Abstract
Chlorella vulgaris (a freshwater microalga) and Dunaliella tertiolecta (a marine microalga) were grown for bulk harvest, and their biomass was tested as feedstock for electricity production in cubic two-chamber microbial fuel cells (MFCs) at 37°C. The anode inoculum was anaerobic consortium from a municipal sewage sludge digester, enriched separately for the two microalgal biomass feedstocks. After repeated subculturing of the two anaerobic enrichments, the maximum power density obtained in MFCs was higher from C. vulgaris (15.0 vs. 5.3 mW m−2) while power generation was more sustained from D. tertiolecta (13 vs. 9.8 J g-1 volatile solids). Anolytes of algal biomass-fed MFCs also contained substantial levels of butanol (8.7–16 mM with C. vulgaris and 2.5–7.0 mM with D. tertiolecta), which represents an additional form of utilizable energy. Carryover of salts from the marine D. tertiolecta biomass slurry resulted in gradual precipitation of Ca and Mg phosphates on the cathode side of the MFC. Polymerase chain reaction-denaturing gradient gel electrophoresis profiling and sequencing of bacterial communities demonstrated the presence of Wolinella succinogenes and Bacteroides and Synergistes spp. as well as numerous unknown bacteria in both enrichments. The D. tertiolecta enriched consortium contained also Geovibrio thiophilus and Desulfovibrio spp. Thus, the results indicate potential for combining fermentation and anaerobic respiration for bioenergy production from photosynthetic biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of biomass-based fuels and energy carriers has been widely studied due to finite petroleum supplies and concerns for environmental effects caused by fossil fuel utilization. Microalgae may prove an alternative to terrestrial crops as they have higher photosynthetic efficiencies, higher yields and growth rates, and lower cultivation area requirements. They may also be cultivated in brackish and saline waters and in ponds, channels or photobioreactors constructed in arid or agriculturally uncultivable land areas [1, 2]. Biochemical conversion of algal biomass to energy carriers via anaerobic microbial metabolism does not require cost-intensive dewatering of the biomass [3]. In addition, algal biomass has high content of lipids, starch, and proteins and does not contain recalcitrant lignin, thus making it amenable to anaerobic digestion processes [1, 2, 4].
Microbial fuel cells (MFCs) convert chemically bound energy into electricity via anaerobic microbial respiration that couples with anode as the final electron acceptor. Many MFC studies have been conducted using model substrates such as acetate, butyrate, and glucose [5, 6]. Some studies have reported the use of complex substrates such as cellulose [7], domestic wastewater [8], paper recycling wastewater [9], and solid animal manure [10]. Power production varies greatly due to differences in MFC configurations and electrogenic microbial consortia [11]. MFCs have been operated with pure cultures or mixed cultures on the anode. Pure cultures have relatively predictable metabolic capabilities but require aseptic process conditions, are prone to process disturbances, have relatively low power outputs, and are usually unable to degrade complex substrates such as plant or algal biomass [12]. Microalgal biomass is potential feedstock for electrogenic bacteria on the anode as electricity production has been reported from suspensions of powderized macro- and microalgal biomass [13], natural marine plankton suspensions [14, 15], and effluent of an anaerobic microalgal biomass digester [16].
The purpose of this work was to utilize the biomass of two microalgal species, freshwater Chlorella vulgaris and marine Dunaliella tertiolecta, as feedstocks in cubic two-chamber MFCs. These were inoculated with microbial consortia derived from a municipal sewage sludge digester and enriched separately for several passages with the two microalgal biomass slurries as the feedstock. The anodic microbial communities were characterized using polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) profiling. Previous MFC studies with algal biomass have focused on power output without much regard to value-added co-products. The present study is to our knowledge the first to report on concurrent electricity and butanol production from untreated microalgal biomass in MFCs. It is inevitable that MFC systems using plant- or algae-based feedstocks will not accomplish complete mineralization of biomass residues. Thus, it is worthwhile to explore the possibility that metabolic pathways in MFC systems be manipulated to generate metabolites that have fuel value or other useful industrial properties. This study was initiated to address this proof-of-concept, an important aspect in the development of sustainable biorefinery approaches.
Experimental
Algal Biomass and Inoculum Enrichment
C. vulgaris (UK strain 211/11B, Culture Collection of Algae and Protozoa, SAMS Research Services Ltd., Oban, Argyll, UK) and Dunaliella tertiolecta (strain SAG 13.86, Sammlung von Algenkulturen Göttingen, Germany) were grown photoautotrophically and harvested with chitosan and NaOH flocculation, respectively [17]. C. vulgaris biomass contained 36, 13, and 8 % proteins, lipids and sugars on dry weight basis, respectively. The corresponding biomass composition of D. tertiolecta was 15, 11, and 4 % [17]. These values fall within the generally broad range reported for microalgal biomass composition [17].
