Abstract
Objective
We aimed to determine whether NaF-PET/CT or FDG-PET/CT can detect abdominal aortic molecular calcification and inflammation in patients with rheumatoid arthritis (RA).
Methods
In this study, 18 RA patients (4 women, 14 men; mean age 56.0 ± 11.7) and 18 healthy controls (4 women, 14 men; mean age 55.8 ± 11.9) were included. The controls were matched to patients by sex and age (± 4 years). All subjects of this study underwent NaF-PET/CT scanning 90 min following the administration of NaF. FDG-PET/CT imaging was performed 180 min following intravenous FDG injection. Using OsiriX software, the global mean standardized uptake value (global SUVmean) in abdominal aorta was calculated for both FDG and NaF. The NaF SUVmean and FDG SUVmean were divided by the blood pool activity providing target-to-background ratios (TBR) namely, NaF-TBRmean and FDG-TBRmean. The CT calcium volume score was obtained using a growing region algorithm based on Hounsfield units.
Results
The average NaF-TBRmean score among RA patients was significantly greater than that of healthy controls (median 1.61; IQR 1.49–1.88 and median 1.40; IQR 1.23–1.52, P = 0.002). The average CT calcium volume score among RA patients was also significantly greater than that of healthy controls (median 1.96 cm3; IQR 0.57–5.48 and median 0.004 cm3; IQR 0.04–0.05, P < 0.001). There was no significant difference between the average FDG-TBRmean scores in the RA patients when compared to healthy controls (median 1.29; IQR 1.13–1.52 and median 1.29; IQR 1.13–1.52, respectively, P = 0.98).
Conclusion
Quantitative assessment with NaF-PET/CT identifies increased molecular calcification in the wall of the abdominal aorta among patients with RA as compared with healthy controls, while quantitative assessment with FDG-PET/CT did not identify a difference in aortic vessel wall FDG uptake between the RA and healthy control groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is associated with increased cardiovascular morbidity and mortality [1,2,3]. Cardiovascular disease (CVD) is the leading cause of premature death in patients suffering from RA [4, 5]. The exact pathogenesis of increased risk of CVD in RA patients has not yet been determined. The increased risk cannot be wholly explained by traditional cardiovascular risk factors and may be related to disease-related inflammation [6, 7]. For this reason, conventional methods used for CVD risk stratification such as the Framingham risk score (FRS) suffer from limitations in risk stratification in this population. However, no standard technique has been successfully employed to reliably identify RA patients with active atherosclerotic disease. As such, finding a tool to detect and quantify the active process of atherosclerosis is warranted.
Imaging techniques can noninvasively provide information about atherosclerotic calcification in the arterial wall, which is a strong predictor of CV events [8]. For instance, computed tomography (CT) is a conventional imaging modality by which vascular macro-calcifications can be visualized. However, the observation of macro-calcifications on CT imaging does not provide information about ongoing calcification or the extent of inflammation, and may not be a reliable predictor of future cardiovascular events [9]. Conversely, molecular imaging techniques provide us with the ability to gain insight into the physiological nature of pathology. Nearly 20 years ago, the role of 18F-fluorodeoxyglucose (FDG) in detecting inflammation of vasculature was suggested [10]. Several authors have highlighted the role of FDG in identifying the existing inflammation in the plaques within the arterial wall [11,12,13]. Lately, the credibility of this radiotracer in cardiovascular assessment has been challenged by the unfavorable results generated from the CAMONA study indicating an association between thoracic aortic uptake of NaF, but not FDG, and the 10-year FRS [14, 15]. A growing body of research proposes that NaF-PET/CT is capable of detecting the active calcification within the vascular wall [14, 16,17,18,19] and might be superior to FDG-PET/CT in cardiovascular risk assessment. Therefore, it is important to evaluate the role of these two radiotracers in high-risk groups that would benefit from imaging biomarkers such as patients with RA.
This study was designed to assess whether NaF and FDG as markers of active calcification and inflammation, respectively, can sensitively discriminate levels of abdominal aorta (AA) calcification and inflammation between RA patients and normal subjects.
