Abstract
Hydrogen is a clean energy vector that could help to face the current environmental issues of greenhouse gas emissions and, over a longer time scale, to replace the depleting nonrenewable fuels. Biological production by fermentation of waste and residues has the potential to surrogate the current technologies of production of this gas. In this chapter we report a summary of the fermentative pathways related to hydrogen production in the thermophilic microorganisms of the genera Thermotoga and Pseudothermotoga that embrace several marine species with the highest hydrogen yields among eubacteria. The contribution includes a brief review of dark fermentation (DF) and capnophilic lactic fermentation (CLF), the two processes related to hydrogen synthesis in these organisms, together with a discussion of new data concerning the distribution of CLF in these bacteria. The data show a varied scenario with different metabolic capabilities spread across the two genera. Under standard conditions, CLF is active only in few species of Thermotoga genus. The study underlines the great potential of these microbes in the valorization of agro-food waste and production of fuel and chemicals. In particular, the metabolic and biochemical diversity of Thermotoga and Pseudothermotoga species, together with their resilience to different environmental conditions, suggests the possibility to overtake many of the bottlenecks related to operational factors such as substrates, temperature, pH, hydraulic retention time, and hydrogen partial pressure.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The steadily increasing use of renewable forms of energy is envisaged to mitigate climate risks, but effective reduction of the concentration of greenhouse gases entails carbon sequestration strategies and transition to fuels that are not carbon based. Hydrogen (H2) is commonly considered a secure and environmentally safe alternative to traditional energy vectors based on fossil fuels. Utilization of hydrogen by combustion engine or fuel cell produces heat and electricity with pure water as only waste. Nevertheless, hydrogen production requires manufacturing by processes that are currently energy-consuming and polluting . The access to this hydrogen does not solve the environmental issue, and it is clear that only production of the energy vector from renewable sources can be sustainable in the long term.
Hydrogen gas is also the side product of metabolism of a few microorganisms through biological transformations that include photobiological water splitting and fermentation of biomass. The latter process, which comprises photo- and dark fermentation, is particularly interesting because it is entitled of high productivity and allows use of residual material such as agro-food waste. Hyperthermophilic and extremely thermophilic bacteria and archaea are promising candidates for the development of dark fermentation (DF) -based technologies because the rate and yield are higher than in mesophilic processes . In the last years, we reported that substitution of N2 with CO2 in the headspace of a standard culture of Thermotoga neapolitana DSMZ 4359 induces shift from DF to an unprecedented process that we named capnophilic lactic fermentation (CLF) after the mandatory presence of CO2 and increase of lactic acid production [1, 2]. Differently from DF, CLF shows a so far unexplained deviation from the Thauer rule [3] with simultaneous synthesis in good yields of hydrogen and lactic acid [2, 4]. Very recently we noticed the acquisition of a novel phenotype and genotype of our lab strain of T. neapolitana that we have proposed as a new subspecies named T. neapolitana subsp. capnolactica with regard to the improved ability to produce lactic acid under capnophilic conditions [5].
T. neapolitana is a marine , organotroph eubacterium that ferments sugars to hydrogen under strictly anaerobic conditions. The microorganism belongs to the order Thermotogales that embraces a family of extremely thermophilic eubacteria that have been mostly isolated from marine geothermally heated environments across the globe, including shallow and deep-sea marine hydrothermal systems [6]. The order comprises the emended genus Thermotoga and the new genus Pseudothermotoga gen. nov. [7]. The aim of the present study is to give an overview of the previous research on T. neapolitana and to report unpublished results on the occurrence of CLF and DF in the other members of the Thermotoga and Pseudothermotoga genera maintained under standard (TnN2) and capnophilic (TnCO2) conditions.
2 Species and Culture Conditions
2.1 Taxonomy
Thermotogales (phylum Thermotogae, class Thermotogae) are anaerobic, Gram-negative, rod-shaped bacteria encapsulated by a unique “toga”-like outer membrane. These bacteria are strict organotrophs, fermenting preferentially simple and complex carbohydrates or complex organic matter. Genomic data from 17 members of the phylum support the division of the current genus Thermotoga in two evolutionary distinct taxonomic genera. On the basis of genome sequence data and genome-derived characteristics, Bhandari and Gupta [7] have proposed a reclassification of the genus Thermotoga . According to this work, the former genus Thermotoga should retain only the species Thermotoga maritima, Thermotoga neapolitana, Thermotoga petrophila, Thermotoga naphthophila, Thermotoga sp. EMP, Thermotoga sp. A7A, and Thermotoga sp. RQ2, while the other Thermotoga species (Thermotoga lettingae, Thermotoga thermarum, Thermotoga elfii, Thermotoga subterranea, and Thermotoga hypogea) are moved to a new genus, Pseudothermotoga gen. nov. [7]. According to molecular analysis [8], the recently reported Thermotoga caldifontis and Thermotoga profunda should be also included in the Thermotoga genus.
The genus Thermotoga embraces bacteria that have an optimal growth temperature in the range of 77–80 °C [7]. Together with members of the order Aquificales, these bacteria show the highest growth temperatures known so far. The genus Pseudothermotoga includes species that generally grow in the range of 65–70 °C. All members of the order produce hydrogen , but extent can change from one strain to another. The biogas is formed as final product and is released to protect the microorganisms [9].
