Abstract
Purpose
Crossed cerebellar diaschisis (CCD) has been widely studied in hemispheric stroke but is less characterized in epilepsy. In this study, we used 18F-FDG PET/CT to investigate the risk factors for CCD and its prognostic value for intractable epilepsy caused by focal cortical dysplasia (FCD).
Methods
One hundred medically intractable epilepsy patients pathologically diagnosed with FCD postoperatively were included and classified into two groups: CCD+ and CCD−. PET/CT images were analyzed qualitatively by visual assessment and semi-quantitatively using the absolute asymmetry index (|AI|). Clinical factors, including age, sex, body mass index (BMI), age at seizure onset, epilepsy duration, seizure type, seizure frequency, electroencephalography (EEG) and brain magnetic resonance imaging (MRI), were retrospectively assessed from medical records. Follow-up outcomes were evaluated according to the Engel classification at 3, 6, 12, 24 and 36 months postoperatively.
Results
Of the 100 patients, 77 (77.0%) were classified as CCD−, and 23 (23.0%) were classified as CCD + . CCD+ patients had a higher number of lobes involved on PET (3.61 ± 2.16 vs 2.26 ± 1.01, P < 0.001) than CCD- patients. CCD- patients showed more negative MRI results than CCD + patients (P = 0.02). At 12 months postoperatively, 70.7% (29/41) of CCD- patients and 30.8% (4/13) of CCD + patients presented a favourable prognosis (P = 0.02). Significant differences in the average |AI| values in the posterior frontal and anterior temporal lobes were found between CCD+ and CCD− patients (P < 0.05).
Conclusion
The number of lobes involved on PET, structural anomalies on MRI, the |AI| values in the posterior frontal and anterior temporal lobes may be predisposing factors for CCD. CCD occurrence may help predict the prognosis of FCD patients at 12 months postoperatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epilepsy is the most common, chronic, serious neurological disease, with 65 million people affected worldwide [1]. However, almost 30% of seizures cannot be controlled with antiepileptic drugs [2], reflecting medically intractable epilepsy. Focal cortical dysplasia (FCD) is one of the most common causes of medically intractable epilepsy, which can be effectively treated by surgery [3]. However, a significant portion of patients continue experiencing seizures following surgery; thus, preoperative evaluation of the epileptogenic zone (EZ) is particularly important. Magnetic resonance imaging (MRI), positron emission tomography (PET)/computed tomography (CT) and electroencephalogram have been widely used for pre-surgical localization of the EZ. The sensitivity of 18F-FDG PET, as a form of functional imaging, for localizing the EZ has been reported to be 85%–94% [4, 5]. 18F-FDG, as the most commonly used PET tracer, was used to measure glucose metabolism related to the synaptic and neuronal activity of brain tissue [6]. Interictal 18F-FDG PET often showed hypometabolism of the EZ. In addition to the EZ, hypometabolism is sometimes displayed in remote sites, such as the contralateral cerebellum and ipsilateral thalamus, which is called "diaschisis".
In 1914, von Monakow coined the term “diaschisis” to describe the neurophysiological changes that occur distant to a focal brain lesion, and many years later, in 1981, Baron described the occurrence of crossed cerebellar diaschisis (CCD) after hemispheric stroke [7]. CCD is a phenomenon including decreases in metabolism and blood flow in the cerebellar hemisphere contralateral to a supratentorial lesion. In the following decades, this concept attracted clinical interest, with researchers attempting to investigate associated biomarkers and the mechanism and clinical role of CCD using single-photon emission computed tomography (SPECT), PET or MRI. These studies focused mainly on cerebrovascular diseases, especially cerebral infarction. Although many studies on the CCD phenomenon have been conducted in other supratentorial lesions, such as epilepsy, tumors, and cerebral trauma, most published literature on the topic includes case reports with limited patient populations. Biersack first reported three cases of CCD in epilepsy patients using 99mTc-HMPAO SPECT before and during the Wada test in 1987 [8]. To date, no systematic analysis of medically intractable epilepsy caused by FCD with CCD using 18F-FDG PET/CT has been performed, and no study has detected associations between CCD and surgery outcomes. This study was designed to clarify relevant factors for CCD and investigate the relationship between CCD occurrence and the prognosis of epilepsy surgery.
