Abstract
Significant amounts of cell wall degrading (CWD) enzymes are required to degrade lignocellulosic biomass into its component sugars. One strategy for reducing exogenous enzyme production requirements is to produce the CWD enzymes in planta. For this work, various CWD enzymes were expressed in maize (Zea mays). Following growth and dry down of the plants, harvested maize stover was tested to determine the impact of the expressed enzymes on the production of glucose and xylose using different exogenous enzyme loadings. In this study, a consolidated pretreatment and hydrolysis process consisting of a moderate chemical pretreatment at temperatures below 75°C followed by enzymatic hydrolysis using an in-house enzyme cocktail was used to evaluate engineered transgenic feedstocks. The carbohydrate compositional analysis showed no significant difference in the amounts of glucan and xylan between the transgenic maize plants expressing CWD enzyme(s) and the control plants. Hydrolysis results demonstrated that transgenic plants expressing CWD enzymes achieved up to 141% higher glucose yield and 172% higher xylose yield over the control plants from enzymatic hydrolysis under the experimental conditions. The hydrolytic performance of a specific xylanase (XynA) expressing transgenic event (XynA.2015.05) was heritable in the next generation, and the improved properties can be achieved even with a 25% reduction in exogenous enzyme loading. Simultaneous saccharification and fermentation of biomass hydrolysates from two different transgenic maize lines with yeast (Saccharomyces cerevisiae D5A) converted 65% of the biomass glucan into ethanol, versus only a 42% ethanol yield with hydrolysates from control plants, corresponding to a 55% improvement in ethanol production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocelluosic biomass is an attractive feedstock for the production of biofuels, chemicals, and bioproducts. Biomass provides many benefits, including abundant availability, low cost, sustainability, and the fact that it is not used as a source of food [1–3]. Today, biomass resources envisioned for use in a biorefinery consist of forestry and agricultural residues (corn stover, bagasse, wheat straw, waste wood, and forest trimmings), energy crops (switchgrass, sorghum, and poplar), waste paper, or municipal solid waste [3]. To convert biomass into renewable energy and biochemicals, bioprocesses convert a portion of the biomass into simple sugars, which are converted into biofuels or other bioproducts [1]. The key to the economic success of a biorefinery is to produce soluble sugars at low cost for fermentation or catalytic conversion.
In the past decades, enzymatic hydrolysis processes have attracted increasing interest due to their more selective hydrolysis and the formation of less inhibitory by-products [4]. However, the cost of sugar production through biological conversion is expensive due to the costs of biomass pretreatment and enzymatic hydrolysis. It is known that plant cell walls are recalcitrant to enzymatic hydrolysis because the heterogeneity, chemical composition, and structural features of the cell wall polysaccharides make them inaccessible to hydrolytic enzymes [5]. As a result, enzymatic hydrolysis requires a pretreatment that can make plant cell walls accessible. The molecular mechanisms involved in pretreatment processes include modifying or loosening the cell wall structure, delignifying or removing hemicelluloses, and disrupting the crystalline structure of cellulose [6–8]. The leading pretreatment technologies typically employ harsh conditions that rely on high temperatures (150–220°C) and extreme pHs (very acidic or very alkaline) [6, 7], which requires expensive up-front capital equipment. The extreme pretreatment conditions inevitably cause sugar degradation, resulting in reduced sugar yield and the formation of compounds that are toxic in fermentation, requiring additional steps for detoxification, separation, and neutralization. As a result, the biomass pretreatment becomes the second most expensive step for a biorefinery [8]. In addition to the high pretreatment costs, the high costs of exogenous enzyme loadings, slow hydrolysis rate, and the limited supply of enzymes are hurdles for the commercialization of these processes. Although many scientific efforts have been made to modify current pretreatment technologies [9–15] or introduce new pretreatment chemicals such as ionic liquids [16], and to improve enzyme cocktails [17], the fundamental cost challenges in sugar production from biomass remain unchanged. To fully realize commercial use of lignocellulosic materials for fuel and chemical production, new technologies are necessary to enable economically competitive production processes.
Heterologous expression of cell wall degrading (CWD) enzymes in plants provides a significantly lower cost alternative to the industrial production of enzymes by fermentation [18–20], and significant progress has been made in plant expression of enzymes at reduced costs for commercial use [21–33]. To address the issue of high cost in both pretreatment and enzyme production, we have developed technologies to express CWD enzymes in maize and other plants using Agrobacterium-mediated transformation as described in the literature [34–36]. In contrast to previous strategies to create processing value through plant expression of enzymes, the strategy investigated in this work relies on harvesting the entire plant biomass, containing plant-expressed CWD enzymes, for use as a feedstock in sugar production. Since the hydrolytic enzymes are expressed within the plant, it is anticipated that the mass transfer resistance to enzyme diffusion will be minimized and the non-selective adsorption/binding of enzymes to lignin and other molecules will be reduced. As a result, the transgenic plant biomass with hydrolytic traits can be processed without harsh pretreatments to improve cellulose accessibility for exogenously added enzymes. The expression of different classes of CWD enzymes in a single plant should create a low-cost sugar platform for biofuel and biochemical production.