2.2 Strain Origin
Thermotoga and Pseudothermotoga species have been isolated from geothermally heated environments across the globe, including continental solfatara springs of low salinity, shallow and deep-sea marine hydrothermal systems, and high-temperature marine and continental oil fields, and have been extensively studied for insights into the basis for life at elevated temperatures and for biotechnological opportunities (e.g., biohydrogen production, biocatalysis). Indeed the species T. maritima had been originally isolated from a geothermally heated, shallow marine sediment at Vulcano, Italy [10]. The second species of this genus, T. neapolitana, was obtained from a submarine hot spring at Lucrino near Naples, Italy [11, 12]. Members of the marine T. maritima-T. neapolitana group are widespread within high-temperature ecosystems, such as shallow submarine hydrothermal systems . The isolates from deep-subsurface petroleum reservoirs were T. petrophila and T. naphthophila isolated from the Kubiki oil reservoir production fluid in Niigata (Japan; [13]). T. caldifontis and T. profunda are the newest members of the family [8]. Both species were isolated from terrestrial hot springs in Japan. The optimum growth conditions for strain T. profunda were 60 °C at pH 7.4, and those for strain T. caldifontis were 70 °C at pH 7.4. Further Pseudothermotoga isolates from oil production wells were described as P. elfii [14], Pseudothermotoga subterranea (East Paris Basin; [15]), and Pseudothermotoga hypogea (Africa; [16]) and represent a third ecological group originating from subsurface ecosystems and adapted to levels of salinity intermediate between those of marine species and those of terrestrial species. Pseudothermotoga thermarum was isolated in 1989 from continental solfataric springs with low ionic strength in Lake Abbe, Djibouti [17]. Despite the terrestrial origin, the bacterium is able to grow at low levels of salinity. Pseudothermotoga lettingae was isolated from a sulfate-reducing bioreactor , where methanol was the only carbon source [18].
2.3 Culture Conditions
The following strains of Thermotoga and Pseudothermotoga genera used in this study T. maritima DSMZ 3109T, T. neapolitana DSMZ 4359T, T. petrophila DSMZ 13995T, T. naphtophila DSMZ 13996T, T. caldifontis DSMZ 23272T, T. profunda DSMZ 23275T, P. thermarum DSMZ 5069T, P. elfii DSMZ 9442T, P. subterranea DSMZ 9912T, P. hypogea DSMZ 11164T, and P. lettingae DSMZ 14385T were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). T. neapolitana subsp. capnolactica was originally bought as T. neapolitana DSMZ 4359T and maintained in our laboratory under saturating concentrations of CO2 for several years [5].
Except for T. neapolitana subsp. capnolactica, all the other strains were reactivated from lyophilized samples in Tn medium [19] supplemented with 28 mM (0.5% w/v) glucose and 0.4% (w/v) yeast extract/tryptone by using 120 mL sealed flasks with working volume of 30 mL, without agitation and with pH adjustment to optimal value for each bacterium by means of 1 M NaOH. Anaerobic cultures were grown at optimal temperature for each bacterium (60 °C for T. profunda; 70 °C for T. caldifontis, P. subterranea, P. hypogea, P. lettingae, P. elfii; 80 °C for T. neapolitana, T. neapolitana subsp. capnolactica, T. maritima, T. naphtophila, T. petrophila, and P. thermarum) under N2 atmosphere. Fermentation test batches were inoculated (6% v/v) at room temperature with precultures grown overnight. Before each experiment, culture media were degasified by N2 or CO2 bubbling. Samples were regularly taken (1.5 mL every 24 h) from the cultures to monitor the main fermentation parameters including optical density at λ = 540 nm (O.D.), dry cell weight (mg mL−1), glucose consumption, production of organic metabolites (acetic acid, lactic acid, alanine, valine, ethanol, 2,3-butanediol), hydrogen, and CO2. After each sampling, hydrogen was removed by flushing into culture media pure nitrogen or carbon dioxide for 3 min, and pH was adjusted to optimal value for each bacterium by means of 1 M NaOH [1]. All the cultures were performed in triplicate.
3 Fermentative Hydrogen Production by T. neapolitana
Originally isolated from shallow submarine hot spring near Lucrino in the Bay of Naples in 1986 [11, 12], T. neapolitana is a Gram-negative bacterium that grows between 55 and 90 °C with an optimal growth temperature of 80 °C. An overview on T. neapolitana has been reported in [20]. The species is well-recognized producer of hydrogen from sugar fermentation. In the following paragraphs, we briefly summarize the production of hydrogen by the two main fermentation pathways , dark fermentation and capnophilic lactic fermentation, that are currently known to operate in this eubacterium and in its subspecies, T. neapolitana subsp. capnolactica.
3.1 Dark Fermentation (DF) by T. neapolitana
In analogy with the other members of the genus, T. neapolitana displays a wide metabolic ability to use a large variety of substrates, including sulfur compounds [14]. Conversion of glucose to pyruvate is only due to Embden-Meyerhof-Parnas (EMP) glycolytic pathway, but it has been calculated that about 15% of hydrogen is due to fermentation of protein source [19]. According to the classical model of dark fermentation, glucose oxidation to acetate can theoretically produce 4 mol of hydrogen per mole of sugar [3]. As fermentative hydrogen production is a mean to dispose of electrons, there is a direct relationship between the biogas yield and the type of the organic products that are concurrently released during the process. As shown in Fig. 6.1, yield is optimized only when all glucose is converted to acetate, because there is no leak of electrons in other reactions. The entire pathway is thermodynamically driven by formation of ATP, but when hydrogen accumulates and consumption of NADH stops, pyruvate is diverted away from acetate production. In this case lactic acid or alanine is a common side product of the process [6]. According to the mechanism of Fig. 6.1, no hydrogen is produced when these metabolites are the only products of the fermentation.
