, Lois E, Serdar A (1995) Effects of fatty acid derivatives on the ignition quality and cold flow of diesel fuel. J Am Oil Chem Soc 72:433–437
Thi P, Borrel-Flood C, Vieira da Silva J, Justin AM, Mazliak P (1985) Effects on lipid metabolism in cotton leaves. Phytochemistry 24:723–727
Acknowledgements
This study was funded by the Canary Islands Agency for Research, Innovation and the Information Society as part of the BIOAC project and by Programa de Cooperación Transnacional Azores, Madeiras y Canarias (PCT-MAC 2007-2013, FEDER). The support and assistance provided by the halophyte and extremophile algae maintenance and collection services of the Canary Islands Technological Institute were essential to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza Guzmán, H., de la Jara Valido, A., Freijanes Presmanes, K. et al. Quick estimation of intraspecific variation of fatty acid composition in Dunaliella salina using flow cytometry and Nile Red. J Appl Phycol 24, 1237–1243 (2012). https://doi.org/10.1007/s10811-011-9768-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-011-9768-y