Previous studies on plant expression of enzymes have investigated a variety of enzymes and plant species but have not focused on process integration based on the novel activities contained within the transgenic plants. Montalvo-Rodriguez et al. [37] conducted a study on autohydrolysis of plant polysaccharides in transgenic tobacco expressing hyperthermophilic enzymes. They found that direct conversion of plant tissue into free sugar was evident using whole plant extracts. A study by Oraby et al. [38] using ammonium fiber expansion pretreatment technology, has demonstrated that about 22% of the cellulose from maize biomass and 30% of the cellulose from rice straw biomass were converted into glucose in transgenic plants expressing E1 (endoglucanase from Acidothermus cellulolyticus). Brunecky et al. [39] demonstrated that in planta expression of E1 reduced cell wall recalcitrance in tobacco and maize when pretreating the transgenic plants using 1% sulfuric acid at elevated temperatures. Autohydrolysis of plant xylans by an apoplastic expression of thermophilic bacterial endoxylanase conducted by Borkhardt et al. [40] showed that the extracts containing plant-expressed xylanase could break down oat spelt xylan to oligomers, primarily xylotriose and xylobiose, and free xylose. However, a systematic study on the effect of plant-expressed CWD enzymes regarding direct processing of plants for sugar production has not been reported. This paper presents data on the hydrolysis of transgenic plant biomass carrying in planta CWD enzymes through the use of a direct, moderate, consolidated pretreatment, and hydrolysis process.
Materials and Methods
Production of Transgenic Maize Plants
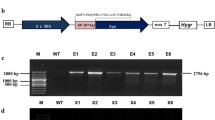
Agrobacterium-mediated transformation of immature maize embryos was performed as described previously [35, 41]. Briefly, the expression cassettes for enzyme genes were cloned into the KpnI-EcoRI sites of the pAG2004 vector to generate an intermediate vector capable of recombining with the pSB1 vector in triparental mating in Agrobacterium tumefaciens strain LBA4404 using procedures reported previously [36, 42–44]. Maize (Zea mays cultivars HiII, A188 or B73) stock plants were grown in a greenhouse under 16 h of daylight at 28°C. Immature zygotic embryos were isolated from the kernels and inoculated with the Agrobacterium solution containing the genes of interest. After inoculation immature embryos were grown in a tissue culture process for 10–12 weeks. Well-developed seedlings with leaves and roots were sampled for PCR analysis to identify transgenic plants containing the genes of interest. PCR positive and rooted plants were rinsed with water to wash off the agar medium and transplanted to soil and grown in the greenhouse to generate seeds and stover.
Plant Stover Preparation
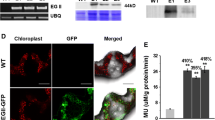
In this study, wild-type and transgenic maize stover were used as biomass substrate for processing. The wild-type control (A×B) represents untransformed A×B maize plants, while transgenic controls TGC.2004 and TGC.4000 used in this study stand for transgenic A×B maize plants generated through the process of A. tumefaciens mediated transformation with the vectors pAG2004 or pAG4000. Transgenic plants expressing enzymes, initially selected by genotyping and enzyme activity in green leaf tissues, were further assayed to confirm enzyme activity in stover [34–36] before the actual hydrolysis test was performed. Harvested greenhouse maize stover was dried in a forced air incubator at 37°C for 1–2 weeks. After drying, the stover was cut manually to 1.0–1.5-in. pieces and then milled using an UDY cyclone mill (Model 014, UDY Corporation, Fort Collins, CO) with a 0.5-mm screen.
Chemicals and Enzymes
Sugar standards (glucose, xylose, arabinose, galactose, mannose, and cellobiose) were purchased from Acros Organics (Morris Plains, NJ). All other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO). Endoglucanase (C8546), β-glucosidase (49291), and endoxylanase (X2753) were all purchased from Sigma (St. Louis, MO). The cellobiohydrolase (E-CBHI) was purchased from Megazyme (Wicklow, Ireland). Accellerase™ 1500 and Accellerase™ XY were generous gifts from Genencor International (Rochester, NY). The yeast (Saccharomyces cerevisiae D5A) was obtained from the American Type Culture Collection (Manassas, VA).
Biomass Carbohydrate Compositional Analysis
Prior to carbohydrate compositional analysis, 3.0 g of air-dried milled stover were refluxed with 90% (v/v) ethanol using a glass Soxhlet extraction system (Fisher Scientific, Pittsburgh, PA) to remove the ethanol-extractable materials by following NREL standards (NREL/TP-510-42619). The extractives-free stover was subject to a two-step acid hydrolysis (NREL/TP-510-42618), which first hydrolyzes at 30°C with 1.5 mL of 72% (w/w) sulfuric acid per 0.16–0.18 g (air dry weight) for 60 min, followed by 121°C for 1 h with supplementation of 42.0 mL of water. After acid hydrolysis, sodium hydroxide and calcium hydroxide were added to adjust the pH to between 4 and 9 and all samples were filtered through 0.2 μm PVDF filters (Fisher Scientific) for high-performance liquid chromatography (HPLC) analysis.
Consolidated Process with Moderate Pretreatment and Saccharification
To evaluate the effect of plant-expressed CWD enzymes on stover hydrolysis, a consolidated process was developed for this study, which consists of a mild pretreatment followed by enzymatic hydrolysis without inter-stage washing of the pretreated biomass. The consolidated process removes any washing/separation/detoxification steps and allows an integrated pretreatment and simultaneous saccharification and fermentation (SSF) process.