Dark fermentation (DF) in T. neapolitana. The marine bacterium operates synthesis of hydrogen from sugar fermentation. Pyruvate is the key intermediate of the process and derives from Embden-Meyerhof-Parnas glycolytic pathway. Hydrogenase (Hyd) is represented by trimer composed of Hydα, Hydβ, and Hydγ. The final organic product of dark fermentation is acetate even if other minor compounds are also released in the medium. The scheme illustrates the direct correlation between acetate synthesis and hydrogen production. The largest yield of hydrogen cannot exceed 4 mol/mol of glucose (Thauer limit) and can be achieved only when acetate is the product of the process (see main text). Fdred = reduced ferredoxin; Fdox = oxidized ferredoxin. Protons and other side products and cofactors are omitted for simplicity
Synthesis of lactate is under control of lactate dehydrogenase (LDH) and requires concomitant reoxidation of NADH to NAD+. Lactate levels reported during fermentation by Thermotoga species vary from trace amounts up to levels rivaling those of acetate [21,22,23,24]. On the other hand, the bacterium lacks the key genes necessary for further reduction of acetyl-CoA to ethanol and butyrate [25,26,27].
Evolution of hydrogen is an efficient way to dissipate excess reductant as a diffusible gas during microbial fermentation and photobiological processes. Synthesis of the gas by T. neapolitana is mostly due to the characteristics of the heterotrimeric [FeFe]-hydrogenase . Hydrogenases (H2ase) constitute a family of metalloenzymes that reduce protons to hydrogen by low-potential electrons. These enzymes play a central role in energy metabolism of many bacteria and archaea but are also found in a few eukaryotes [28]. Hydrogenases are traditionally classified in three groups according to the structure of the catalytic site: [FeFe]-hydrogenases, [NiFe]-hydrogenases, and [Fe]-hydrogenases [28,29,30]. Sequence analysis on the three proteins that form the hydrogenase of T. maritima, which has more than 90% homology with that of T. neapolitana, suggests a [Fe-Fe]-heterotrimer. The α subunit (HydA) is a protein that contains the catalytic site, which is termed H cluster , composed of three 4Fe-4S and two 2Fe-2S clusters [23]. This is one of the most complex iron-containing structures characterized to date and requires the specific action of three highly conserved proteins to be assembled [31, 32]. Other three 4Fe-4S clusters and one 2Fe-2S cluster are also present in the β-subunit (HydB), whereas the hydrogenase γ-subunit (HydC) contains one 2Fe-2S cluster. According to the model suggested by Schut and Adams [23], HydA transfers electrons to H+ from NADH through the β-subunit and from reduced ferredoxin through the γ-subunit. However, despite the detailed proposal of the active site, how the endergonic reaction of hydrogen production is accomplished under physiological conditions has been vague for a long time.
Reduction of H+ is an energetically unfavorable reaction that is typically influenced by environmental conditions such as pH, cell growth rate, and hydrogen partial pressure. In 2008, a new mechanism of driving endergonic redox reactions was proposed for the synthesis of butyric acid from crotonyl-CoA in Clostridium kluyveri by simultaneous transfer of electrons from NADH (E0′ = −320 mV) to ferredoxin (E0′ = −450 mV) and crotonyl-CoA (E0′ = −10 mV) [33]. The process is thermodynamically favored because the flux of electrons is “bifurcated” toward two acceptors with different redox potentials in order to conserve the total energy of the system [34]. Analogously, the hydrogenases of T. maritima and T. neapolitana are suggested to be electron-bifurcating enzymes that couple the endergonic reduction of H+ to hydrogen by NADH with the exergonic reduction of H+ to hydrogen by reduced ferredoxin [23]. The trimeric hydrogenase complex of these bacteria is not able to produce hydrogen with the oxidation of reduced Fd or of NADH when each was used as the sole electron donor. Only, the presence of both biological reductants promoted efficient hydrogen production with yield close to the Thauer limit. Because the hydrogenase of T. neapolitana uses both NADH and reduced ferredoxin as electron donors, hydrogen production may be influenced by factors that affect both reductants. Furthermore, the composite mechanism of this hydrogenase is consistent with the complexity of the trimeric structure, which is much greater than that of the typical Fd-dependent , single subunit [FeFe]-hydrogenase found in Clostridium spp.
3.2 Capnophilic Lactic Fermentation (CLF) by T. neapolitana
A continuous inflow of gases, mainly nitrogen (N2), is the most commonly reported method for removing hydrogen from the reactor headspace and increasing productivity [35, 36]. Sparging pure cultures of T. neapolitana with CO2 significantly increases the rate of both glucose consumption and hydrogen production even if there is no improvement of the overall productivity and molar yield that remained substantially unchanged in comparison with standard N2 conditions [1]. Paradoxically, CO2 stimulated also synthesis of lactic acid. Feeding experiments with labeled precursors clearly proved that at least part of exogenous CO2 was biologically coupled with acetyl-CoA to give lactic acid when the cultures were stripped by CO2 gas or enriched in sodium bicarbonate. The process recycled both glycolysis-derived acetyl-CoA and exogenous acetate. In this latter case, the overall outcome was a conversion of equimolar concentration of acetate and CO2 into lactic acid. The fermentative CO2-dependent synthesis of lactic acid and hydrogen was named capnophilic lactic fermentation (CLF), and as suggested in Fig. 6.2, it put forward the possibility to fully convert sugar to lactic acid (or other reduced derivatives of pyruvate ) without affecting hydrogen synthesis by means of an additional consumption of reducing equivalents deriving from other cellular processes [2].