Materials and methods
Study design
As part of a prospective cross-sectional study conducted between 2012 and 2014 at the Philadelphia VA Medical Center, 19 patients who met the 2010 American College of Rheumatology classification criteria for RA were recruited. One subject did not undergo NaF-PET/CT imaging and was excluded from our study, therefore, a total of 18 RA patients (4 women, 14 men; mean age 56.0 ± 11.7) were included. The controls were matched to patients by sex and age (4 women, 14 men; mean age 55.8 ± 11.9). Patients were not included if they had evidence of active malignancy, metabolic bone disease, or recent computed tomography (CT) imaging within 6 months due to concerns about radiation exposure. 11 patients were receiving methotrexate, 9 were receiving prednisone, and 8 were receiving biological drugs. 9 patients had a history of hypertension and 4 had a history of diabetes mellitus. Approval was obtained from the Philadelphia VA Medical Center Internal Review Board, and all work was performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Eighteen healthy control subjects, age- and sex-matched to RA patients with available FDG-PET/CT and NaF-PET/CT, were selected from a pool of subjects who were recruited from the general population as part of a prospective study conducted at Odense University Hospital in Denmark (NCT01724749) [14]. Subjects were not included if they had a history of cardiovascular disease, malignant neoplasm within the past 5 years, deep vein thrombosis or acute pulmonary embolism, physical or mental disability, state of immunodeficiency or in treatment with immunosuppressive drugs, history of alcohol abuse, illicit drug use or drug abuse, significant mental illness, and unstable or recently diagnosed autoimmune disease. The control subjects were not known to have any history of risk factors, such as HTN, dyslipidemia, DM, aortic aneurysm. Approval for this study was obtained from the Danish National Committee on Health Research Ethics and the Philadelphia VA Medical Center IRB.
Image acquisition
All subjects fasted for at least 6 h before the procedure and the study was performed after a confirmed blood glucose concentration below 8 mmol/L. Whole-body static FDG-PET/CT scans were obtained 180 min after tracer administration (4.0 MBq/kg or 0.11 mCi/kg). The acquisition time was 3.5 min/bed. The NaF-PET/CT scans were obtained 90 min following the tracer injection with an acquisition time of 2.5 min/bed. Scans of RA subjects were performed on a Biograph 64 Hybrid PET/CT Imaging System (Siemens Medical Solutions, Inc. Malvern, USA), and scans of healthy subjects were performed on integrated PET/CT scanners (GE Discovery 690, VCT, RX, and STE). PET attenuation correction was performed with the CT data.
Quantitative image analysis
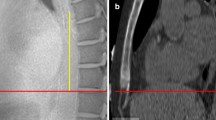
Whole artery analysis was attained by drawing regions of interests (ROI) around the abdominal aorta wall borders on axial slices using Osirix MD 9.0 software (Fig. 1). The abdominal aorta was defined from the slice containing the ostium of the celiac artery and ending with the last slice before the aortic bifurcation. The NaF and FDG uptake was quantified by calculating SUVmean using the following equation:
The NaF-SUVmean and FDG-SUVmean calculated for the AA were divided by the tracer blood pool activity (the average of NaF/FDG uptake in two consecutive slices in superior vena cava) giving us NaF-TBRmean and FDG-TBRmean (TBR: target-to-background ratio).
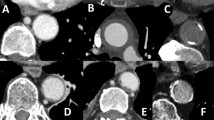
Lastly, the CT calcium volume score was obtained on unfused CT images of FDG-PET/CT. The threshold for detecting the arterial calcium was set at 130 HU (Fig. 2).