Moderate Pretreatment
We developed a mild chemical pretreatment with 0.18 M ammonium bisulfite at pH 8.1. For evaluating plant stover hydrolysis, 20.0 mg milled corn stover was added to 2-mL microcentrifuge tubes with pretreatment chemical solution at a liquor-to-solid (L/S) ratio of 10 or less. The pretreatment was incubated in a shaker at 350 rpm and a temperature of 55–75°C for 16 h. For plant stover hydrolysis evaluation, all the chemical pretreatments were run at 55°C for 16 h. The pretreated material was subject to enzymatic hydrolysis without inter-stage washing.
Enzymatic Hydrolysis
The pretreated stover was subject to direct enzymatic hydrolysis in 1x Britton-Robinson polybuffer [45] with 0.02% sodium azide. The enzymatic hydrolysis was conducted at 2% (w/v) solids content, pH 4.9, 50°C in a New Brunswick shaker (New Brunswick Scientific, Edison, NJ) at 150 rpm for varying times (0–144 h), specifically 72 h for plant stover hydrolysis evaluation. A full in-house enzyme cocktail (FCt) comprising major individual enzyme component (endoglucanase, cellobiohydrolase, β-glucosidase, and endoxylanase (Sigma-Aldrich and Megazyme)) was used for plant stover evaluation with a loading of 10 filter paper units (FPU) per gram glucan. In conjunction, two types of enzymatic hydrolysis were run in parallel: FCt and the enzyme cocktail minus the plant-expressed enzyme (Ct-PE), i.e., enzyme cocktail lacking endoxylanase (Ct-Xyn) or endoglucanase (Ct-EG) or both depending on the enzyme expressed in plant. Hydrolysis was also done with the commercial Accellerase™ enzymes. Accellerase™ 1500 was loaded at 12.5 FPU/g glucan with Accellerase™ XY at 0.1 mL/g dry mass. Glucose and xylose yields (% of theoretical) were expressed as a percentage of total glucose and xylose produced over the glucose and xylose content in each original substrate. Error bars in the graphs are the standard deviation of the mean from three replicate assays.
Simultaneous Saccharification and Fermentation
The yeast (S. cerevisiae D5A) inoculation culture was grown to an OD600 of 0.5 in YPD (10 g/L yeast extract, 20 g/L peptone and 20 g/L dextrose) at 30°C and 250 rpm. The cells were harvested by centrifugation (3,000×g for 5 min) and re-suspended in a 1× YP (10 g/L yeast extract and 20 g/L peptone).
SSF experiments were performed in 250-mL Erlenmeyer glass flasks with a working volume of 50 mL, consisting of 3.0 g (oven dry weight) pretreated biomass, 1× Britton-Robinson buffer, 10× YP (100 g/L yeast extract and 200 g/L peptone), yeast, and hydrolytic enzymes. The flasks were sealed by a rubber stopper with an airlock. The experiments were started by adding yeast and enzymes (Accellerase™ 1500 at 12.5 FPU/g glucan with Accellerase™ XY at 0.1 mL/g dry mass) and were incubated at 35°C and 120 rpm for 7 days. Samples were withdrawn after 0, 24, 48, 72, 144, and 168 h and analyzed for ethanol and sugars as described below.
Analysis of Fermentable Sugars and Ethanol
The hydrolysate samples were heated at 90°C for 20 min to stop hydrolysis and then centrifuged at 10,000×g, following which the supernatants were clarified by filtration through 0.2 μm PVDF filters (Fisher Scientific). Monosaccharide and disaccharide concentrations were determined by HPLC, using a Shimadzu LC-20AD binary pump with LC solutions software (Shimadzu, Kyoto, Japan). Sugar concentrations were determined using an Aminex HPX-87P sugar column (Bio-Rad Laboratories, Hercules, CA) operating at 0.6 mL/min and 80°C with degassed water as the mobile phase. Ethanol concentration in fermentation broth was analyzed using an Aminex HPX-87H Column (Bio-Rad Laboratories) acid column operating at 0.6 mL/min, 60°C with 0.004 M sulfuric acid as the mobile phase. Peak areas for all samples, analyzed with an RI detector (RID 10AD), were integrated and the values were compared to standard curves for quantification.
Results
Carbohydrate Compositional Analysis
The stover from transgenic plants were characterized in terms of their structural carbohydrate composition and sugar content to examine any significant changes caused by genetic modification. The glucan and xylan content from a set of transgenic plants, whether expressing a CWD enzyme or lacking a transgene (TGC), are similar to the wild-type control plants (A×B) (Table 1). A Student’s t test shows there is not a significant difference in the amount of glucan between transgenic maize events expressing CWD enzymes and wild-type maize or between transgenic maize events expressing CWD enzymes and transgenic control events that do not express a CWD enzyme with a P value of 0.90 and 0.14, respectively. The corresponding P values from a t test on xylan content are 0.57 and 0.36, respectively.
Effect of Plant-Expressed CWD Enzymes on Biomass Hydrolysis
For each transgenic plant stover, FCt and Ct-PE enzymatic hydrolyses were run in parallel against a transgenic control plant stover. For the hydrolysis, two criteria as demonstrated in Fig. 1 have been set for evaluation: (1) total sugar yield and (2) sugar yield difference between hydrolysis with FCt versus Ct-PE.