Capnophilic lactic fermentation (CLF) in T. neapolitana. The process is an anaplerotic pathway that is activated by increase of the concentration of CO2 in the reactor. As depicted in the scheme, acetyl-CoA deriving from sugar catabolism (see Fig. 6.1) undergoes to reductive carboxylation by reverse catalysis of pyruvate:ferredoxin oxidoreductase (PFOR). Newly generated pyruvate is then reduced to lactic acid by lactic dehydrogenase. The pathway is able also to use exogenous acetate. Hydrogen production is not affected by the capnophilic route, thus allowing simultaneous synthesis of lactic and hydrogen in deviation of the Thauer limit. Fdred = reduced ferredoxin; Fdox = oxidized ferredoxin. Protons and other side products and cofactors are omitted for simplicity
The transition from acetate production (dissimilation) to acetate utilization, also named acetate switch, is a common facet in the metabolism of many bacteria [37]. It is noteworthy that, according to acetyl-CoA synthetase (ACS) mechanism , acetate assimilation is an endergonic process that requires one mole of ATP and one mole of CoA per each mole of acetate that is imported and utilized in the bacterial cell. Assimilation of CO2 in T. neapolitana is committed to synthesis of lactic acid and, like in acetogenic bacteria and methanogens [38, 39], is dependent on pyruvate:ferredoxin oxidoreductase (PFOR) . The experiments established the presence of PFOR in strict occurrence with pyruvate synthase activity and production of lactic acid. Labelling of lactic acid during sugar fermentation unambiguously demonstrates that the catabolic and biosynthetic reactions can occur at the same time. However, pyruvate synthase levels were detectable for a longer time in cultures exposed to CO2 thus suggesting that the two processes could be independently regulated by either glucose or CO2. This is consistent with the apparent absence of cross talking between hydrogen production and capnophilic lactic fermentation under CO2-enriched conditions [2].
In Thermotogales reductive carboxylation of acetyl-CoA (Ac-CoA) likely requires the pool of ferredoxin that is also involved in hydrogen production. The role of ferredoxin as efficient reductant in pyruvate synthesis has been demonstrated in vitro with Clostridium thermoaceticum [40] and suggested in vivo for methanogenic archaea, such as Methanosarcina barkeri [41]. It is noteworthy that the sequence of pyruvate oxido-reductase of this last organism has a good relation to those of T. neapolitana and T. maritima [2]. CLF is an example of biological sequestration of CO2 by coupling with an exogenous substrate (acetate, glucose, etc.) and release of the end product (lactic acid) outside of the cell. Incorporation of radioactivity from 14C-glucose into cells proved that little of this carbon is used in primary metabolic pathways. Analogously, no carbon deriving from CO2 or HCO3 − is apparently fixed in the microbial biomass. This is not too surprising but makes a difference between CLF and mechanisms of fixation known in other anaerobes. In fact, unlike autotrophic [42, 43] and heterotrophic [44] pathways for CO2 assimilation, the capnophilic metabolism of T. neapolitana implies complete excretion of CO2 after “incorporation” in lactic acid and no synthesis of primary metabolites (e.g., proteins, sugars, or lipids). Recently, the first mathematical model of this process has been reported to describe the kinetics of the formation of lactate and hydrogen [4]. The model can effectively predict that about 90% of lactate was produced via the CLF pathway.
4 Anaerobic Sugar Fermentation in Thermotoga and Pseudothermotoga Genera
4.1 Bacterial Growth and Tolerance to CO2
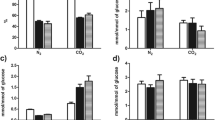
The lyophilized strains of Thermotoga and Pseudothermotoga genera were reactivated and grown on glucose in Tn medium enriched with yeast extract and tryptone [19]. Growth was measured as density at λ = 540 nm (O.D.) and also monitored as wet and dry cell weight. The choice to use the same medium for the different species reflects our decision to make homogeneous the response. This condition is probably not optimal for every strain, and the resulting results do not indicate the best fermentation performance.
As reported in Fig. 6.3, the bacteria showed the common ability to growth on glucose under both N2 (TnN2, standard DF) and CO2 (TnCO2, CLF) sparging. Stimulatory effect of CO2 on the growth of obligate anaerobes is widely recognized [45] even if CO2 is considered an inhibitor of biochemical processes, and, with this aim, the gas is used as a bactericide in food packaging and carbonated beverages.
Stripping with CO2 significantly increases hydrogen yields in mixed cultures due to the removal of hydrogen [46]. A major complication with using CO2 as stripping gas is the formation of bicarbonate accompanied by acidification of the medium and increased osmotic pressure. Acidification has been shown not to be necessarily the primary origin for growth inhibition by CO2 since analogous reduction of the pH in N2 atmospheres did not have the same inhibitory effect. However, higher solute concentrations can lead to growth inhibition and cell lysis. Bicarbonate and carbonate can also react with amino groups forming carbamates, which can affect the function of proteins. In addition, at low external pH, CO2 can diffuse into the cell and lowers the intracellular pH by forming bicarbonate and thus reducing the growth rate. Finally, elevated partial CO2 pressure might inhibit catabolism by reversing the biological decarboxylating reactions.
CO2 atmosphere did not inhibit fermentation and growth of Thermotoga and Pseudothermotoga species despite previous studies report acidification of the medium and increase of the osmotic pressure [47]. This was not unexpected since other hyperthermophilic bacteria, e.g., Pyrococcus furiosus , can grow optimally in the presence of high salt concentrations for the ability to produce osmoprotectants , such as mannosylglycerate, di-myo-inositol phosphate , and glutamate [48].
4.2 Glucose Consumption and Hydrogen Yield Under N2 and CO2 Conditions
Response in Thermotoga and Pseudothermotoga species to CO2-enriched atmosphere was monitored by glucose consumption and production of hydrogen and organic metabolites (acetic acid, lactic acid, and alanine; valine, ethanol, and 2,3-butanediol) according to the methods described by Dipasquale et al. [1].