The CT calcium volume score was determined within the drawn ROIs (a). Voxels with the Hounsfield unit (HU) of 130 and above were delineated (b). A 3D growing region algorithm with a lower Hounsfield unit (HU) threshold of 130 was assigned on unfused CT images (c). The CT calcium volume score was calculated by summing the areas of calcification in each slice and multiplying the result by the slice thickness
Statistical analysis
A non-parametric test (Mann–Whitney U test) was used to assess the differences of NaF-TBRmean, FDG-TBRmean and CT calcium volume score between the RA patients and the healthy controls. Spearman correlation assessed the relationship between NaF-TBRmean and FDG-TBRmean, age and CT calcium volume score in the RA group. Moreover, the association between the disease activity (DAS28-CRP) and NaF-TBRmean and FDG-TBRmean was assessed by spearman correlation. The statistical analysis for this paper was generated using SPSS (SPSS Inc., Chicago, IL, USA; Version 25). A P value of less than 0.05 was considered as statistically significant.
Results
All 18 RA patients regardless of age had AA calcification on CT scan, while no calcification was detected in the AA in seven healthy controls. The average NaF-TBRmean scores among RA patients were significantly greater than that of healthy controls (median 1.61; IQR 1.49–1.88 and median 1.40; IQR 1.23–1.52; P = 0.002). The average CT calcium volume score among RA patients was also significantly greater than that of healthy controls (median 1.96 cm3; IQR 0.57–5.48 and median 0.004 cm3; IQR 0.04–0.05, P < 0.001). There was no difference between the average FDG-TBRmean scores in the RA patients when compared to healthy controls (median 1.29; IQR 1.13–1.52 and median 1.29; IQR 1.13–1.52, P = 0.98) (Fig. 3). There was no statistically significant correlation between NaF-TBRmean and FDG-TBRmean (ρ = − 0.18, P = 0.46), whereas there was a positive correlation between NaF-TBRmean and CT calcium volume score in RA patients (ρ = 0.62, P = 0.005) (Fig. 4). An inverse but statistically insignificant trend was found between FDG-TBRmean and CT calcium volume score (ρ = − 0.31, P = 0.20). Moreover, a significant positive correlation was observed between age and CT calcium volume score (ρ = 0.59, P = 0.008) but not NaF-TBRmean (ρ = 0.40, P = 0.09).
Box plot comparison of NaF and FDG uptake in the abdominal aorta as well as CT calcium volume score of RA patients and the matched healthy controls. NaF-TBRmean and CT calcium volume score were significantly higher among RA patients than that of matched healthy controls (P = 0.002, P < 0.001, respectively). FDG-TBRmean was also higher in RA group but the difference was not statistically significant (P = 0.98)
In healthy controls, there was no association between NaF-TBRmean/FDG-TBRmean and age or CT calcium volume score (All P > 0.05). However, there was a positive correlation between age and CT calcium volume score (ρ = 0.50, P = 0.03).
There was no correlation between DAS28-CRP score and NaF-TBRmean/FDG-TBRmean (all P > 0.05).
Discussion
In this study, we hypothesized that the assessment of AA calcification may be more sensitive than the assessment of inflammation for evaluating the atherosclerotic process in RA patients using molecular imaging techniques. We found that the AA calcification in RA patients as determined by NaF-PET/CT was higher than that of healthy controls at both microscopic and macroscopic levels. It is worth noting that all 18 RA subjects had detectable AA macro-calcification on CT, including younger subjects. In contrast, the assessment of AA inflammation by FDG-PET/CT did not show any difference between the RA group and the healthy controls. Overall, these findings support the hypothesis that NaF-PET/CT might more effectively identify RA-related inflammatory changes to the vasculature compared to FDG-PET/CT.
Patients with RA are known to have higher incidence of atherosclerosis [20]. Previous reports have also demonstrated that RA patients develop early-onset and extensive vascular calcification [21, 22], findings confirmed in our study. Prior studies have shown that AA calcification is a strong predictor of future cardiovascular events [8]. However, a growing body of evidence suggests that there is a substantial difference between micro-calcification and macro-calcification in the process of atherosclerosis [23]. Macro-calcification (assessed by CT) occurs when vascular smooth muscle cells promote fibrosis and undergo osteogenic transdifferentiation which stabilizes the plaque by acting as a barrier towards inflammation [23]. In contrast, micro-calcification is the initial deposition of calcium in response to pro-inflammatory stimuli which might cause further inflammation and instability of the plaque [23]. Therefore, it has been hypothesized that measuring the active micro-calcification in vasculature may provide more useful information about the atherosclerotic calcification process rather than macro-calcification.