Materials and methods
Subjects
All patients from our institution are informed that their medical data can be rendered anonymous and used for scientific purposes. No opinion of our China ethic committee was required in this study, because of its retrospective nature and because only routine investigations were performed. Patients with medically intractable epilepsy who had undergone 18F-FDG PET/CT preoperatively between April 2015 and July 2018 were retrospectively reviewed. One hundred (30 females and 70 males; mean age ± standard deviation (SD), 24.18 ± 9.22 years) patients with medically intractable epilepsy caused by FCD were included in this study. We classified all the patients into two groups depending on the PET/CT images: CCD positive (CCD+) and CCD negative (CCD−). Patients were pathologically diagnosed with FCD postoperatively (lesion resection). Clinical factors, including age, sex, body mass index (BMI), age at seizure onset, epilepsy duration, seizure type, seizure frequency, electroencephalography (EEG), and brain MRI, were reviewed. Epileptic seizure types were classified as “motor onset” or “non-motor onset” according to the 2017 International League Against Epilepsy (ILAE) guidelines [9]. Seizure frequency was classified as daily, weekly, monthly and yearly [10]. Epileptiform discharges revealed by EEG were classified as focal (seizures originating in a single lobe) or non-focal (seizures originating in more than two lobes, bilateral seizures, or generalized seizures).
The inclusion criteria were as follows: clinical diagnosis of epilepsy [11]; failure of more than two kinds of antiepileptic drugs; epileptic seizure-free status in excess of 24 h before 18F-FDG PET/CT [12]; and available follow-up data for the Engel classification of postoperative outcomes [13]. The exclusion criteria were as follows: 18F-FDG PET/CT image showed a bilateral hypometabolism; diabetes, hypertension, smoking, cerebrovascular disease, such as infarct or cerebrovascular stenosis found by MRI, magnetic resonance angiography (MRA) or computed tomography angiography (CTA), pathology-detected FCD with, tumors, heterotopic gray matter, cerebrovascular malformation and so on, and a clinical history of febrile convulsion or head trauma.
PET/CT imaging
All PET/CT scans were performed on an uMI 510 PET/CT scanner (United Imaging Healthcare, Shanghai, China). 18F-FDG was produced on site in a radiochemistry laboratory, and the radiochemical purity of the tracer was greater than 98%. Interictal 18F-FDG PET/CT brain scans were acquired 40 min after intravenous injection of the radiotracer 18F-FDG (3.7 MBq/kg). All patients were instructed to fast for 4–6 h before injection. After injection, the patients were asked to keep their eyes closed and remain quiet during the 40-min uptake period. The scan protocol included low-dose CT acquisition, which was used for attenuation correction and localization. The CT image parameters were a slice thickness of 3.0 mm and a scanning time of 11.4 s. A single PET bed position was subsequently acquired for 15 min. Iterative reconstruction was performed with a matrix size of 128 × 128 and a slice thickness of 2.44 mm.
Qualitative analysis
Two nuclear medicine physicians analyzed the 18F-FDG PET/CT images by visual assessment. The brain was classified into 14 regions: six in each supratentorial cerebral hemisphere (anterior and posterior frontal, anterior and posterior temporal, parietal and occipital lobes) and one in each cerebellar hemisphere [14]. Each brain region was assessed separately. We defined hypometabolic brain regions as brain regions showing decreased radiotracer uptake in more than two positions and two continuous images compared with the contralateral symmetrical areas. When a diagnostic disagreement occurred between the two readers, a discussion ensued until a consensus was reached.