T. neapolitana subsp. capnolactica, T. neapolitana DSMZ 4359, T. maritima, T. profunda, P. thermarum, and P. subterranea showed the highest consumption rate of glucose and fermented almost completely the sugar under N2 and CO2 conditions (Table 6.1). Except for T. maritima, P. hypogea, and P. lettingae, use of CO2 improved the efficiency of glucose consumption even if the effect was quantitatively different from one species to another. The most evident increase was recorded with T. naphtophila, T. petrophila, T. caldifontis, T. profunda, and P. subterranea, which are all species isolated from oil deposit or deep hot springs where presumably CO2 concentration is higher than in other habitats [8, 13, 15]. All strains also produced hydrogen, but the molar yield (mol hydrogen/mol glucose) in these experimental conditions was significantly higher than 2 only with T. neapolitana subsp. capnolactica, T. petrophila, and T. caldifontis (Table 6.1).
Despite the increase of glucose consumption, almost all species lowered hydrogen production under TnCO2 conditions, and only T. maritima, P. elfii, and P. lettingae showed substantially unvaried yield of the energy vector (Table 6.1). T. neapolitana subsp. capnolactica was the only species to increase H2 yield moving from N2 to CO2. In a few species, production of hydrogen did not reach 50% of the theoretical Thauer limit [3], but it is noteworthy that these experiments were not carried out under optimized culture conditions.
4.3 Organic Metabolite Production Under N2 and CO2 Conditions
1H-NMR analysis of the fermentation broths from TnN2 and TnCO2 experiments revealed that all the tested strains produced a mixture in which acetic acid, lactic acid, and alanine were the predominant and most recurring metabolites (Table 6.2). Despite our expectation on CLF metabolism, CO2 atmosphere did not induce a generalized increase of lactic acid to the detriment of acetic acid. Only P. subterranea showed a significant change in the fermentation products, but this was mostly driven by synthesis of L-alanine . Production of this amino acid is a trait that Thermotogales share with members of the archaeal order Thermococcales. Therefore, it has been proposed that L-alanine production from sugar fermentation is a remnant of an ancestral metabolism that these microbes have inherited from their common ancestor [9]. The species P. hypogea, P. elfii, P. lettingae, and P. subterranea showed a significant increase of alanine production under CO2-enriched atmosphere (Table 6.2). This process was associated to reduction of hydrogen in P. hypogea as probably consequence of a shift of glucose fermentation from acetate to pyruvate and then alanine. The other three species maintained a constant level of hydrogen from N2 to CO2, but we did not observe any reduction in acetate synthesis, thus suggesting that CLF does not operate in these bacteria. It is to note that the constant level of acetate in comparison with N2 cultures suggests that synthesis of alanine is probably independent of DF. The investigation of the mechanism for the biosynthesis of alanine is behind the scope of this work, but the most plausible explanation is derivation of this amino acid from protein catabolism. Amino acids and peptides of the medium, such as the hydrolates of yeast extract and tryptone, have been already shown to contribute to total hydrogen production and biomass formation in T. neapolitana [19, 49].
In addition to acetic acid, lactic acid, and alanine, P. hypogea, P. elfii, and P. lettingae produced valine, while the broth of the other strains contained also ethanol and 2,3-butanediol. T. neapolitana subsp. capnolactica, T. neapolitana DSMZ 4359, T. profunda, P. subterranea, and P. thermarum were the major producers of these products, whereas the highest valine level was found in T. profunda. However, increase of organic acid production under CO2 conditions was found only in T. naphtophila, T. caldifontis, P. elfii, and P. subterranea (Table 6.3). A few species responded to CO2 by reducing the fermentation process.
Analysis of the single components made evident that in most species the acetic acid yields decreased under TnCO2 condition in comparison with TnN2, but only in T. neapolitana subsp. capnolactica, P. hypogea and T. naphtophila, there was a positive effect on lactic acid. Oddly lactic acid production resulted to be lower under capnophilic conditions than TnN2 in the cultures of T. neapolitana DSMZ 4359, T. maritima, T. profunda, P. subterranea, and P. lettingae. The ratio of lactic acid versus acetic acid (LA/AA) showed the highest values with T. neapolitana subsp. capnolactica, T. naphtophila, T. petrophila, T. profunda, and T. hypogea under capnophilic conditions (Fig. 6.4), whereas it remained unchanged or even slightly decreased in comparison with TnN2 in the other strains.
Taking in consideration also glucose consumption and hydrogen yield, T. neapolitana subsp. capnolactica , T. naphtophila and, at a lesser extent, T. petrophila were the only species to give increase of LA/AA ratio with no or little detriment of hydrogen production. Apparently the original strain of T. neapolitana DSZM 4359 does not show the same performance. Our finding of T. neapolitana subsp. capnolactica is rather recent, but we cannot exclude that the mutation with the consequent increase of lactic acid production occurred earlier. Thus, in consideration of the present results , it is very likely that most of our previous reports [1, 4, 19, 50] were due to this new subspecies instead of the original DSZM 4359 strain.
According to the CLF model [1, 2], the key enzymatic step of the pathway is the reductive addition of CO2 to acetyl-CoA catalyzed by an enzyme of the PFOR family. Sequence analysis of the four proteins that compose PFOR showed a very high identity in the three species of Thermotoga genus (Table 6.4). This was in agreement with the results in production of lactic acid in these species under CO2 and indirectly supported the assumption about the role of this enzyme in the regulation of the CLF pathway.