Initially, the application of NaF-PET/CT was limited to malignant skeletal disease [24, 25] due to its ability to portray calcium metabolism in the bone [26]. An increasing number of investigations have revealed the feasibility of this modality in detection of extra-skeletal calcification [27,28,29,30] such as calcification of atherosclerotic plaques [19, 31, 32]. This calcification is mainly the micro-calcification which is undetectable on structural imaging techniques such as CT (Fig. 5). Although some studies have shown the link between FDG uptake and atherosclerotic disease [33,34,35,36], recent studies propose that NaF-PET/CT might be a better alternative for cardiovascular assessment [37, 38].
Our findings were in line with previous studies as we demonstrated that global assessment with NaF-PET/CT could sensitively discriminate AA calcification between a high-risk group and healthy controls, whereas, FDG-PET/CT could not. Recently, Arani and colleagues evaluated the association between NaF and FDG uptake in the AA and cardiovascular risk factors [38]. They utilized the same methodology as the current study which has been shown to be more accurate in detecting molecular calcification compared to conventional methods of PET quantification [37, 39]. They observed that the global tracer uptake value for NaF not FDG was higher in patients with chest pain than healthy controls [38]. Additionally, the global NaF uptake in the AA was positively correlated with age and 10-year Framingham risk score while the FDG uptake was not. Blomberg et al. observed a positive correlation between the unfavorable cardiovascular risk profile and thoracic aortic micro-calcification as determined by NaF-PET/CT but not arterial inflammation as determined by FDG-PET/CT [14]. The negative results for FDG-PET/CT in our study are consistent with these prior reports and may have four explanations. First, FDG is a non-specific radiotracer and the amount of FDG uptake within the ROIs in the aorta may be influenced by other structures such as vascular smooth muscle. Second, the inflammatory phase in the vessel wall is likely much shorter than the post-inflammatory phase, limiting the time frame to image the vessel at the inflammatory phase [38]. Furthermore, it has been shown that the inflammation in the atherosclerotic plaque is waxing and waning as determined by FDG-PET/CT and calcification and inflammation are not necessarily present at the same time [40, 41]. Lastly, as some RA patients were receiving RA medications, these drugs may have attenuated the FDG uptake in the atherosclerotic plaques.
Our findings confirmed the previous reports that the NaF uptake in the aorta is positively correlated with CT calcification [14, 42]. There was an inverse correlation between the AA FDG uptake and CT calcification in RA patients. However, this correlation was not statistically significant (P = 0.20). Additionally, this study showed that in RA patients, there is a positive correlation between age and macro-calcification but not micro-calcification. This was an expected finding since the macro-calcification observed on CT is a cumulative process and the volume of macro-calcification is expected to increase with age. However, our findings suggest that the process of active micro-calcification in RA patients is not related to age, and disease-related factors such as inflammation or other unknown factors may instead drive micro-calcification in this population.
This study is limited by small sample size. The female to male ratio of RA patient has been reported to be 3:1 [43]. In our study, we included 14 male patients and 4 females. Further prospective studies with larger sample sizes and with the same female/male ratio as the general population are needed to validate our results and assess the correlation between NaF uptake and different cardiovascular factors. Another limitation was the lack of histological data to correlate with NaF and FDG uptake. The NaF and FDG uptake values are reported to indicate the arterial micro-calcification and inflammation, however the association between uptake of these radiotracers and the observed histology needs further evaluation. Another consideration is the adjacency of spine and abdominal aorta which raises concern for spill over from spine to aorta. We used SUVmean for our measurements which indicates the average of all voxels within the ROI and is less affected by the contamination from spine as opposed to SUVmax which is highly prone to be altered as it is a value of one voxel. However, this adjacency should still be considered even though we believe using SUVmean minimizes the effect of any contamination from the adjacent tissues. Finally, because of ethical concerns about radiation in healthy controls, this study utilized previously collected controls. Similar protocols were used to obtain images and extract data. However, it should be considered as two different machines were used, the values might be affected due to the existing challenge of cross-calibrating different machines.