Anaerobic cultures were enriched from a sample of an anaerobic digester operating at 35°C and treating primary and secondary sludge from a municipal activated sludge process in the Viinikanlahti wastewater treatment plant (City of Tampere, Finland). Cultures were first enriched separately with the two algal feedstocks for CH4 production as a series of batch incubations at 37°C with 5 g volatile solids (VS) L−1 algal biomass [17]. The cultures were further enriched in cubic two-chamber MFCs (37°C, 120 rpm) by transferring the culture suspension from the anode compartment to the next enrichment step. Enrichments were batch incubations of 15 days in step 1 and 28 days in step 2 with 5 g VS L−1 algal biomass. Then the enrichment was continued in the MFCs in fed-batch mode with an initial algal biomass loading of 5 g VS L−1 and addition of 2.5 g VS L−1 during each feed cycle in 2- to 4-day intervals in enrichment steps 3 to 6. These fed-batch enrichment steps were incubated for 16, 12, 16, and 13 days, respectively. Thus, the enrichment cultures went through six passages in MFCs with microalgal biomass as the feedstock. These consortia were developed separately for the two algal biomass types. The C. vulgaris-fed consortium is hereafter referred to as U-C and D. tertiolecta-fed consortium as U-D. The enrichment procedure is described in detail in Table 1.
MFC Configuration and Experimental Conditions
The cubic two-chamber MFCs consisted of two polycarbonate or poly(methyl methacrylate) halves separated by an Ultrex proton-exchange membrane (CMI-7000, Membranes International, Ringwood, NJ) (Fig. S1 in the Electronic Supplementary Material (ESM)). The working volumes of both anode and cathode were 75 mL. Graphite plate electrodes (4.6 × 2.7 × 0.6 or 5 × 2.9 × 0.6 cm, McMaster-Carr, Aurora, OH) were used in both chambers and they were pretreated as described by Bond and Lovley [18] before and between each MFC run. Modified Zehnder medium was used as the anolyte [19, 20]. One liter of medium contained 0.4 g KH2PO4, 0.4 g Na2HPO4, 0.3 g NH4Cl, 0.3 g NaCl, 0.1 g CaCl2·2H2O, 0.1 g CaCl2·2H2O, 0.1 g MgCl2·6H2O, 4 g NaHCO3, 2 mg FeCl2·4H2O, 50 μg H3BO3, 50 μg ZnCl2, 38 μg CuCl2·2H2O, 41 μg MnCl2·2H2O, 50 μg (NH4)6Mo7O24·4H2O, 50 μg AlCl3, 50 μg CoCl2·6H2O, 50 μg NiCl2·6H2O, 0.5 mg EDTA, 0.24 g Na2S·9H2O, 26.3 μg Na2SeO3·5H2O, 32.9 μg NaWO4·5H2O, 0.5 g cysteine-HCl, and 0.2 g yeast extract and vitamin solution according to Karlsson et al. [19]. Thus, cysteine was both the oxygen scavenger and assimilatory sulfur source. K-ferricyanide (50 mM K3Fe(CN)6) in phosphate buffer (100 mM Na2HPO4, pH 7.0) was used as the catholyte. Ferricyanide was used in this study as it is more soluble in water and thus a more efficient electron acceptor at the cathode than dissolved O2. The anode compartment was flushed with nitrogen prior to the experiments. The MFCs were inoculated (10 %, v/v) with the enrichment culture and operated at 37°C and 120 rpm shaking in fed-batch mode with feed cycle every 2 days. During each feeding, catholyte was replaced by fresh K-ferricyanide, sample was taken from the anolyte and the sample volume replaced by fresh medium and feed. If the anode pH was below 7.0, it was adjusted to 7.0 ± 0.2 with degassed 1 M NaOH.
A fixed external resistance (R) of 100 Ω was connected between the electrodes and the closed circuit potentials of the MFCs were recorded every 2 min with an Agilent 34970A data logger. The power density was calculated according to the equation, \( P = I \times V/A\), where V is voltage (V), I (I = V/R) the current (amp), and A the surface area of the anode (m2). Polarization characteristics of the MFCs were determined by varying the resistance (between 1 MΩ and 5 Ω) using a variable resistor box from the open circuit voltage stepwise in 5-min intervals. The internal resistance (R i) of the fuel cells was estimated according to the equation \( {R_{\text{i}}} = \left( {{V_{\text{o}}} - {V_{\text{r}}}} \right)/I \), where V o is the open-circuit potential and V r the potential across the external resistance. Coulombic efficiency was calculated by comparing the actual coulombs produced (by integrating the current over time) to the theoretical production of coulombs based on removal of total chemical oxygen demand (CODtot), using a conversion factor of 8 g COD per mole of electrons [21, 22]. The conversion factor is derived from the stoichiometric ratio of 4 mol e−/mol O2 reduced (4e−/32 g O2 = 1e−/8 g O2). The energy content of the produced electricity was calculated by integrating the power over time for the highest electricity producing batch cycle.