5 Challenges
Dark fermentation can achieve a maximum H2 conversion of 33%, i.e., four molecules of H2 per molecule of glucose. Based on thermodynamics , the process with mesophilic fermentation requires almost double the amount of feedstock compared to operation with thermophilic bacteria to produce the same amount of hydrogen. In general, only extreme thermophilic bacteria and archaea are able to use most of the reducing equivalents formed during glycolysis for the production of hydrogen, thus approaching the theoretical maximum of 4 mol H2 per mol of hexose [3]. Recently, we reported that a combined process based on T. neapolitana under CLF conditions and Rhodopseudomonas palustris 42OL produces 9.4 ± 1.3 mol H2 per mol of glucose [50], which is the highest value reported so far by combining dark and photofermentation.
In addition to the thermodynamic factor , other important aspects do favor the fermentation yields in thermophilic cultures [51]. In particular, due to higher hydrolysis activity, better pathogenic destruction, and less risk of biological contamination, extreme thermophilic fermentation seems also attractive for complex feedstock, such as lignocellulosic residues or agricultural and food residues . However, development of these processes at industrial level is so far hampered by inherent drawbacks, such inoculum production, low cell density, and tolerance.
Capnophilic lactic fermentation (CLF) , as previously reported in T. neapolitana subsp. capnolactica and here described in T. naphtophila and, at a lesser extent, T. petrophila, may become the cornerstone for economically attractive biotechnological production of lactic acid from carbohydrate-rich feedstocks such as agro-food waste. However, this process needs optimization of upstream and downstream processes including the use and treatment of different substrates, the definition of the operational parameters, the reactor design, and the separation of the fermentation products. CLF process can be more economically sustainable if the reactors can handle with high organic loading rate (OLR) and with a short hydraulic retention time (HRT) . These issues can be targeted either by using immobilized biomass or by applying other bioreactor configurations such as packed bed reactor (PBR) or fluidized bed reactor (FBR) .
Hydrogen yield approaching the theoretical maximum value (i.e., Thauer limit) of 4 mol hydrogen/mol glucose can be achieved only if the flux electrons produced by glucose oxidation are directed to reduce protons (Fig. 6.1). Nevertheless, in practice, these reducing equivalents are also used for biosynthetic purposes and the formation of other fermentation products, i.e., lactate. Manipulation of the redox balance has been demonstrated to push the metabolism of mesophilic bacteria toward formation of organic acids. Evidence is that ferredoxin-NADH knob plays an important role for lactic production under CLF condition. Therefore, targeting the redox balance of Thermotoga and Pseudothermotoga species might constitute a promising metabolic strategy to improve the fermentation yields.
CLF pathway represents a first example of biological sequestration of CO2 by coupling with an exogenous substrate (acetate, glucose, etc.) and release of the end product (lactic acid) outside of the cell. Since T. neapolitana does not convert CO2 to the reduced organic compounds required for cell metabolism, the above mechanism is not related to the autotrophic fixation known in other anaerobes . The process appears to be unprecedented, but its regulation is largely unknown to date. However, the pathway offers the possibility to convert directly CO2 into chemicals, which could become an economically feasible option after further improvement. Recently this approach has been explored for production and secretion of isobutyraldehyde and isobutanol directly from CO2 by the genetically modified cyanobacterium Synechococcus elongatus [52]. The application of CLF and Thermotoga and Pseudothermotoga species in biotechnological processes will depend on the full elucidation of the molecular and biochemical characteristics of the process, as well as on selection and engineering of the productive species.
Finally, production of lactate independent of hydrogen suggests that CLF may trigger or be related to metabolic pathways other than glycolysis . One of the possibilities is the contribution of peptides and the effects of sparged CO2 on peptide catabolism. Accurate redox and carbon balances are necessary to provide convincing data about the role of peptide in the lactate synthesis from acetate. Fermentation of Thermotoga and Pseudothermotoga strains is a challenging system in this regard, due to the dependence on peptides in the medium that makes it difficult to determine just how much acetate, hydrogen, or lactate is made as part of peptide versus glucose metabolism . New culture conditions must be designed to allow these measurements and to perform studies committed to estimate the energy and stoichiometry of the fully coupled reactions.
6 Conclusions
Thermotoga and Pseudothermotoga genera embrace a small but heterogeneous group of thermophilic and hyperthermophilic bacteria . To date the scientific and biotechnological attention for these microorganisms has been driven by the capability to produce hydrogen and, at lower extent, to the possibility to clean sites contaminated with petrochemicals and industrial wastes . The recent discovery of taxonomically related mesophilic members ( Mesotoga prima and Mesotoga infera ) [53, 54] and the finding of an apparently unprecedented pathway for synthesis of lactic acid from acetate and CO2 clearly opened the use of these organisms in other biotechnological sectors. In particular, after further improvement, the fermentative transformation of agro-food residues combined with hydrogen production seems to be suitable to design a platform for the valorization of waste and CO2, with recovery of both energy and value-added products. As outlined in this study, only a few members of the two genera show the capability to perform capnophilic lactic fermentation. However, all the species produce energy carriers and fuels, such as hydrogen and ethanol, and chemicals like acetic acid, butyric acid, and lactic acid, among others. In this context, these microorganisms appear as an attractive solution that will reduce those residues and increase the global efficiency of biomass-based production of energy and valuable chemicals, interlinked in a biorefinery concept .
