Abstract
Background
Cardiac resynchronization therapy (CRT) reduces morbidity and mortality in chronic systolic heart failure. About 20% of implanted patients are considered as “non-responders”. This study aimed to evaluate gated myocardial perfusion single-photon emission computed tomography (GMPS) phase parameters as compared to echocardiography in the assessment of predictors for response to CRT before and after CRT activation.
Methods
Forty-two patients were prospectively included during 15 months. A single injection of 99mTc-tetrofosmin was used to acquire GMPS phase pre- and post-CRT activation. Indicators of positive CRT response were improvement of functional status and 15% reduction in left ventricular end-systolic volume at 3 months.
Results
Phase parameters at baseline were similar in the two groups with no influence of perfusion data. Phase parameters after CRT activation were significantly improved in the responders’ group (Δ Bandwidth −19° ± 24° vs. 13° ± 31°, p = 0.001; Δ SD −20° ± 30° vs. 26° ± 46°, p = 0.001; Δ Entropy −11 ± 12 vs. 2 ± 6%, p = 0.001). Feasibility and reproducibility were higher for GMPS.
Conclusion
Acute phase modifications after CRT activation may predict response to CRT immediately after implantation, but not at baseline, even when adjusted to perfusion data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The treatment of cardiac heart failure (HF) took a major step forward with the development of cardiac biventricular pacing, or cardiac resynchronization therapy (CRT). Several studies showed that CRT improves quality of life, symptoms of heart failure (HF), exercise capacity and survival rate [1, 2]. CRT results in reverse remodeling by reducing left ventricular (LV) volumes, and improvement of left ventricular ejection fraction (LVEF) [3]. Current selection criteria for implantation, supported by international practice guidelines, are limited to symptoms, QRS duration, and LVEF <35% [4].
However, about one-fifth of the patients meet these criteria but do not respond to this invasive therapy (non-responders) [1, 5, 6]. For more than 10 years, cardiac mechanical dyssynchrony assessed by different imaging modalities has been studied to improve the selection of patients who would benefit from CRT. Despite many studies and several criteria based on 2D, 3D, Doppler tissue imaging or speckle-tracking [7–9], trans-thoracic echocardiography (TTE) is still not able to define predictors of response to CRT, accurately. This is mostly due to the poor reproducibility and feasibility of these parameters. However, since the PROSPECT [10] and EchoCRT [11] trials, new dyssynchrony parameters were found and seem to be more accurate [8, 12].
Gated myocardial perfusion single-photon emission computed tomography (SPECT) (GMPS) is used to assess myocardial perfusion but also provides information on regional wall thickening. Phase analysis derived from GMPS can assess left ventricular mechanical dyssynchrony [13–15]. Using dedicated software, GMPS phase analysis may predict response to CRT but sensitivity and specificity remain insufficient. Myocardial perfusion data explored by GMPS on the same exam were not studied as additional information to make phase analysis more accurate.
First, we investigated whether mechanical dyssynchrony parameters assessed by GMPS phase analysis, before and just after CRT activation, may predict response to CRT including perfusion data, as compared with TTE parameters. Then, we compared reproducibility and feasibility of SPECT with TTE to assess dyssynchrony.
Materials and methods
Population
During the study period, 142 patients were admitted in our center for CRT implantation; selection criteria for CRT implantation were those described in the 2013 European guidelines [4], and consisted in depressed LVEF <35%, NYHA class ≥2, and prolonged QRS duration (>120 ms). Patients with permanent right ventricle stimulation were also included. Exclusion criteria were permanent atrial fibrillation, non-ambulatory class 4 NYHA, and refusal of consent; 42 patients were finally included. All the patients gave their consent to participate in this study, approved by the local ethics committee (No. 80-1114).
Study protocol
Before CRT device implantation, all patients had a clinical evaluation status using NYHA class and exercise testing (6 min test walk and ventilation gas exchange analysis on cycle ergometer). Then TTE was performed to assess left ventricle function and dyssynchrony parameters. At the end of the CRT implantation procedure, the left lead was left inactive deliberately. The day after, GMPS was performed and phase dyssynchrony parameters acquired before and after activation of the left lead (CRT activation), using a single injection of radiotracer. The response to CRT was evaluated 3 months after implantation with a new clinical evaluation status, exercise testing and TTE.
Echocardiography
Patients were imaged in the left lateral decubitus position with a commercially available system (Vivid E9 General Electric Healthcare). A 1.5–3.6 MHz transducer was used. Baseline TTE included standard gray-scale and color TDI in the parasternal (short and long axis) and apical (2, 3, and 4 chambers) views with a high frame rate (at least 35 frame/s). Patients with more than 2 non-analyzable segments were excluded from the TTE analysis. The TDI and speckle-tracking analysis were performed on a post-treatment workstation (Echopac version 11, GE Vingemed). For each patient, atrioventricular, interventricular and intraventricular dyssynchrony was explored by TTE. Left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDV), septal-posterior wall motion delay (SPWMD, M mode measured by parasternal short-axis view), left ventricular filling time (LVFT) measured by trans-mitral Doppler, interventricular mechanical delay (IVMD) defined as the difference between left and right ventricular preejection intervals, and intraventricular dyssynchrony left lateral wall contraction (LLWC) defined as the presence of overlap between the end of lateral wall contraction (via M mode) and onset of LV filling were measured.