In addition to non-pretreated C. vulgaris or D. tertiolecta biomass slurry, electricity production was tested with glucose, pre-digested C. vulgaris and pre-digested D. tertiolecta (biomass after 4–6 weeks of anaerobic digestion). Algal biomass was pre-digested in anaerobic serum bottles with sewage sludge as the inoculum as previously described by Lakaniemi et al. [17]. Aliquots of C. vulgaris and D. tertiolecta biomass were added to the MFCs initially at 5 and subsequently 2.5 g VS L−1 during each feed batch cycle, and the corresponding additions of glucose were 5 and 2.5 g L−1, respectively. For the pre-digested algal biomass, the initial feedstock concentration was 1.2 and 2.1 g VS L−1 and subsequently 0.6 and 1.05 g VS L−1 of pre-digested C. vulgaris and pre-digested D. tertiolecta, respectively. Controls without substrate and without inoculum were also included in the experiments.
Analytical Methods
The pH of anode solution was measured with a WTW pH 3301 pH meter and WTW pH SenTix 41 electrode. The formation of volatile fatty acids (VFAs; including acetate, propionate, butyrate, isobutyrate, valerate, and caproate) and alcohols (ethanol and butanol) was analyzed with a Perkin Elmer Clarus 500 GC system equipped with an HP-5MS column and a flame ionization detector. The temperatures of injector and detector were 250 and 280°C, respectively. Oven temperature was held at 50°C for 3 min, increased from 50 to 100°C at the rate of 20°C min−1, from 100 to 150°C at 10°C min−1 and finally held at 150°C for 5 min. Chemical oxygen demand (COD) was analyzed with dichromate method according to standard SFS 5504 [23]. CODtot was based on unfiltered samples that included all soluble metabolites as well as feedstock biomass, bacteria, cellular debris and digestion products. Gas formation on the anode was measured by connecting tedlar bags (Zefon International, Ocala, FL) to the anode chamber. The gas volume in the gas bags was determined with water displacement method. The gas composition in the gas bags (H2, CH4 and CO2) was measured using Shimadzu gas chromatograph GC-2014 equipped with Porapak N column (80/100 mesh) and a thermal conductivity detector. The oven, injector and detector temperatures were 80, 110 and 110°C, respectively. Nitrogen was used as carrier gas at a flow rate of 20 mL min−1. Conductivity of the anode solution was measured with a WTW LF95 conductivity meter. Samples of precipitates formed on cathode electrode and cathode side of the membrane in MFCs fed with D. tertiolecta biomass were air dried, mounted on Al-stubs, and carbon coated for examination with Philips XL-30 scanning electron microscope (SEM) equipped with ion microprobe for elemental analysis. Concentration of chloride ions was analyzed with Dionex DX-120 ion chromatograph equipped with AS40 auto sampler and IonPac AS23 (4 × 250 mm) anion exchange column. Concentration of sodium ions was analyzed with inductively coupled plasma emission-mass spectrometry according to industry standard DIN EN ISO 17294.
Microbial Community Analyses
Duplicate samples of suspended cultures (1.5 mL) were taken from the anode chamber at the end of the MFC experiment and stored at −20°C. Prior to DNA extraction samples were pelleted by centrifugation (10,000×g, 5 min) and the supernatant was removed. DNA was extracted from the pellets with PowerSoilTM DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA). The extracted DNA sample was used as a template for PCR. Partial bacterial 16S rRNA genes of the community DNA were amplified by using primer pair GC-BacV3f [24] and 907r [25] as described by Lakaniemi et al. [17]. DGGE was performed with INGENYphorU2×2-system (Ingeny International BV, Goes, The Netherlands) using 8 % polyacrylamide gels with denaturing gradient from 30 to 70 % as described by Lakaniemi et al. [17] (100 % denaturing solution contains 7 M of urea and 40 % formamide). The dominant bands were excised from the gels, eluted in 20 μL of sterile water at +4°C overnight, stored at -20°C and reamplified for sequencing. Sequencing was conducted at Macrogen Inc. (Seoul, Korea). Sequence analyses were performed with BioEdit-software and online BLAST software tool.
Results
Enrichment of Electrochemically Active Microbial Community
In first two batch enrichment steps, current generation remained below 0.09 mA with both microalgal feedstocks. Thereafter, fed-batch operation mode was started, and the current increased up to 0.40 mA with D. tertiolecta biomass and U-D, whilst the current still remained very low (0.04 mA) with C. vulgaris and U-C. In the beginning of enrichment step 4, the C. vulgaris MFC was inoculated with 1:1 ratio of anode solution from C. vulgaris and U-C MFC and anode solution from D. tertiolecta and U-D MFC from the previous enrichment step to enhance the current generation from C. vulgaris. This increased the current generation up to 0.28 mA from C. vulgaris. During enrichment steps 5 and 6, current generation remained at similar levels as in previous steps, being at maximum 0.32 and 0.42 mA from C. vulgaris and D. tertiolecta, respectively.