References
Dipasquale L, d’Ippolito G, Fontana A (2014) Capnophilic lactic fermentation and hydrogen synthesis by Thermotoga neapolitana: an unexpected deviation from the dark fermentation model. Int J Hydrog Energy 39:4857–4862
d’Ippolito G, Dipasquale L, Fontana A (2014) Recycling of carbon dioxide and acetate as lactic acid by the hydrogen-producing bacterium Thermotoga neapolitana. ChemSusChem 7:2678–2683
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Pradhan N, Dipasquale L, d’Ippolito G, Fontana A, Panico A, Pirozzi F, Lens PNL, Esposito G (2016) Model development and experimental validation of capnophilic lactic fermentation and hydrogen synthesis by Thermotoga neapolitana. Water Res 99:225–234
Pradhan N, Dipasquale L, d’Ippolito G, Panico A, Lens PNL, Esposito G, Fontana A (2017) Hydrogen and lactic acid synthesis by the wild-type and a laboratory strain of the hyperthermophilic bacterium Thermotoga neapolitana DSMZ 4359T under capnophilic lactic fermentation conditions. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2017.05.052
Frock AD, Notey JS, Kelly RM (2010) The genus Thermotoga: recent developments. Environ Technol 31:1169–1181
Bhandari V, Gupta RS (2014) Molecular signatures for the phylum (class) Thermotogae and a proposal for its division into three orders (Thermotogales, Kosmotogales ord. nov. and Petrotogales ord. nov.) containing four families (Thermotogaceae, Fervidobacteriaceae fam. nov., Kosmotogaceae fam. nov. and Petrotogaceae fam. nov.) and a new genus Pseudothermotoga gen. nov. with five new combinations. Anton van Leeuwenhoek 105:143–168
Mori K, Yamazoe A, Hosoyama A, Ohji S, Fujita N, Ishibashi J, Kimura H, Suzuki K (2014) Thermotoga profunda sp. nov. and Thermotoga caldifontis sp. nov., anaerobic thermophilic bacteria isolated from terrestrial hot springs. Int J Syst Evol Microbiol 64:2128–2136
Huber R, Hannig M (2006) Chapter 12.1. Thermotogales. Prokaryotes 7:899–922
Huber R, Langworthy TA, Konig H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch Microbiol 144:324–333
Belkin S, Wirsen CO, Jannasch HW (1986) A new sulfur-reducing, extremely thermophilic eubacterium from a submarine thermal vent. Appl Environ Microbiol 51:1180–1185
Jannasch HW, Huber R, Belkin S, Stetter KO (1988) Thermotoga neapolitana sp. nov. of the extremely thermophilic, eubacterial genus Thermotoga. Arch Microbiol 150:103–104
Takahata Y, Nishijima M, Hoaki T, Maruyama T (2001) Thermotoga petrophila sp. nov. and Thermotoga naphthophila sp. nov., two hyperthermophilic bacteria from the Kubiki oil reservoir in Niigata, Japan. Int J Syst Evol Microbiol 51:1901–1909
Ravot G, Magot M, Fardeau ML, Patel BKC, Prensier G, Egan A, Garcia JL, Ollivier B (1995) Thermotoga elfii sp. nov, a novel thermophilic bacterium from an African oil-producing well. Int J Syst Bacteriol 45:308–314
Jeanthon C, Reysenbach AL, L’Haridon S, Gambacorta A, Pace NR, Glenat P, Prieur D (1995) Thermotoga subterranea sp. nov., a new thermophilic bacterium isolated from a continental oil reservoir. Arch Microbiol 164:91–97
Fardeau ML, Ollivier B, Patel BKC, Magot M, Thomas P, Rimbault A, Rocchiccioli F, Garcia JL (1997) Thermotoga hypogea sp. nov., a xylanolytic, thermophilic bacterium from an oil-producing well. Int J Syst Bacteriol 47:1013–1019
Windberger E, Huber R, Trincone A, Fricke H, Stetter KO (1989) Thermotoga thermarum sp. nov. and Thermotoga neapolitana occurring in African continental solfataric springs. Arch Microbiol 151:506–512
Balk M, Weijma J, Stams AJM (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol 52:1361–1368
d’Ippolito G, Dipasquale L, Vella FM, Romano I, Gambacorta A, Cutignano A, Fontana A (2010) Hydrogen metabolism in the extreme thermophile Thermotoga neapolitana. Int J Hydrog Energy 35:2290–2295
Pradhan N, Dipasquale L, d’Ippolito G, Panico A, Lens PN, Esposito G, Fontana A (2015) Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int J Mol Sci 16:12578–12600
Johnston J, Mayo MC, Khare A (2005) Hydrogen: the energy source for the 21st century. Technovation 25:569–585
Khanna N, Das D (2013) Biohydrogen production by dark fermentation. WIREs Energy Environ 2:401–421
Schut GJ, Adams MWW (2009) The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: a new perspective on anaerobic hydrogen production. J Bacteriol 191:4451–4457
Wu SY, Hung CH, Lin CN, Chen HW, Lee AS, Chang JS (2006) Fermentative hydrogen production and bacterial community structure in high-rate anaerobic bioreactors containing silicone-immobilized and self-flocculated sludge. Biotechnol Bioeng 93:934–946
Eriksen NT, Nielsen TM, Iversen N (2008) Hydrogen production in anaerobic and microaerobic Thermotoga neapolitana. Biotechnol Lett 30:103–109
Kyazze G, Martinez-Perez N, Dinsdale R, Premier GC, Hawkes FR, Guwy AJ, Hawkes DL (2006) Influence of substrate concentration on the stability and yield of continuous biohydrogen production. Biotechnol Bioeng 93:971–979
Munro SA, Zinder SH, Walker LP (2009) The fermentation stoichiometry of Thermotoga neapolitana and influence of temperature, oxygen, and pH on hydrogen production. Biotechnol Prog 25:1035–1034
Greening C, Biswas A, Carere CR, Jackson CJ, Taylor MC, Stott MB, Cook GM, Morales SE (2016) Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J 10:761–777