Semi-quantitative analysis
Semi-quantitative analysis was completed by two experienced scientists (one from the nuclear medicine department and one from united imaging) who were double-blinded to the results of the qualitative analysis. The region of interest (ROI) of each brain region was drawn along the outline of the gray matter cortex and mirrored to the contralateral side manually (see Fig. 1). The mean SUV of each ROI was quantitatively measured and recorded. The difference between the abnormal side and the normal side of the brain cortex is expressed by the absolute asymmetry index (|AI|). We adopted the average count of each ROI. The |AI| was calculated as follows: |AI| =|(left—right)/([left + right]/2)|. CCD was considered positive if the |AI| of a certain zone was greater than 0.1 [15].
Follow-up
After surgery, all patients were followed up from 3 to 36 months via outpatient visits or telephone interviews. According to the Engel classification, seizure outcomes were classified into classes I–IV as follows [16]: Engel class I: completely seizure free, auras only, or convulsions with drug withdrawal only; Engel class II: rare seizures; Engel class III: meaningful improvement; and Engel class IV: no significant improvement or worse. Engel class I had a good prognosis, and Engel classes II–IV had a poor prognosis.
Statistical analysis
All data were analyzed using SPSS software (version 21.0; IBM SPSS Statistics). Unless otherwise stated, values are reported as the mean ± SD. A comparison of sex, MRI, EEG, seizure type, seizure frequency, FCD type, and the prognosis of epilepsy surgery between CCD+ and CCD− patients was also performed using the χ2 test. A two-sample t test was used to compare age, BMI, onset age, epilepsy duration and hypometabolic regions on PET images between CCD+ and CCD− patients. The |AI| values in different brain regions among CCD+ and CCD− patients were compared using the independent-sample Mann–Whitney test. A P value less than 0.05 (P < 0.05) was considered statistically significant.
Results
Patients were classified as having three types of FCD according to the pathology results. The most common type was FCD I (64/100, 64.0%), followed by FCD II (25/100, 25.0%) and FCD III (11/100, 11.0%). The EZ defined by surgery was most frequently located in a single lobe of the cerebrum; for example, almost 50.0% (45/90) of seizures began in the temporal lobe, 39.0% (35/90) began in the frontal lobe, 10.0% (9/90) began in the parietal lobe, and only 1.0% (1/90) began in the occipital lobe. Ten patients had epileptic foci originating in two lobes of the brain (7 began in the temporal and frontal lobes, 2 began in the temporal and occipital lobes, and 1 began in the parietal and occipital lobes).
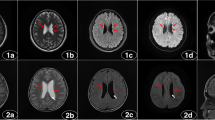
PET/CT reading and statistical analysis were used to classify CCD; 77 patients (77.0%) were classified as CCD− (mean age 24.0 ± 9.7 years), and 23 patients (23.0%) were classified as CCD+ (mean age 25.0 ± 7.5 years). In total, we found 220 hypometabolic regions by qualitative analysis of the interictal PET/CT images. The most common location of hypometabolic regions was the anterior temporal lobe (82/220, 37.3%) (Fig. 2), followed by the anterior frontal lobe (52/220, 23.6%), then successively parietal (31/220, 14.1%), posterior frontal (30/220, 13.6%), posterior temporal (16/220, 7.3%), and occipital (9/220, 4.1%).
Male, 27 years, age of onset 20 years, and duration of epilepsy 7 years. Interictal 18F-FDG PET/CT images show hypometabolism in the left anterior temporal lobe (ellipse; a axial, b coronal) and contralateral cerebellum (ellipse; c axial, d coronal) on PET images, without significant abnormalities on CT images (e–h). The pathology after surgery was FCD IIb in the left anterior temporal lobe
Comparison between clinical characteristics and the presence of CCD
CCD+ patients had more lobes involved on PET than CCD− patients (P < 0.001). MRI showed more negative results in CCD− patients than in CCD+ patients (P = 0.02) (Table 1). No significant differences in age, sex, BMI, onset age, epilepsy duration, EEG type, seizure type seizure frequency or FCD type were found between CCD+ and CCD− patients (all P > 0.05).