The sum of VFAs and alcohols at the end of each MFC enrichment step increased up to step 5, being 26.7 and 11.7 mM at highest with C. vulgaris and U-C, and D. tertiolecta and U-D, respectively (Fig. S2A in the ESM). Butanol and propionate were the main metabolites detected under these conditions.
Electricity Production
In the electricity production assay, electricity was produced from C. vulgaris with U-C, D. tertiolecta with U-D, glucose with U-C, glucose with U-D as well as from pre-digested D. tertiolecta with U-D (Fig. 1). In contrast, no current was produced from pre-digested C. vulgaris with U-C.
Current over time in fed-batch MFCs with C. vulgaris and U-C (A), D. tertiolecta and U-D (B), glucose and U-C (C), glucose and U-D (D), and pre-digested D. tertiolecta and U-D (E). U-C=C. vulgaris-fed enrichment culture, U-D=D. tertiolecta-fed enrichment culture. Black graph represents MFC A, and grey graph MFC B of the duplicate treatments. Only one replicate was included with the pre-digested D. tertiolecta and U-D
Repeated cycles of substrate addition resulted in a rapid current increase (<5 min) in MFCs fed with glucose and a slower current increase (<11 h) in MFCs fed with C. vulgaris, D. tertiolecta, or pre-digested D. tertiolecta (Fig. 1). In glucose-fed MFCs the current dropped steeply after the maximum, whereas in MFCs fed with algal biomass or pre-digested D. tertiolecta the decrease in the current was slower. The current was at the minimum prior to the next feed batch cycle.
The maximum power density was higher from C. vulgaris with U-C than from D. tertiolecta with U-D (Table 2). Similarly, the maximum power density was higher from glucose with U-C than from glucose with U-D (Table 2). The maximum power density was highest from the pre-digested D. tertiolecta with U-D, but this power was attained only once and all the other current peaks were much lower (Table 2; Fig. 1E). Current peaks after each feeding were wider from D. tertiolecta or glucose with U-D than from C. vulgaris or glucose with U-C (Fig. 1). With C. vulgaris and U-C the voltage decreased to near zero between the feed cycles whereas with D. tertiolecta and U-D the current remained above 0.07 mA at all times. Thus, although higher maximum power densities were obtained with enrichment culture U-C, electricity production was more sustained with enrichment culture U-D. When the energy content of the produced electricity was calculated by integrating the power over time during the highest electricity producing batch cycle, the produced energy was clearly higher from D. tertiolecta than from C. vulgaris (Table 2). Electricity produced from pre-digested D. tertiolecta and from glucose with either enrichment culture was lower than that from the algal biomasses.
COD Removal and Coulombic Efficiency
The relative removal of CODtot with enrichment culture U-C was 18.4 and 17.6 % from C. vulgaris biomass and glucose, respectively. The corresponding values for D. tertiolecta and glucose with enrichment culture U-D were 16.8 and 7.7 %. Although the CODtot removal was generally higher with U-C than U-D, the electrons were more efficiently utilized in electricity generation with U-D based on the coulombic efficiencies (Table 2). Relatively low removals of CODtot were obtained with glucose as the electron donor. Anaerobic glucose oxidation, involving some fermentation, was fast and resulted in accumulation of VFAs at relatively high rates and decrease in pH, which inhibited further metabolism of these intermediate products seen also as low overall COD removal.
Metabolic Products
Methane and hydrogen were not detected in headspace samples of the MFCs. The overall concentrations of VFAs and alcohols increased in the MFCs fed with algal biomass or glucose at the beginning of the incubation (Fig. S2B in the ESM). Metabolite concentrations stabilized to 23.6–28.9 and 11.5–14.2 mM in C. vulgaris- and D. tertiolecta-fed MFCs by day 9 but continued to increase in glucose-fed MFCs (Fig. S2B in the ESM). In algal biomass-fed MFCs, the main metabolites (in a descending order of concentration) were butanol (Fig. 2A) > propionate > butyrate, while in glucose-fed MFCs they were butanol (Fig. 2B) > butyrate > propionate. In C. vulgaris-fed MFCs, the concentration of butanol was 16.1 mM at maximum and 12.7 mM in the end of the experiment, whereas in D. tertiolecta-fed MFCs the corresponding values were 7.0 and 2.5 mM (Fig. 2A). In the MFC fed with pre-digested D. tertiolecta, only low levels (<0.1 mM) of ethanol and propionate were produced. Acetate was not detected under any experimental conditions.
Other MFC Parameters
Anode solution conductivities were on average 9.8, 15.4, 13.9, and 6.6 mS cm−1 in the MFCs fed with C. vulgaris, D. tertiolecta, pre-digested D. tertiolecta, and glucose, respectively. Internal resistances of the MFCs were calculated from the region of constant voltage drop in the center of the polarization curves representing ohmic losses (data not shown). Internal resistances (±standard error) were 136 ± 2, 310 ± 100, 131 ± 0, 2,780 ± 890, and 2,130 ± 33 Ω for MFCs with C. vulgaris and U-C, D. tertiolecta and U-D, pre-digested D. tertiolecta and U-D, glucose and U-C, and glucose and U-D, respectively.