For criterion 1, higher sugar yields are expected from enzyme-expressing plants; for criterion 2, a minimal difference is preferred. Glucose and xylose yields from the in-house cocktail hydrolysis on a pretreated transgenic plant expressing xylanase A (XynA.2015.05) and a pretreated transgenic control plant that does not express a CWD enzyme (TGC.4000.12) are seen in Fig. 1. The glucose and xylose yields from enzymatic hydrolysis of XynA.2015.05 met both criteria. Therefore, this transgenic plant expressing XynA is identified as a candidate with improved hydrolysis performance and used for further study.
After identifying a transgenic event as a leading candidate in its first generation (T0), seeds from this event were planted to grow second-generation (T1) plants. The second-generation stover (XynA.2015.05T1) demonstrated similar glucose and xylose yields to the first generation plant (XynA.2015.05T0), both of which are better than the control plant stover (Fig. 2).
To further explore the effect of in planta expressed enzyme(s) on hydrolysis, time course hydrolysis experiments for selected candidate transgenic plants were conducted. The kinetic changes in glucose yield from FCt hydrolyses of a pretreated transgenic plant XynA.2015.05T1 and a pretreated control plant TGC.2004.08.02 follows a typical profile: a rapid initial increase followed by a slow rising phase at 24 h and a final plateau after 48 h (Fig. 3). The XynA.2015.05T1 demonstrates consistently improved hydrolysis compared with the control plant through the time course. In addition to the higher glucose yields achieved from the enzyme-expressing plants, the results from the time course of hydrolysis also show a higher initial slope in the glucose production (Fig. 3), indicating rapid initial hydrolysis with the transgenic plants than the control plants.
Transgenic plants expressing other xylanase enzymes were also assessed for hydrolysis characteristics. XynB.2063.17 is a transgenic plant expressing xylanase B (XynB). In-house Ct-Xyn hydrolysis of XynB.2063.17 achieved 28.7% higher glucose yield and 86.7% higher xylose yield than TGC.4000.11, a transgenic control, following the same pretreatment and hydrolysis methods (Fig. 4). The improved xylose yield from the expression of XynB was also demonstrated by the difference in xylose yield between the FCt and Ct-Xyn hydrolyses (Fig. 4b).
Enzymatic hydrolysis of plants expressing an endoglucanase (EG) with the in-house cocktail are also examined. For EGA-expressing maize events, both EGA.2049.02 and EGA.2049.10 achieved 48.7–126.9% higher glucose yield compared with the transgenic control plant (TGC.4000.12) (Fig. 5a). The difference in glucose yield between Ct-EG and FCt hydrolysis is negligible for EGA.2049.10 but about 29.1% lower for TGC.4000.12. Similar hydrolysis observations in glucose yield (criterion 2) also existed for EGA.2049.02. In addition, the time courses of enzymatic hydrolysis with the in-house Ct-EG shows the glucose yield of EGA.2049.10 is over 40% higher than that of TGC.4000.11 consistently (data not shown).
Another endoglucanase-expressing plant, the EGB expressing transgenic plant (EGB.2042.03) shows 63.6% higher glucose yield from Ct-EG hydrolysis than the transgenic control (TGC.2004.8.02) (Fig. 5b). The glucose yield from Ct-EG hydrolysis of EGB.2042.03 is only 12.2% lower than from FCt hydrolysis, while the number is 23.0% for TGC.2004.8.02.
We also attempted to express two or more enzymes simultaneously in maize. XynA/AccA/B.2096.01 and XynA/AccA/B.2096.05 are two maize plants that express XynA and accessory enzymes (Acc). The hydrolysis yield from Ct-Xyn hydrolysis on both pretreated events is 80.4% and 93.5% higher than the control plant (TGC.2004.8.02), respectively (Fig. 6a). The xylose yields from Ct-Xyn hydrolysis of the pretreated transgenic tissues are 143.4% and 172.1% higher respectively compared with the control plant (Fig. 6b), which may be attributed to a synergistic effect of multiple enzymes.
Glucose and xylose yields from enzyme hydrolysis with in-house cocktail of pretreated multiple enzyme-expressing plants and pretreated transgenic control plants. a, b Transgenic plants expressing XynA and accessory enzymes (Acc; XynA/AccA/B.2096.01 and XynA/AccA/B.2096.05) versus a transgenic control plant (TGC.2004.8.02). c, d A transgenic plant expressing XynA and EGA (EGA/XynA.2242.09) versus a transgenic control plant (TGC.4000.12)
EGA/XynA.2242.09 is a maize event that expresses both EGA and XynA. The effect of in planta XynA on hydrolysis can also be observed from the improved glucose and xylose yields from Ct-Xyn hydrolysis of pretreated EGA/XynA.2242.09 relative to that of the pretreated control plant (TGC.4000.12) (Fig. 6c, d), which is 50.1% higher in glucose yield and 29.8% higher in xylose yield from Ct-Xyn hydrolysis. The effect of in planta EGA on biomass hydrolysis is noted from the difference in glucose yield between FCt and Ct-EG hydrolysis as well as Ct-Xyn-EG hydrolysis (Fig. 6c, d), which is 102.7% and 140.9% higher than the control, respectively. The similar processing performance is also observed from EGA/XynA.2242.09T1 plants, second generation plants grown from seed of the original EGA/XynA.2242.09. Time courses of hydrolysis with in-house FCt, Ct-EG, and Ct-Xyn on pretreated EGA/XynA.2242.09 also confirmed the effect of the expressed endoglucanase and xylanase with the consistently higher glucose yields than those of the control plant throughout the time course (data not shown).