Correlations between the Engel class and CCD
The 100 FCD patients with medically intractable epilepsy were followed up at 3, 6, 12, 24 and 36 months postoperatively. Sixty-five patients were classified as Engel class I, 12 as Engel class II, 13 as Engel class III, and 10 as Engel class IV. In summary, 65.0% of the patients presented a favorable prognosis, and 35.0% of the patients presented a poor prognosis.
Fifty-four patients were followed up at 12 months after surgery: 41 were classified as CCD−, and 13 were classified as CCD+ . At 12 months postoperatively, 70.7% (29/41) of CCD− patients had a good prognosis, whereas 30.8% (4/13) of CCD+ patients had a good prognosis (P = 0.02) (Fig. 3). Thirteen patients were followed up at 3 months after surgery, 14 patients were followed up at 6 months after surgery, 16 patients were followed up at 24 months after surgery, and 3 patients were followed up at 36 months after surgery. No significant differences at 3, 6, 24, or 36 months were observed between CCD− and CCD+ patients (all P > 0.05). Two voluntary patients were call back for postoperative FDG PET and MRIs, one showed CCD positive (Fig. 4) and one showed CCD negative (Fig. 5) on preoperative FDG PET.
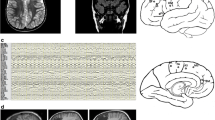
Female, 52 years, age of onset 6 years, and duration of epilepsy 46 years. Interictal 18F-FDG PET images before surgery show hypometabolism in the left anterior temporal lobe and contralateral cerebellum (red arrow), without significant abnormalities on MRI images. The pathology after surgery (anterior temporal lobe lesion resection) was FCD Ic in the left anterior temporal lobe. After 1 year of the surgery, interictal 18F-FDG PET images show metabolic defect of the surgery area (red arrow), hypometabolism in the left adjacent temporal lobe (white arrow), but the contralateral cerebellum show normal metabolism, the MRI just show postoperative changes (red arrow). Follow-up after 1 year of surgery is Engel class III
Male, 45 years, age of onset 15 years, and duration of epilepsy 30 years. Interictal 18F-FDG PET images before surgery show hypometabolism in the right anterior temporal lobe (red arrow), without significant abnormalities on MRI images. The pathology after surgery (anterior temporal lobe lesion resection) was FCD I in the right anterior temporal lobe. After 2 years of the surgery, interictal 18F-FDG PET images show metabolic defect of the surgery area (red arrow), hypometabolism in the right adjacent temporal lobe, right anterior front and contralateral cerebellum (white arrow), the MRI just show postoperative changes(red arrow). Follow-up after 2 years of surgery is Engel class IV
Semi-quantitative PET analysis
No significant difference in the |AI| was noted between the two experienced scientists (P = 0.66). We found significant differences in the average |AI| values in the posterior frontal and anterior temporal lobes (P < 0.05) between CCD+ and CCD− patients, but no significant difference was found in the anterior frontal, parietal, posterior temporal or occipital lobe (Fig. 6).
Discussion
We retrospectively analyzed the results of the interictal 18F-FDG PET/CT brain scans of 100 FCD patients. CCD was observed in 23% of FCD patients in this study. This rate is lower than the previously reported prevalence of CCD in patients with ictal epileptic seizures and after stroke [15, 17,18,19]. In addition, our study demonstrated that the number of lobes involved on PET, structural anomalies on MRI, the |AI| in the posterior frontal and anterior temporal lobes may be predisposing factors for CCD; the presence of CCD may affect the prognosis of FCD patients at 12 months postoperatively. To the best of our knowledge, this is the first interictal 18F-FDG PET/CT study to investigate the incidence of CCD, identify risk factors for seizure-related CCD, and determine the correlation between CCD and prognosis in patients with FCD confirmed by postoperative pathology.