Initially, the pH of anode solution decreased in all MFCs from days 0 to 3. Subsequently, the pH remained relatively constant (near 7.0) with C. vulgaris and D. tertiolecta (Fig. 3A). The pH of anode solution varied more in the beginning with pre-digested D. tertiolecta, but after day 9 the pH stabilized to near 7.5. Anode pH changed more with glucose (Fig. 3B). During each feeding the pH was adjusted to 7.0 ± 0.2 with NaOH, but it dropped to as low as pH 5.7 during incubation indicating VFA production.
The average Cl- concentration in the anode solutions was 1.2, 8.1, and 4.7 g L−1 and Na+ concentration was 1.6, 4.1, and 2.6 g L−1 in MFCs fed with C. vulgaris, D. tertiolecta and pre-digested D. tertiolecta, respectively.
Microbial Community Composition
Suspended cultures were repeatedly transferred to the anode compartment during enrichment. Their bacterial compositions were profiled using PCR-DGGE. Both anaerobic consortia had been originally enriched separately with C. vulgaris and D. tertiolecta biomasses for CH4 production [17]. The cultures were further enriched for electricity production, again separately for the two algal biomass types. The DGGE profiles of U-C and U-D were dissimilar prior to further enrichment for electricity production and developed increasingly differently in the MFCs (Fig. 4).
Bacterial community profiles of the enrichment cultures U-C and U-D. Samples were taken from MFCs fed with C. vulgaris and D. tertiolecta, respectively. MFCs were run in duplicate (A, B) and duplicate samples for microbial community analyses were taken from each MFC. See Table 3 for the labeled bands
Some bacterial 16S rDNA sequences amplified from the MFC enrichments matched uncultured bacteria with no genus or species-level information (Fig. 4; Table 3). The matches with species level information in the U-C enrichment were a Bacteroides sp. (band 1), Wolinella succinogenes (band 3), an uncultured Synergistes sp. (band 4) and C. vulgaris (band 5). In the U-D enrichment they were a Bacteroides sp. (bands 1 and 8), W. succinogenes (band 3), an uncultured Synergistes sp. (band 4), Geovibrio thiophilus (band 10), a Roseobacter sp. (band 11), and a Desulfomicrobium sp. (band 14).
Precipitate Formation in the MFCs
Two types of precipitates were gradually formed on the cathode electrode and the cathode side of the membrane in the MFCs that were fed with D. tertiolecta biomass and pre-digested D. tertiolecta (Fig. 5A). Microprobe analysis with SEM revealed that many precipitates contained Mg, P, and O (P1 in Fig. 5B) and some contained Ca, P, and O (P2 in Fig. 5B).
Discussion
This study demonstrated electricity production in MFCs from untreated C. vulgaris and D. tertiolecta biomass. Electricity was also produced from anaerobically pre-digested D. tertiolecta, but not from pre-digested C. vulgaris. No optimization of the MFC design was undertaken in this study, but the anaerobic inoculum was extensively enriched for these experiments. The sequential enrichment increased current generation from 0.02 to 0.76 mA with C. vulgaris and from 0.03 to 0.53 mA with D. tertiolecta. Differences in electricity production were likely related to different chemical compositions and cell wall structures in the two microalgal biomass stocks. C. vulgaris had a higher content of proteins, lipids and carbohydrates than D. tertiolecta, but lower conductivity due to carry-over of salts with the D. tertiolecta-biomass. C. vulgaris has a rigid cell wall which is relatively recalcitrant unlike the fragile D. tertiolecta, which is prone to lyse. Thus, current peaks were higher with C. vulgaris but current generation was more sustained with D. tertiolecta. Inhibition of electricity production by salt water ions was not apparent.
During pre-digestion, methanogenic conversion of D. tertiolecta was incomplete, whilst C. vulgaris was more efficiently converted to CH4 [17]. Conversely, pre-digested C. vulgaris was less amenable to electricity production than pre-digested D. tertiolecta. Thus most of the chemical energy content of C. vulgaris was already converted to CH4 during pre-digestion before the MFC experiments.
In this study glucose was used as a positive control to establish that the electrogenic culture in the MFCs was metabolically active, but no effort was made to define the corresponding kinetics or maximum power yields with glucose. The conditions in the MFCs were designed for electricity production from algal biomass based feedstock rather than glucose. Glucose was metabolized fast by the mixed population in the anodic chamber as shown by the rapid current increase in glucose-fed MFCs. This resulted in accumulation of VFAs faster than they were further utilized by the consortium. VFA accumulation lowered the anodic pH and thus inhibited the electrogenic activity. Similar phenomenon was observed in a previous methanogenic study [26], where the pH of positive controls with both acid-hydrolyzed reed canary grass (5.76 g L−1 soluble sugars) and glucose (5 g L−1) decreased to as low as pH 3.9–4.3 due to excessive accumulation of VFAs. Consequently, methane production remained low despite subsequent pH adjustment to above 6.0 [26]. In glucose-fed MFCs, the conductivity of anode solution was also lower than in MFCs fed with algal biomass. As a consequence, the internal resistance in the glucose-fed MFCs was significantly higher (approximately 2,100–2,800 Ω) than in MFCs fed with C. vulgaris, D. tertiolecta, or pre-digested D. tertiolecta (130–400 Ω), resulting in a lower maximum power density. Internal resistance is generally lower with soluble substrates than with particulate substrates [10] while suboptimal pH and low conductivity have been reported to increase internal resistance and thus reduce power generation of an MFC [9, 11].