When increasing pretreatment temperature from 55°C to 65°C and 75°C with hydrolysis using Accellerase™ enzymes, the transgenic plants expressing XynA and EGA (XynA/EGA.2242.09.01 and XynA/EGA.2242.09.07) can achieve up to 83.5–89.1% glucose yield and 50.0–64.3% xylose yield while control plants achieved only 63.0–76.6% glucose yield and 35.7–45.3% xylose yield (Fig. 7).
Effect of pretreatment temperature on glucose (a) and xylose (b) yields from enzyme hydrolysis of pretreated transgenic plants expressing enzymes (XynA/EGA.2242.09.01 and XynA/EGA.2242.09.07) and pretreated control plants (A×B and TGC.4000.11). Enzymes: 0.2 ml Accellerase™ 1500/g stover + 0.1 ml Accellerase™ XY/g stover
Enzyme Loading Reduction and Fermentability
Since the transgenic plants expressing CWD enzyme(s) demonstrated higher hydrolysis yields and more rapid kinetics in hydrolysis over the control plants under the same processing conditions, we examined the impact from reducing exogenous enzyme loadings. Figure 8 presents the hydrolysis results for XynA.2015.5T1 against a control plant TGC.2004.8.4 at varying external enzyme loadings (FCt, 0.75 FCt, 0.50 FCt. 0.25 FCt, 0.10 FCt, and 0 FCt) using the in-house cocktail. Under the experimental conditions, XynA.2015.15T1 achieved over 60% glucose yield with 0.75 FCt but the transgenic control only achieves ∼50% glucose yield with even FCt, demonstrating the potential of reducing exogenous enzyme loadings with the transgenic material while achieving similar sugar hydrolysis yields.
To evaluate the fermentability of the hydrolysates that are produced from transgenic plants expressing CWD enzymes, a SSF experiment was performed using S. cerevisiae D5A. Figure 9 shows the ethanol concentration from SSF of enzyme-expressing transgenic plants EGA.2049.10 and EGA/XynA.2242.09 as well as control plants at an initial solids content of 6% biomass. With the moderately pretreated biomass, SSF of the enzyme-expressing plants (EGA.2049.10 and EGA/XynA.2242.09) produced ethanol at a concentration of 8.0 g/L (∼65% ethanol yield (% of theoretical)) versus 4.5 g/L (∼42% ethanol yield (% of theoretical)) for the control plants, approximately 55% higher ethanol production compared with the control plants.
Discussion
Recently, the expression of CWD enzymes in planta has received increasing attention from industry because it provides a low-cost alternative enzyme source that can be delivered in feedstock crops [20]. Significant advances in the expression of cellulases and hemicellulases in crop plants have been achieved in the past decades [19]. Positive results have been observed when extracting heterologous enzymes from transgenic crops and then adding them to pretreated biomass to convert the biomass into fermentable sugars [37–39]. Since the transgenic plants themselves can be used as biomass feedstocks, the next step was to process them directly and examine the effect of expressing CWD enzymes in planta on biomass hydrolysis.
Biomass Carbohydrate Composition
In planta enzyme expression uses the plant as a “factory” rather than microbial fermentation to produce industrial CWD enzymes. This strategy has the additional advantage of delivering the enzymes directly in the biomass feedstocks for fermentable sugar production. Park et al. [46] found that the overexpression of xyloglucanase in poplar would promote not only cellulose degradation but also the production of cellulose in plants. Therefore, in planta enzyme expression actually provides an opportunity that can simultaneously produce low-cost enzymes and biomass feedstocks with hydrolytic traits for low-cost production of fermentable sugars.
Hydrolysis Evaluation of CWD Enzyme-Expressing Plants
Methodology for Plant Biomass Hydrolysis Evaluation
One of the goals of expressing CWD enzymes in planta is to eliminate or reduce the severity of biomass chemical pretreatment. To determine which enzyme(s) support improved hydrolysis performance, two hydrolysis criteria were established for initial screening of transgenic events. The total sugar yield obtained from processing was the first criterion to be considered because it directly affects the yield of final products, the productivity, and operational cost. With the expressed CWD enzymes, it is expected that enzyme-expressing transgenic plants can achieve better overall hydrolysis than control plants under the same processing conditions as demonstrated from the total glucose and xylose yields in Fig. 1. The second criterion, the sugar yield difference between FCt and Ct-PE, represents an effect of plant-expressed enzymes on hydrolysis. When using transgenic plants as biomass feedstocks, it is expected that exogenous enzyme(s) in the enzyme cocktail can be partially or completely replaced by the expressed CWD enzyme(s) to achieve similar or equal hydrolysis, which can be indicated by a reduced or eliminated difference in sugar yield between FCt and Ct-PE hydrolysis (Fig. 1a). Applying these criteria, we were able to identify CWD expressing transgenic plants with good hydrolysis performance. Some of the plants chosen for this study included xylanase-expressing events: XynA.2015.05 and XynB.2063.17, endoglucanase-expressing plants: EGA.2049.10 and EGB.2042.03, and plants expressing multiple enzymes: XynA/AccA/B.2096.01, and EGA/XynA.2242.09 plants.