Our results suggest that the number of lobes involved on PET is related to the appearance of CCD. Previous studies have uncovered possible risk factors associated with the presence of stroke-related CCD, but conflicting results still exist. A recent survey of 108 patients with ischaemic stroke within 6 h of onset found no significant differences in the distribution of supratentorial ischaemic lesions between CCD+ and CCD− patients using whole-brain volume perfusion CT (CTP) [20]. Kim et al. [21] hypothesized that the location rather than the extent of a lesion may be the primary determinant for the occurrence of CCD in patients with cerebral infarction. They found that CCD+ was significantly higher when infarctions were located in the frontoparietal lobes or the deep middle cerebral artery territory compared to other regions (11/19 vs 1/7, P = 0.048). Kunz et al. [22] found no significant association between CCD + and infarction volume (P = 0.972). Conversely, Jeon et al. [23] demonstrated that the supratentorial ischaemic volume on each CTP map did not differ significantly between CCD+ and CCD− groups (P > 0.05), but a correlation analysis of the supratentorial ischaemic volume on each CTP map showed a positive and significant linear correlation (P < 0.05) between CCD+ and ischaemic volume. In our study, the CCD+ group had a higher number of hypometabolic regions on PET than the CCD− group (P = 0.00), which can be regarded as equal to the volume of the EZ. However, the range of hypometabolic regions on PET is always greater than that in the EZ [5, 24]; thus, these results do not imply the real volume of the EZ. In the future, we need quantitative methods to calculate the real volume of the EZ.
We found that the average |AI| values in the posterior frontal and anterior temporal lobes in the CCD+ group were significantly higher than those in the CCD− group. Jeon et al. [23] found that the supratentorial degree of perfusion reduction and the infratentorial asymmetry index were strongly and significantly (P < 0.05) correlated with each other in CCD+ patients. Kunz et al. [22] showed that CCD+ patients had larger supratentorial cerebral blood flow deficits than CCD− patients (median: 164 ml vs. 115 ml; P = 0.001) by CT perfusion imaging among acute ischaemic stroke patients. To date, most studies on CCD have focused on cerebral perfusion, and studies on cerebral metabolism are lacking. Cerebral blood flow perfusion and cerebral glucose metabolism reflect different brain physiological states, and a direct integrated study of brain perfusion and metabolism should be performed.
To date, this study is the first to investigate the prognostic value of CCD in FCD patients postoperatively; however, pioneering studies have explored its clinical significance in ischaemic patients. Sin et al. [15] hypothesized that CCD was associated with poor motor recovery after 6 months in patients with haemorrhagic stroke assessed with the Fugl-Meyer Assessment (FMA) and the Korean version of the Modified Barthel Index (K-MBI) score, which is consistent with Small et al. [25] who concluded that the occurrence of CCD had a close association with motor recovery using functional MRI. Sebök et al. [19] included 25 cases with symptomatic unilateral cerebrovascular steno-occlusive disease and concluded that CCD+ patients were in poorer clinical condition than CCD− patients after a 3-month follow-up: National Institutes of Health Stroke Scale (NIHSS) 2 vs 0, P = 0.02; modified Rankin Scale (mRS) 1 vs 0, P = 0.04. Other authors demonstrated that CCD has no prognostic value for stroke outcomes. Zhang et al. [20] found no difference in the NIHSS score between CCD+ and CCD− patients with acute ischaemic stroke, which is similar to the result obtained by Kunz et al. [22]. In summary, CCD is not only a concomitant symptom in diseases such as cerebral infarction and epilepsy but also an important indicator for functional recovery and prognosis. We need a more exact evaluation index in addition to the Engel class to explore the value of CCD in patients with epilepsy at baseline and during follow-ups.