Frey M (2002) Hydrogenases: hydrogen-activating enzymes. Chembiochem 3:153–160
Vignais PM, Billoud B, Meyer J (2001) Classification and phylogeny of hydrogenases. FEMS Microbiol Rev 25:455–501
Albertini M, Vallese F, Di Valentin M, Berto P, Giacometti GM, Costantini P, Carbonera D (2014) The proton iron-sulfur cluster environment of the [FeFe]-hydrogenase maturation protein HydF from Thermotoga neapolitana. Int J Hydrog Energy 39:18574–18582
Peters JW, Schut GJ, Boyd ES, Mulder DW, Shepard EM, Broderick JB, King PW, Adams MWW (2015) [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim Biophys Acta 1853:1350–1369
Herrmann G, Jayamani E, Mai G, Buckel W (2008) Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol 190:784–791
Buckel W, Thauer RK (2013) Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim Biophys Acta Bioenerg 1827:94–113
Mizuno O, Dinsdale R, Hawkes FR, Hawkes DL, Noike T (2000) Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresour Technol 73:59–65
Valdez-Vazquez I, Ríos-Leal E, Carmona-Martínez A, Munos-Paez KM, Poggi-Varaldo HM (2006) Improvement of biohydrogen production from solid wastes by intermittent venting and gas flushing of batch reactors headspace. Environ Sci Technol 40:3409–3415
Wolfe AJ (2005) The acetate switch. Microbiol Mol Biol Rev 69:12–50
Butler CS, Lovley DR (2016) How to sustainably feed a microbe: strategies for biological production of carbon-based commodities with renewable electricity. Front Microbiol 7:1–6
Martin WF (2012) Hydrogen, metals, bifurcating electrons, and proton gradients: the early evolution of biological energy conservation. FEBS Lett 586:485–493
Furdui C, Ragsdale SW (2000) The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood-Ljungdahl pathway. J Biol Chem 275:28494–28499
Bock AK, Schonheit P, Teixeira M (1997) The iron-sulfur centers of the pyruvate:ferredoxin oxidoreductase from Methanosarcina barkeri (Fusaro). FEBS Lett 414:209–212
Berg IA, Ramos-Vera WH, Petri A, Huber H, Fuchs G (2010) Study of the distribution of autotrophic CO2 fixation cycles in Crenarchaeota. Microbiology 156:256–269
Braakman R, Smith E (2012) The emergence and early evolution of biological carbon-fixation. PLoS Comput Biol 8:e1002455
Werkman CH, Wood HG (2000) Heterotrophic assimilation of carbon dioxide. Adv Enzymol Relat Areas Mol Biol 74:135–182
Reilly S (1980) The carbon dioxide requirements of anaerobic bacteria. J Med Microbiol 13:573–579
Kim S-H, Han S-K, Shin H-S (2004) Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int J Hydrog Energy 29:1607–1616
Willquist K, Claassen PAM, van Niel EWJ (2009) Evaluation of the influence of CO2 on hydrogen production by Caldicellulosiruptor saccharolyticus. Int J Hydrog Energy 34:4718–4726
Willquist K, Zeidan AA, van Niel EWJ (2010) Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: an efficient hydrogen cell factory. Microb Cell Factor 9:89–105
Cappelletti M, Bucchi G, Mendes JDS, Alberini A, Fedi S, Bertin L, Frascari D (2012) Biohydrogen production from glucose, molasses and cheese whey by suspended and attached cells of four hyperthermophilic Thermotoga strains. J Chem Technol Biotechnol 87:1291–1301
Dipasquale L, Adessi A, d’Ippolito G, Rossi F, Fontana A, De Philippis R (2015) Introducing capnophilic lactic fermentation in a combined dark-photo fermentation process: a route to unparalleled H2 yields. Appl Microbiol Biotechnol 99:1001–1010
Li C, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol 37:1–39
Atsumi S, Higashide W, Liao JC (2009) Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat Biotechnol 27:1177–1180
Hania WB, Postec A, Aüllo T, Ranchou-Peyruse A, Erauso G, Brochier-Armanet C, Hamdi M, Ollivier B, Saint-Laurent S, Magot M, Fardeau ML (2013) Mesotoga infera sp. nov., a mesophilic member of the order Thermotogales, isolated from an underground gas storage aquifer. Int J Syst Evol Microbiol 63:3003–3008
Nesbø CL, Bradnan DM, Adebusuyi A, Dlutek M, Petrus AK, Foght J, Doolittle WF, Noll KM (2012) Mesotoga prima gen. nov., sp. nov., the first described mesophilic species of the Thermotogales. Extremophiles 16:387–393
Acknowledgments
This work is based upon research supported by the PON01_02740 project “Sfruttamento Integrato di Biomasse Algali in Filiera Energetica di Qualità” (SIBAFEQ), Programma Operativo Nazionale—Ricerca e Competitività 2007–2013 and the European Horizon-2020 project “Biological routes for CO2 conversion into chemical building blocks BioRECO2VER)” (Project ID: 760431). The authors are especially grateful to FERRERO SPA and SEPE SRL for the ideative contribution and technical support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Dipasquale, L., Pradhan, N., d’Ippolito, G., Fontana, A. (2018). Potential of Hydrogen Fermentative Pathways in Marine Thermophilic Bacteria: Dark Fermentation and Capnophilic Lactic Fermentation in Thermotoga and Pseudothermotoga Species. In: Rampelotto, P., Trincone, A. (eds) Grand Challenges in Marine Biotechnology. Grand Challenges in Biology and Biotechnology. Springer, Cham. https://doi.org/10.1007/978-3-319-69075-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-69075-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-69074-2
Online ISBN: 978-3-319-69075-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)