Dominant anaerobic metabolic pathways of microalgal biomass degradation were different in the MFCs as compared with hydrogenic and methanogenic incubations described in a previous study with the same original inoculum [17]. In previous experiments, main soluble metabolites in methanogenic enrichment cultures were acetate and propionate [17], whereas in the MFC cultivations of this study they were butanol, propionate and butyrate. The lack of acetate may be due to the absence of acetogens or presence of electrogenic organisms rapidly oxidizing acetate.
Neither hydrogen nor methane was detected in the anode headspace. The reason for the lack of methanogenesis in anode chambers is not clear, especially since the inocula for MFCs were developed with methanogenic consortia. It is plausible that the biodegradation of algal biomass feedstock did not proceed to H2 and acetate production in amounts that would support detectable CH4 formation. In glucose-fed MFCs methanogenesis was likely limited by the low pH. At near neutral pH values, in the microalgal biomass-fed MFCs, the absence of methanogenesis may also have been due to low levels of oxygen, which may have diffused to the anode chamber from non-gas tight cathode [8, 27]. Some fermentative and electrogenic bacteria are facultative anaerobes and thus less susceptible to oxygen inhibition than methanogens, which are strictly anaerobic organisms [27, 28]. Oxygen diffusion to the anode may also have been an additional reason for the low coulombic efficiencies attained in these systems [27].
The precipitation of Ca and Mg phosphates on the cathode and the cathode side of the cation exchange membrane in MFCs resulted from carryover of Ca2+ and Mg2+ ions in the D. tertiolecta slurry. Salt concentrated biomass and new catholyte with phosphate buffer added to the system during each feed cycle lead to gradual precipitation as Ca2+ and Mg2+ ions were translocated to the cathode chamber through the cation exchange membrane. Ca and Mg phosphate precipitation would also be possible in salt water algae-fed MFCs with biocathodes, if the catholyte contains phosphate buffer. For example, Jeremiasse et al. [29] reported formation of Ca-phosphate precipitates on graphite felt biocathode of a two-chamber microbial electrolysis cell and postulated that the precipitate gradually decreased the current density. Although ferricyanide cathodes were used successfully in this proof-of-concept study, they would not be amenable to commercial MFC applications due to unsustainable nature of ferricyanide as it requires chemical regeneration from ferrocyanide back to ferricyanide [21].
Rabaey et al. [30] have reported that both suspended and attached bacteria perform efficient electron transfer. In this study, suspended (planktonic) bacteria were characterized by analysis of 16S rDNA sequence. The U-C consortium contained Bacteroides spp., W. succinogenes and Synergistes spp. None of these were detected in the preceding methanogenic enrichment cultures that were the original inocula of C. vulgaris-fed MFC cultures [17]. Bacteroides spp., W. succinogenes, and Roseobacter spp. in the U-D consortium were already identified in the original inocula for D. tertiolecta-fed MFCs. Synergistes spp., G. thiophilus, and Desulfomicrobium spp. were not detected in the original inoculum [17]. Bacteroides spp. and W. succinogenes in the electrogenic U-C enrichment culture may also have their origin in the U-D enrichment as it was used to boost electricity production in C. vulgaris-fed MFCs. W. succinogenes [31], Synergistes spp. [32], and Geovibrio spp. [7] have been previously reported in bioelectrochemical systems, but their specific roles in electricity generation are not clear. Bacteroides spp. transfer electrons to Fe(III) [33], and Desulfomicrobium spp. are sulfate-reducers [34] that contain cytochromes with the ability to link electron transfer to the anode electrode [35]. Roseobacter spp. are strictly aerobic bacteria, which are generally found in marine environments and likely originated from the D. tertiolecta slurry [36], but the sequence here may represent a related species capable of growing under anaerobic conditions.