Conclusion
Quantitative assessment with NaF-PET/CT may be a useful approach to identify excess micro-calcification in the abdominal aorta among at-risk patients with RA. Quantitative assessment with FDG-PET/CT did not identify a difference between the RA and healthy control groups. Further prospective studies are needed to confirm the potential role of NaF-PET/CT to diagnose, monitor and assess treatment response in patients at high risk for atherosclerosis.
References
Crowson CS, Liao KP, Davis JM III, Solomon DH, Matteson EL, Knutson KL, et al. Rheumatoid arthritis and cardiovascular disease. Am Heart J. 2013;166(4):622–628.e621.
Turesson C, Jarenros A, Jacobsson L. Increased incidence of cardiovascular disease in patients with rheumatoid arthritis: results from a community based study. Ann Rheum Dis. 2004;63(8):952–5.
Santos MJ, Vinagre F, Silva J, Gil V, Fonseca J. Cardiovascular risk profile in systemic lupus erythematosus and rheumatoid arthritis: a comparative study of female patients. Acta Reumatologica Portuguesa. 2010;35(3):325–32.
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers (Primer). 2018;4:18001.
England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res. 2016;68(1):36–45.
Dessein PH, Joffe BI, Veller MG, Stevens BA, Tobias M, Reddi K, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol. 2005;32(3):435–42.
Boyer J-F, Gourraud P-A, Cantagrel A, Davignon J-L, Constantin A. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Jt Bone Spine. 2011;78(2):179–83.
Gonçalves FB, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98(13):988–94.
Sheikine Y, Akram K. FDG–PET imaging of atherosclerosis: do we know what we see? Atherosclerosis. 2010;211(2):371–80.
Vallabhajosula S, Fuster V. Atherosclerosis: imaging techniques and the evolving role of nuclear medicine. J Nucl Med. 1997;38(11):1788.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825–31.
Chen W, Bural GG, Torigian DA, Rader DJ, Alavi A. Emerging role of FDG-PET/CT in assessing atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2009;36(1):144–51.
Wassélius JA, Larsson SA, Jacobsson H. FDG-accumulating atherosclerotic plaques identified with 18 F-FDG-PET/CT in 141 patients. Mol Imaging Biol. 2009;11(6):455.
Blomberg BA, de Jong PA, Thomassen A, Lam MG, Vach W, Olsen MH, et al. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging. 2017;44(2):249–58.
Nakahara T, Strauss HW. From inflammation to calcification in atherosclerosis. Eur J Nucl Med Mol Imaging. 2017;44(5):858–60.
de Oliveira-Santos M, Castelo-Branco M, Silva R, Gomes A, Chichorro N, Abrunhosa A, et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects–a subclinical atherosclerosis imaging study with 18F-NaF PET-CT. Atherosclerosis. 2017;260:41–6.
Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59(17):1539–48.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by 18 F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495.
Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51(6):862–5.
Skeoch S, Bruce IN. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol. 2015;11(7):390.
Paccou J, Renard C, Liabeuf S, Kamel S, Fardellone P, Massy ZA, et al. Coronary and abdominal aorta calcification in rheumatoid arthritis: relationships with traditional cardiovascular risk factors, disease characteristics, and concomitant treatments. J Rheumatol. 2014;41(11):2137–44.
Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–53.
Pugliese G, Iacobini C, Fantauzzi CB, Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238(2):220–30.
Sachpekidis C, Goldschmidt H, Hose D, Pan L, Cheng C, Kopka K, et al. PET/CT studies of multiple myeloma using 18 F-FDG and 18 F-NaF: comparison of distribution patterns and tracers’ pharmacokinetics. Eur J Nucl Med Mol Imaging. 2014;41(7):1343–53.
Blau MO, Nagler WI, Bender MA. Fluorine-18: a new isotope for bone scanning. J Nucl Med. 1962;1:3.