Plants Expressing Xylanase
Xylan is known to be the dominant hemicellulose in hardwoods, agricultural residues, and perennial grasses. Xylan is a heteropolymeric biopolymer that consists of a repeating β-1,4-linked xylose backbone decorated with branch groups and may be cross-linked to lignin by aromatic esters [47]. Xylan destruction and removal often benefits the hydrolysis of cellulose into fermentable sugars. In a typical hydrolytic enzyme cocktail, xylanases are a major class of CWD enzymes required to hydrolyze hemicellulose polymers since they play a key role in making cellulose more accessible to enzymatic hydrolysis. As shown in Figs. 1b, 2b, 4b, and 6b & d, the transgenic plant events expressing XynA or XynB (XynA.2015.05T1, XynB.2063.17, XynA/AccA/B.2096.01, and EGA/XynA.2242.09) demonstrated 29.8–172.1% higher xylose yield from Ct-Xyn hydrolysis than the control plants, indicating the enhanced effect due to the plant-expressed xylanase on biomass xylan hydrolysis. Correspondingly, these transgenic plants also show 50.1–93.5% higher glucose yield from Ct-Xyn hydrolysis than did the control plants. Higher glucose yield for the xylanase-expressing transgenic plants (XynA.2015.05T1) was also observed from the time course hydrolysis of pretreated XynA.2015.05T1 (Fig. 3). Therefore, in planta xylanase expression can be considered as an enzyme pretreatment to improve both biomass hemicellulose and cellulose hydrolysis.
In addition to the better hydrolysis, the transgenic plants expressing CWD enzymes also show more rapid initial hydrolysis than does the control plant (Fig. 3). During growth, the CWD enzymes accumulate, embedded within the plant. Once activated during processing, they can commence catalysis immediately in situ without the need for transport and diffusion. The efficiency of these enzymes is therefore expected to be high because of low resistance from mass transfer and an expected decrease in non-selective binding of the enzymes to lignin or other non-target molecules. The overexpression of plant biomass degrading enzymes in plants does not appear to result in a decrease in cellulose, but rather loosened xyloglucan intercalation, followed by an irreversible wall modification [46]. In addition, the expressed enzymes might be in a proximity to the cell wall polymers, which may directly or indirectly facilitate hydrolysis. All these factors may contribute to the faster hydrolysis for enzyme-expressing plants.
Plants Expressing Cellulase
Lignocellulosic biomass is known to be composed of a matrix with multiple intertwined biopolymers (cellulose, hemicelluloses, lignin, and extractives), which requires several different classes of enzymes to efficiently release fermentable sugars. Among them, cellulase is a key enzyme. Three types of cellulases (endoglucanase, exoglucanase and β-glucosidase) work together to hydrolyze cellulose into glucose. In a typical enzymatic hydrolysis process, the endoglucanase breaks down cellulose chains into oligomers while the exoglucanase hydrolyzes the individual oligomers and the β-glucosidase breaks down cellobiose into monomers of glucose [20]. The endoglucanase-expressing plants (EGA.2049.10 and EGB.2042.03) demonstrated 48.9% and 63.6% higher glucose yield from Ct-EG hydrolysis than did the control plants (Fig. 5), which is also confirmed by the consistently higher glucose yields from Ct-EG hydrolysis of EGA.2049.10 throughout the time course (data not shown). Therefore, both a more rapid and greater extent of hydrolysis has been achieved from the transgenic plants with endoglucanase expression.
Plants Expressing Multiple Enzymes
Since the ultimate goal of our technology is to develop an efficient and inexpensive enzyme production system for rapid and less expensive biomass depolymerization, several key enzymes required in the hydrolytic enzyme cocktail have been expressed in maize. When expressing multiple key enzymes in a single plant, a synergistic hydrolytic effect is expected to be achieved, which is demonstrated by the higher glucose yield from Ct-Xyn-EG hydrolysis of EGA/XynA.2242.09 (Fig. 6c). Improved hydrolysis of transgenic plants that express multiple enzymes was also observed from the glucose and xylose yields for XynA/AccA/B.2096.01 (Fig. 6a, b), which is most likely caused by a synergistic effect.
Heritability of Hydrolytic Traits
The XynA.2015.05 plant was identified as a good first generation (T0 plant) hydrolysis candidate. Similar to XynA.2015.05T0, the second generation of 2015.05 (XynA.2015.05T1) also demonstrated better hydrolysis than the control, which is 55.3% higher in glucose yield and 101.6% higher in xylose yield from Ct-Xyn hydrolysis than the control plant (TGC.4000.12) (Fig. 2), indicating that the enzyme based hydrolytic trait is heritable from one generation to the next. The heritability of these traits is also demonstrated by the hydrolysis results of pretreated EGA/XynA.2242.09T0 (Fig. 6c, d), EGA/XynA.2242.09.01T1 and EGA/XynA.09.07T1 (Fig. 7).