The underlying pathophysiologic mechanism of CCD due to cerebral infarction is functional disruption of the cortico-ponto-cerebellar (CPC) pathways [26, 27]. Recently, Hong demonstrated that FCD lesions can influence whole-brain network integrity using structural equation modeling [28]. Our study showed that CCD+ patients showed more significant structural anomalies on MRI than CCD− patients (P < 0.05). We thought that these findings were closely related to the CPC pathways. Massaro [29] found CCD in patients with status epilepticus by MRI and attributed the finding to excessive neuronal transmission caused by prolonged excitatory synaptic activity from the supratentorial hemisphere to the contralateral cerebellum through the CPC pathway, resulting in increased energy metabolism and cerebral blood flow. Moreover, the possible mechanism of CCD found in stroke patients might be different from that in intractable epilepsy. We assume that CCD in intractable epilepsy patients is a prolonged effect which may be related to cellar changes in the cerebellum due to repeated blood flow and metabolic disturbance during epilepsy. In the next step, we will analyze differences in fiber tracts in CPC pathways between CCD+ and CCD− epilepsy patients using integrated 18F-FDG PET/MR-DTI and we hope that more patients will come to do FDG PET after surgery to further explore the mechanism of CCD.
Some limitations exist in this retrospective research. First, the checkout offset cannot be avoided completely. Second, in this study, we performed only a semi-quantitative analysis to evaluate the severity of CCD and not a quantitative analysis. Despite these limitations, the population in this retrospective study was sufficiently large to investigate the predictors and clinical value of CCD and may provide a basis for further prospective research.
Conclusion
The number of lobes involved on PET, structural anomalies on MRI, the |AI| values in the posterior frontal and anterior temporal lobes may be predisposing factors for CCD. The CCD phenomenon may be helpful for predicting the prognosis of FCD patients at 12 months postoperatively.
References
Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–98.
Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017;377(17):1648–56.
Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135(5):1348–69.
Ding Y, Zhu Y, Jiang B, Zhou Y, Jin B, Hou H, et al. 18f-FDG Pet and high-resolution MRI co-registration for pre-surgical evaluation of patients with conventional MRI-negative refractory extra-temporal lobe epilepsy. Eur J Nucl Med Mol Imaing. 2018;45(9):1567–72.
Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, part 1: sporadic temporal and extratemporal lobe epilepsy. J Nucl Med. 2013;54(10):1775–81.
Rocher AB, Chapon F, Blaizot X, Baron J, Chavoix C. Resting-state brain glucose utilization as measured by pet is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 2003;20(3):1894–8.
Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137(9):2408–22.
Biersack HJ, Linke D, Brassel F, Reichmann K, Kurthen M, Durwen HF, et al. Technetium-99m HM-PAO Brain Spect in epileptic patients before and during unilateral hemispheric anesthesia (Wada Test): report of three cases. J Nucl Med. 1987;28(11):1763–7.
Fisher RS, Cross JH, D’souza C, French JA, Haut SR, Higurashi N, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531–42.
Ding Y, Zhu Y, Jiang B, Zhou Y, Jin B, Hou H, et al. F-FDG PET and high-resolution MRI co-registration for pre-surgical evaluation of patients with conventional MRI-negative refractory extra-temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 2018;45(9):1567–72.
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Buch K, Blumenfeld H, Spencer S, Novotny E, Zubal IG. Evaluating the accuracy of perfusion/metabolism (SPET/PET) ratio in seizure localization. Eur J Nucl Med Mol Imaging. 2008;35(3):579–88.
Wieser HG, Blume WT, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE commission report. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–6.
Kamouchi M, Fujishima M, Saku Y, Ibayashi S, Iida M. Crossed cerebellar hypoperfusion in hyperacute ischemic stroke. J Neurol Sci. 2004;225(1–2):65–9.