With consortium U-C, the COD removal was generally higher but the coulombic efficiency significantly lower than with consortium U-D. The coulombic efficiencies estimated in this study, 1.4–8.1 %, were low and in the 1.3–5.2 % range previously reported by Zheng and Nirmalakhandan [10] for MFCs fed with solid animal manure. The maximum power densities obtained in this study were also low compared with other complex substrates. For example, the maximum power densities of 55 mW m−2 from cellulose with a two-chamber MFC with ferricyanide cathode [7], 67 mW m−2 from solid animal manure with a single-chamber MFC with air-cathode and brush type anode [10], and 980 mW m−2 from dried C. vulgaris powder with a single chamber MFC with air-cathode and brush type anode [13] have been reported. The recalcitrant nature of untreated C. vulgaris biomass due to its rigid cell wall and the high salinity of D. tertiolecta biomass slurry may limit the anaerobic conversion of the algal biomass stocks [17, 37]. Energy yields as electricity (9.8 and 12.9 J g-VS-1) were orders of magnitude lower than the yields of 10 and 0.86 kJ g-VS-1 recovered as CH4 from C. vulgaris and D. tertiolecta, respectively [17]. It is conceivable that MFC design modification and pretreatment of biomass will lead to improved coulombic efficiency and power density.
In addition to electricity, substantial levels of butanol were detected in the anolytes of MFCs fed with C. vulgaris, D. tertiolecta, or glucose. Finch et al. [38] also reported high butanol concentration in MFC anolytes fed with glucose and inoculated with Clostridium acetobutylicum. Accumulation of high butanol levels retains electrons that would otherwise be shunted in the closed circuit. However, butanol is a prospective candidate as a biofuel and solvent. Based on the butanol concentration in the end of the incubation, the number of feeding cycles and lower heating value of butanol (33.07 kJ g−1) [39], the energy converted to butanol was 1.4 kJ g-VS−1 in C. vulgaris-fed MFCs and 270 J g-VS−1 in D. tertiolecta-fed MFCs. Thus, the energy content of butanol greatly increased the overall energy production of the MFCs and made their energetic yields more comparable with H2 and CH4 production.
Conclusions
This work demonstrated, without optimization of the MFC design, simultaneous electricity and butanol production from untreated biomass of the fresh water microalga C. vulgaris and the marine microalga D. tertiolecta. The maximum power density was higher from C. vulgaris (15 mW m−2), but the power generation was more sustained from D. tertiolecta (13 J g-VS-1). Butanol was produced in the anodes of the MFCs, contributing to the overall energy output of the systems and increasing the estimated energy yield to 1.4 kJ g-VS−1 in C. vulgaris-fed MFCs and 270 J g-VS−1 in D. tertiolecta-fed MFCs. Carry-over of salts with the D. tertiolecta biomass increased solution conductivity but also caused gradual precipitation of Ca and Mg phosphates on the cathode side, which may hinder electricity generation during long-term operation. PCR-DGGE profiling provided matches to bacteria previously described in bioelectrochemical systems.
References
Posten C, Schaub G (2009) Microalgae and terrestrial biomass as source for fuels—a process view. J Biotechnol 142:64–69
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C et al (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res 1:20–43
Lardon L, Hélias A, Sialve B, Steyer JP, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43:6475–6481
Fan LT, Gharpuray MM, Lee YH (1981) Evaluation and pretreatments for enzymatic conversion of agricultural residues. Biotechnol Bioeng Symp 11:29–45
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nature Rev Microbiol 7:375–381
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543
Rismani-Yazdi H, Christy AD, Dehority BA, Morrison M, Yu Z, Tuovinen OH (2007) Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol Bioeng 97:1398–1407
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38:4040–4046
Huang L, Logan BE (2008) Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl Microbiol Biotechnol 80:349–355
Zheng X, Nirmalakhandan N (2010) Cattle wastes as substrates for bioelectricity production via microbial fuel cells. Biotechnol Lett 32:1809–1814
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880
Pham TH, Rabaey K, Aelterman P, Clauwaert P, De Schamphelaire L, Boon N et al (2006) Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng Life Sci 6:285–292
Velasquez-Orta SB, Curtis TP, Logan BE (2009) Energy from algae using microbial fuel cells. Biotechnol Bioeng 103:1068–1076
Reimers CE, Stecher HA III, Westall JC, Alleau Y, Howell KA, Soule L et al (2007) Substrate degradation kinetics, microbial diversity, and current efficiency of microbial fuel cells supplied with marine plankton. Appl Environ Microbiol 73:7029–7040
White HK, Reimers CE, Cordes EE, Dilly GF, Girguis PR (2009) Quantitative population dynamics of microbial communities in plankton-fed microbial fuel cells. ISME J 3:635–646
De Schamphelaire L, Verstraete W (2009) Revival of the biological sunlight-to-biogas energy conversion system. Biotechnol Bioeng 103:296–304
Lakaniemi A-M, Hulatt CJ, Thomas DN, Tuovinen OH, Puhakka JA (2011) Biogenic hydrogen and methane production from Chlorella vulgaris and Dunaliella tertiolecta biomass. Biotechnol Biofuels 4:34
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555
Karlsson A, Ejlertsson J, Nezirevic D, Svensson BH (1999) Degradation of phenol under meso- and thermophilic, anaerobic conditions. Anaerobe 5:25–35
Ejlertsson J, Johansson E, Karlsson A, Meyerson U, Svensson BH (1996) Anaerobic degradation of xenobiotics by organisms from municipal solid waste under landfilling conditions. Antonie van Leeuwenhoek 69:67–74
Logan BE (2008) Microbial fuel cells. John Wiley & Sons Inc., Hoboken, NJ
Zuo Y, Maness PC, Logan BE (2006) Electricity production from steam-exploded corn stover biomass. Energy Fuel 20:1716–1721
SFS (1988) SFS 5504: Determination of chemical oxygen demand (COD Cr) in water with the closed tube method. Oxidation with dichromate. Finnish Standards Association (SFS). Available from: (http://sales.sfs.fi/index.jsp?setLang=1).