Raynor W, Houshmand S, Gholami S, Emamzadehfard S, Rajapakse CS, Blomberg BA, et al. Evolving role of molecular imaging with 18 F-sodium fluoride PET as a biomarker for calcium metabolism. Curr Osteoporos Rep. 2016;14(4):115–25.
Seraj SM, Al-Zaghal A, Østergaard B, Høilund-Carlsen PF, Alavi A. Identification of heterotopic ossification using 18F-NaF PET/CT. Clin Nucl Med. 2019;44(4):319–20.
Al-Zaghal A, Seraj SM, Werner TJ, Gerke O, Høilund-Carlsen PF, Alavi A. Assessment of physiologic intracranial calcification in healthy adults using 18F-NaF PET/CT. J Nucl Med. 2019;60(2):267–71.
Woodhead GJ, Avery RJ, Kuo PH. Atlas of extraosseous findings detected by 18F-NaF PET/CT bone scan. Clin Nucl Med. 2017;42(12):930–8.
Rojulpote C, Borja AJ, Zhang V, Aly M, Koa B, Seraj SM, Raynor WY, Kothekar E, Kaghazchi F, Werner TJ, Gerke O, Høilund-Carlsen PF, Alavi A. Role of 18F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging. 2020;10(1):47–56.
Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med. 2011;52(3):362–8.
Seraj SM, Raynor W, Rojulpote C, Zadeh MZ, Arani L, Werner T, Hoilund-Carlsen PF, Baker J, Alavi A, Hunt S. Assessing the feasibility of NaF or FDG as PET probes to evaluate atherosclerosis in rheumatoid arthritis patients. J Nucl Med. 2019;60(supplement 1):1439.
Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. In: Seminars in nuclear medicine. Elsevier; 2002. p. 70–6.
Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69–77.
Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy J-P, Maunoury C, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008;15(2):209–17.
Arauz A, Hoyos L, Zenteno M, Mendoza R, Alexanderson E. Carotid plaque inflammation detected by 18F-fluorodeoxyglucose-positron emission tomography: pilot study. Clin Neurol Neurosurg. 2007;109(5):409–12.
Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E, et al. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography—a novel concept. Hellenic J Nucl Med. 2011;14(2):114–20.
Arani LS, Gharavi MH, Zadeh MZ, Raynor WY, Seraj SM, Constantinescu CM, et al. Association between age, uptake of 18F-fluorodeoxyglucose and of 18F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hellenic J Nucl Med. 2019;22(1):14–9.
McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, et al. 18 F-NaF and 18 F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging. 2018;45(12):2190–200.
Meirelles GS, Gonen M, Strauss HW. 18F-FDG uptake and calcifications in the thoracic aorta on positron emission tomography/computed tomography examinations: frequency and stability on serial scans. J Thorac Imaging. 2011;26(1):54–62.
Den Harder AM, Wolterink JM, Bartstra JW, Spiering W, Zwakenberg SR, Beulens JW, Slart RH, Luurtsema G, Mali WP, de Jong PA. Vascular uptake on 18 F-sodium fluoride positron emission tomography: precursor of vascular calcification? J Nucl Cardiol. 2020;23:1–1.
Derlin T, Tóth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, et al. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. J Nucl Med. 2011;52(7):1020–7.
Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–82.
Funding
The CAMONA study was funded by the Anna Marie and Christian Rasmussen’s Memorial Foundation, University of Southern Denmark, Odense, Denmark, and the Jørgen and Gisela Thrane’s Philanthropic Research Foundation, Broager, Denmark. The VA study was funded by a Veteran’s Affairs Competitive Pilot Project Fund Award. JFB is supported by a Veteran’s Affairs Clinical Science Research & Development Award (I01 CX001703).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional, national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seraj, S.M., Raynor, W.Y., Revheim, ME. et al. Assessing the feasibility of NaF-PET/CT versus FDG-PET/CT to detect abdominal aortic calcification or inflammation in rheumatoid arthritis patients. Ann Nucl Med 34, 424–431 (2020). https://doi.org/10.1007/s12149-020-01463-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01463-w