Sin DS, Kim MH, Park S, Joo MC, Kim MS. Crossed cerebellar diaschisis: risk factors and correlation to functional recovery in intracerebral hemorrhage. Ann Rehabil Med. 2018;42(1):8–17.
Engel J Jr. Update on surgical treatment of the epilepsies. Summary of the second international palm desert conference on the surgical treatment of the epilepsies (1992). Neurology. 1993;43(8):1612–7.
Won JH, Lee JD, Chung TS, Park CY, Lee BI. Increased contralateral cerebellar uptake of technetium-99m-HMPAO on ictal brain SPECT. J Nucl Med. 1996;37(3):426–9.
Won J, Choi DS, Hong SJ, Shin HS, Baek HJ, Choi HC, et al. Crossed cerebellar hyperperfusion in patients with seizure-related cerebral cortical lesions: an evaluation with arterial spin labelling perfusion mr imaging. Radiol Med. 2018;123(11):843–50.
Sebök M, Van Niftrik CHB, Piccirelli M, Bozinov O, Wegener S, Esposito G, et al. Bold cerebrovascular reactivity as a novel marker for crossed cerebellar diaschisis. Neurology. 2018;91(14):E1328–37.
Zhang M, Cao Y, Wu F, Zhao C, Ma Q, Li K, et al. Characteristics of cerebral perfusion and diffusion associated with crossed cerebellar diaschisis after acute ischemic stroke. Jpn J Radiol. 2020;38(2):126–34.
Kim SE, Choi CW, Yoon B, Chung JK, Roh JH, Lee MC, et al. Crossed-cerebellar diaschisis in cerebral infarction: technetium-99m-HMPAO SPECT and MRI. J Nucl Med. 1997;38(1):14–9.
Kunz WG, Sommer WH, Höhne C, Fabritius MP, Schuler F, Dorn F, et al. Crossed cerebellar diaschisis in acute ischemic stroke: impact on morphologic and functional outcome. J Cereb Blood Flow Metab. 2017;37(11):3615–24.
Jeon YW, Kim SH, Lee JY, Whang K, Kim MS, Kim YJ, et al. Dynamic CT perfusion imaging for the detection of crossed cerebellar diaschisis in acute ischemic stroke. Korean J Radiol. 2012;13(1):12.
Hartl E, Rémi J, Vollmar C, Goc J, Loesch AM, Rominger A, et al. PET imaging in extratemporal epilepsy requires consideration of electroclinical findings. Epilepsy Res. 2016;125:72–6.
Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125(Pt 7):1544–57.
Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99(11):7699–704.
Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–22.
Hong S, Lee H, Gill R, Crane J, Sziklas V, Bernhardt BC, et al. A connectome-based mechanistic model of focal cortical dysplasia. Brain. 2019;142(3):688–99.
Massaro AM. Teaching neuroimages: crossed cerebellar diaschisis in hemispheric status epilepticus. Neurology. 2012;79(20):E182.
Acknowledgments
This work was funded by grants from the National Key Research and Development Program of China (Grant No. 2016YFC0103909), Project supported by the National Natural Science Foundation of China (Grant No. 81671662) and Beijing Municipal Administration of Hospitals’ Ascent Plan, Code: DFL20180802. The authors and the United Imaging Healthcare (one of the data scientists is an employee of the company) declare that they have no conflict of interest.
Funding
This study was funded by: The National Key Research and Development Program of China (Grant No. 2016YFC0103909). Project supported by the National Natural Science Foundation of China (Grant No. 81671662). Beijing Municipal Administration of Hospitals’ Ascent Plan, Code: DFL20180802.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, Y., Guo, K., Fan, X. et al. Crossed cerebellar diaschisis: risk factors and prognostic value in focal cortical dysplasia by 18F-FDG PET/CT. Ann Nucl Med 35, 719–727 (2021). https://doi.org/10.1007/s12149-021-01613-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01613-8