Enzyme Loading Reductions and Fermentation
It has been demonstrated that the transgenic plants with expressed CWD enzymes achieved significantly higher and more rapid biomass hydrolysis than the control plants with a mild pretreatment. The improved hydrolysis has the potential to be translated into a reduction in the exogenous enzyme loading while still maintaining similar hydrolysis yield (Fig. 8). In planta expression of CWD enzymes demonstrated the production of low-cost sugars from transgenic crops for the production of biofuels, biochemicals, and biomaterials. The rapid initial hydrolysis may decrease hydrolysis times, an advantage for a simultaneous saccharification and fermentation process (Fig. 9) and an opportunity to decrease the requirement for equipment capacity and operation cost.
In planta production of cell wall degrading enzymes is a proposed means to lower the costs associated with fermentable sugar production from biomass. This report is the first description of the direct hydrolysis of transgenic plants with expressed CWD enzymes. The improved hydrolysis results demonstrate a promising approach to produce biomass feedstocks with hydrolytic traits. Current work focuses on multiple enzyme expression with high enzyme accumulation as well as process optimization to achieve desired industrial hydrolysis metrics through a mild pretreatment and significantly reduced exogenous enzyme loading.
Abbreviations
- XynA:
-
Endo-β-1,4-xylanase 229B from Dictyoglomus thermophilum
- XynB:
-
Endo-β-1,4-xylanase from Thermomyces lanuginosus
- EGA:
-
Endo-β-1,4-glucanase from Nasutitermes takasagoensis
- EGB:
-
Endo-β-1,4-glucanase from Acidothermus cellulolyticus
- AccA:
-
Feruloyl esterase A from Aspergillus niger
- AccB:
-
Feruloyl esterase B from Aspergillus niger
- AccA/B:
-
Feruloyl esterase A and feruloyl esterase B from Aspergillus niger
References
Fernando S, Adhikari S, Chandrapal C, Murali N (2006) Biorefineries: current status, challenges, and future direction. Energy & Fuels 20:1727–1737
Langeveld JWA, Dixon J, Jaworski JF (2010) Development perspectives of the biobased economy: a review. Crop Sci 50:S131–S151
Hess JR et al (2003) Roadmap for agricultural biomass feedstock supply in the United States. DOE/NE-ID-11129
Galbe M, Zacchi G (2002) A review of the production of ethanol from softwood. Appl Microbiol Biotechnol 59:618–628
Zhu L, O’Dwyer JP, Chang VS, Granda CB, Holtzapple MT (2008) Structural features affecting biomass enzymatic digestibility. Bioresour Technol 99:3817–3828
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY (2005) Coordinated development of leading biomass pretreatment technologies. Bioresour Technol 96(18):1959–1966
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M et al (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13):4851–4861
Lee SH, Inoue S, Teramoto Y, Endo T (2010) Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process II: effect of hot-compressed water treatment. Bioresour Technol 101(24):9645–9649
Zhu JY, Pan X, Zalesny RS Jr (2010) Pretreatment of woody biomass for biofuel production: energy efficiency, technologies, and recalcitrance. Appl Microbiol Biotechnol 87(3):847–857
Xu J, Cheng JJ (2011) Pretreatment of switchgrass for sugar production with the combination of sodium hydroxide and lime. Bioresour Technol 102(4):3861–3868
Verma P, Watanabe T, Honda Y, Watanabe T (2011) Microwave-assisted pretreatment of woody biomass with ammonium molybdate activated by H(2)O(2). Bioresour Technol 102(4):3941–3945
Zeng J, Singh D, Chen S (2011) Biological pretreatment of wheat straw by Phanerochaete chrysosporium supplemented with inorganic salts. Bioresour Technol 102(3):3206–3214
Zhao X, Zhang L, Liu D (2010) Pretreatment of Siam weed stem by several chemical methods for increasing the enzymatic digestibility. J Biotechnol 5(5):493–504
Liu L, Sun J, Li M, Wang S, Pei H, Zhang J (2009) Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour Technol 100(23):5853–5858
Shill K, Padmanabhan S, Xin Q, Prausnitz JM, Clark DS, Blanch HW (2011) Ionic liquid pretreatment of cellulosic biomass: enzymatic hydrolysis and ionic liquid recycle. Biotechnol Bioeng 108(3):511–520
Banerjee G, Scott-Craig JS, Walton JD (2010) Improving enzymes for biomass conversion: a basic research perspective. Bioenerg Res 3:82–92
Abramson M, Shoseyov O, Shani Z (2010) Plant cell wall reconstruction toward improved lignocellulosic production and processability. Plant Sci 178(2):61–72
Sainz MB (2009) Commercial cellulosic ethanol: the role of plant-expressed enzymes. In Vitro Cell Dev Biol-Plant 45:314–329
Sticklen MB (2008) Plant genetic engineering for biofuel production: towards affordable cellulosic ethanol. Nat Rev Genet 9:433–443
Herbers K, Wilke I, Sonnewald UA (1995) Thermostable xylanase from Clostridium thermocellum expressed at high levels in the apoplast of transgenic tobacco has no detrimental effects and is easily purified. Nat Biotechnol 13:63–66