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Muyzer G, Hottenträger S, Teske A, Waver C (1996) Denaturing gradient gel electrophoresis of PCR-amplified 16S rDNA—a new molecular approach to analyse the genetic diversity of mixed microbial communities. In: Akkermans ADL, van Elsas JD, de Bruijn F. (eds), Molecular microbial ecology manual. Kluwer, Dordrecht, pp. 3.4.4/1–23.
Lakaniemi A-M, Koskinen PEP, Nevatalo LM, Kaksonen AH, Puhakka JA (2011) Biogenic hydrogen and methane production from reed canary grass. Biomass Bioenerg 35:773–780
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686
Li C, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol 37:1–39
Jeremiasse AW, Hamelers HVM, Buisman CJN (2010) Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 78:39–43
Rabaey K, Boon N, Siciliano SD, Vehaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70:5373–5382
Call DF, Wagner RC, Logan BE (2009) Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl Environ Microbiol 75:7579–7587
Borole AP, Hamilton CY, Vishnivetskaya TA (2011) Enhancement in current density and energy conversion efficiency of 3-dimensional MFC anodes using pre-enriched consortium and continuous supply of electron donors. Bioresour Technol 102:5098–5104
Wang A, Liu L, Sun D, Ren N, Lee DJ (2010) Isolation of Fe(III)-reducing fermentative bacterium Bacteroides sp. W7 in the anode suspension of a microbial electrolysis cell (MEC). Int J Hydrogen Energy 35:3178–3182
Dias M, Salvado JC, Monperrus M, Caumette P, Amouroux D, Duran R et al (2008) Characterization of Desulfomicrobium salsuginis sp. nov. and Desulfomicrobium aestuarii sp. nov., two new sulfate-reducing bacteria isolated from the Adour estuary (French Atlantic coast) with specific mercury methylation potentials. Syst Appl Microbiol 31:30–37
Sallez Y, Bianco P, Lojou E (2000) Electrochemical behavior of c-type cytochromes at clay-modified carbon electrodes: a model for the interaction between proteins and soils. J Electroanal Chem 493:37–49
Ruiz-Ponte C, Cilia V, Lambert C, Nicolas JL (1998) Roseobacter gallaeciensis sp. nov., a new marine bacterium isolated from rearings and collectors of the scallop Pecten maximus. Int J Syst Bacteriol 48:537–542
Carver SM, Hulatt CJ, Thomas DN, Tuovinen OH (2011) Thermophilic, anaerobic co-digestion of microalgal biomass and cellulose for H2 production. Biodegradation 22:805–814
Finch AS, Mackie TD, Sund CJ, Sumner JJ (2011) Metabolite analysis of Clostridium acetobutylicum: fermentation in a microbial fuel cell. Bioresour Technol 102:312–315
Laza T, Bereczky Á (2011) Basic fuel properties of rapeseed oil-higher alcohols blends. Fuel 90:803–810
Acknowledgments
We thank Christopher J. Hulatt and David N. Thomas, School of Ocean Sciences, Bangor University, for providing the algal biomass samples. This research was funded by the Finnish Funding Agency for Technology and Innovation (Finland Distinguished Professor Programme, 402/06).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
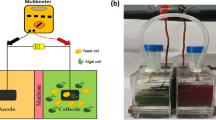
Schematic diagram (A) and photograph (B) of the two-chamber MFC configuration used in this study (http://digitalunion.osu.edu/r2/summer07/nskrinak/assembly.html). (DOC 673 kb)
Fig. S2
Sum of volatile fatty acids (VFAs) and alcohols in the end of the six enrichment steps with C. vulgaris-fed MFCs marked in darker grey and D. tertiolecta-fed MFCs with paler grey (A) and as a time series during the electricity production assay for an MFC with C. vulgaris and U-C (filled diamonds), with D. tertiolecta and U-D (filled squares), with pre-digested D. tertiolecta and U-D (filled triagnles), with glucose and U-C (error marks), and with glucose and U-D (empty circles) (B). (DOC 59 kb)
Rights and permissions
About this article
Cite this article
Lakaniemi, AM., Tuovinen, O.H. & Puhakka, J.A. Production of Electricity and Butanol from Microalgal Biomass in Microbial Fuel Cells. Bioenerg. Res. 5, 481–491 (2012). https://doi.org/10.1007/s12155-012-9186-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9186-2