Dai Z, Hooker BS, Anderson DB, Thomas SR (2000) Expression of Acidothermus cellulolyticus endoglucanase E1 in transgenic tobacco: biochemical characteristics and physiological effects. Transgenic Res 9:43–54
Dai Z, Hooker BS, Anderson DB, Thomas SR (2000) Improved plant-based production of E1 endoglucanase using potato: expression optimization and tissue targeting. Mol Breeding 6:277–285
Ziegelhoffer T, Will J, Austin-Phillips S (1999) Expression of bacterial cellulase genes in transgenic alfalfa (Medicago sativa L), potato (Solanum tuberosum L) and tobacco (Nicotiana tabacum L). Mol Breeding 5:309–318
Patel M, Johnson JS, Brettell RIS, Jacobsen J, Xue GP (2000) Transgenic barley expressing a fungal xylanase gene in the endosperm of the developing grains. Mol Breeding 6:113–124
Ziegler MT, Thomas SR, Danna KJ (2000) Accumulation of a thermostable endo-1, 4-β-d-glucanase in the apoplast of Arabidopsis thaliana leaves. Mol Breeding 6:37–46
Ziegelhoffer T, Raasch JA, Austin-Phillips S (2001) Dramatic effects of truncation and sub-cellular targeting on the accumulation of recombinant microbial cellulase in tobacco. Mol Breeding 8:147–158
Kimura T, Mizutani T, Sakka K, Ohmiya K (2003) Stable expression of a thermostable xylanase of Clostridium thermocellum in cultured tobacco cells. J Biosci Bioeng 95:397–400
Biswas GCG, Ransom C, Sticklen M (2006) Expression of biologically active Acidothermus cellulolyticus endoglucanase in transgenic maize plants. Plant Sci 171:617–623
Hyunjong B, Lee DS, Hwang I (2006) Dual targeting of xylanase to chloroplasts and peroxisomes as a means to increase protein accumulation in plant cells. J Exp Bot 57:161–169
Ransom C, Balan V, Biswas G, Dale B, Crockett E, Sticklen M (2007) Heterologous Acidothermus cellulolyticus 1,4 β-endoglucanase E1 produced within the corn biomass converts corn stover into glucose. Appl Biochem Biotechnol 36:207–220
Yang P, Wang Y, Bai Y, Meng K, Luo H, Yuan T et al (2007) Expression of xylanase with high specific activity from Streptomyces olivaceoviridis A1 in transgenic potato plants (Solanum tuberosum L.). Biotechnol Lett 29:659–667
Linger JG, Adney WS, Darzins A (2010) Heterologous expression and extracellular secretion of cellulolytic enzymes by Zymomonas mobilis. Appl Environ Microbiol 76(19):6360–6369
Gray BN, Bougri O, Carlson AR, Meissner, Pan S, Parker MH, Zhang D, Samoylov V, Ekborg NA, Raab RM (2011) Global and grain-specific accumulation of glycoside hydrolase family 10 xylanases in transgenic maize (Zea mays). Plant Biotechnol J (in press) doi:10.1111/j.1467-7652.2011.00632.x
Negrotto D, Jolley M, Beer S, Wenck AR, Hansen G (2000) The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep 19:798–803
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotech 14:745–750
Montalvo-Rodriguez R, Haseltine C, Huess-LaRossa K, Clemente T, Soto J, Staswick P et al (2000) Autohydrolysis of plant polysaccharides using transgenic hyperthermophilic enzymes. Biotechnol Bioeng 70(2):151–159
Oraby H, Venkatesh B, Dale B, Ahmad R, Ransom C, Oehmke J et al (2007) Enhanced conversion of plant biomass into glucose using transgenic rice-produced endoglucanase for cellulosic ethanol. Transgenic Res 16:739–749
Brunecky R, Selig MJ, Vinzant TB, Himmel ME, Lee D, Blaylock MJ et al (2011) In planta expression of A. cellulolyticus Cel5A endocellulase reduces cell wall recalcitrance in tobacco and maize. Biotechnol Biofuels 4(1):1–10
Borkhardt B, Harholt J, Ulvskov P, Ahring BK, Jørgensen B, Brinch-Pedersen H (2010) Autohydrolysis of plant xylans by apoplastic expression of thermophilic bacterial endo-xylanases. Plant Biotechnol J 8(3):363–374
Ishida Y, Hiei Y, Komari T (2007) Agrobacterium-mediated transformation of maize. Nat Protoc 2:1614
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries ofthe T-DNA. Plant J 6:271–282
Hiei Y, Komari T (2006) Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 85:271–283
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
Britton HTK, Robinson RA (1931) Universal buffer solutions and the dissociation constant of veronal. J Chem Soc 10:1456–1462
Park YW, Baba K, Furuta Y, Iida I, Sameshima K, Arai M et al (2004) Enhancement of growth and cellulose accumulation by overexpression of xyloglucanase in poplar. FEBS Lett 564:183–187
Dodd D, Cann IO (2009) Enzymatic deconstruction of xylan for biofuel production. Glob Change Biol Bioenergy 1(1):2–17
Acknowledgments
We thank Genencor International for its generous gifts of Accellerase™ 1500 and Accellerase™ XY.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., VanFossen, A.L., Pagano, R.M. et al. Consolidated Pretreatment and Hydrolysis of Plant Biomass Expressing Cell Wall Degrading Enzymes. Bioenerg. Res. 4, 276–286 (2011). https://doi.org/10.1007/s12155-011-9138-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-011-9138-2