The TDI data were treated as described by Yu et al. [7] calculating the Ts-SD index, with the standard deviation of the time to peak velocity in 12 LV segments (mid and basal segments).
According to Doltra et al. [12], septal flash was defined as a fast contraction and relaxation (inward/outward motion) of the septum occurring during the isovolumetric contraction period (within the QRS width), as visualized either in 2-dimensional or M-mode images in the parasternal or 4-chamber views.
The strain delay index (SDI) was defined as described by Lim et al. [8] as the mean difference between end-systole strain (ε ES) and peak strain (ε peak) assessed by 2D speckle-tracking analysis, which is supposed to estimate the wasted energy caused by dyssynchrony. For each of the 16 myocardial segments, a strain curve was generated, the εES was defined as the ε value at the time of the end-systole (aortic valve closure), the ε peak was defined as the maximum negative value during cardiac cycle. For a segment that exhibited positive strain or biphasic strain with a peak positive greater than the maximal absolute negative strain, the term (ε peak − ε ES) was entered as zero for the calculation of strain delay index. For non-analyzable segments the 0 value was entered. During this process the time-to-peak strain was used to be compared to the SPECT activation delays.
Gated myocardial perfusion SPECT
Assessment of myocardial dyssynchrony was performed for all patients by myocardial perfusion gated-SPECT after injection of a weight-adjusted dose of 300–400 MBq of 99mTc-tetrofosmin. GMPS was acquired 20–30 min after radiotracer injection using a Symbia T6 (Siemens Healthcare, Erlangen, Germany) double-headed gamma camera equipped with IQ SPECT multifocal collimators. Data were acquired for 180° with 64 frames of 30- and 20-s durations at rest with a 20% window centered on the 140-keV photo peak of 99mTc. A single injection of 99mTc-tetrofosmin was needed to perform two rest GMPS acquisitions; the first was performed before CRT activation, and the second was realized 15–30 min later. Images were analyzed with the Syngo MI Applications software (©Siemens Healthcare, Erlangen, Germany); phase analysis based on myocardial thickening was assessed on QGS 2009 Software (©Cedars-Sinai Medical Center). The calculation process of phase parameters was described before [16, 17]; once timing parameters have been calculated for all sampling points, multiple global and regional LV synchrony measures are computed on the basis of whole-ventricle and used to build a histogram phase angle. The mean and standard deviation (SD) of the histogram are calculated in the standard manner. The bandwidth, β, is defined as the smallest phase-angle range that encompasses 95% of the histogram samples. These different time values were expressed in degrees (range 0°–360°) to compare the patients to each other as it is not influenced by heart rate; however, time values were also expressed in milliseconds to be compared with the TTE parameters, to be more clinically relevant. The entropy (Ә) is a measure of variability computed as previously described [18], normalized to its maximum value for the number of histogram bins used and reported as a percentage. Based on the fact that patients with ischemic cardiomyopathy (ICM) are less improved by CRT [19], especially when the scar burden is high [20]. We made the hypothesis that localization of the ischemic scar could play a role in response to CRT. Indeed, the LV pacing lead used for CRT is generally inserted on the lateral wall of LV; if there is an ischemic scar on the lateral wall, the greater the extent of this scar, the more the myocardial contraction is impaired, and the more likely the response to CRT is to be impaired. The lateral wall myocardial perfusion (LWMP) was calculated as the mean perfusion of the 5 lateral wall segments (2 basal segments, 2 mid-segments and one apical segment of the lateral wall), and expressed as percentage of the maximum. In order to attempt to reach a better accuracy in prediction of the response to CRT, phase parameters were indexed to the perfusion of the lateral wall: bandwidth (°) × LWMP (%), standard deviation (°) × LWMP (%), mean (°) × LWMP (%), entropy (%) ×LWMP (%).
Response to CRT
Response to CRT was evaluated 3 months after device implantation by 2 different methods: functional status or LV remodeling by TTE. First, patients improved there functional status and were classified as “responders” if they improve peak VO2 of at least 10%. Then, LV remodeling after CRT, defined as a reduction in LVESV of ≥15% at 3 months, also defined “responders”. All these criteria were defined in large previous trials [10, 17, 20–23].
Repeatability, reproducibility, feasibility of measurements
For TTE, variability was focused on SPWMD, Ts-SD, and SDI. For GMPS, variability was measured for all synchrony parameters (bandwidth, mean, SD and entropy). Intra-observer and inter-observer variability was assessed on 15 random patients in whom all TTE/GMPS measures could be performed. Feasibility was evaluated on all the patients enrolled in the study. For each parameter, we compared the number of myocardial segments interpretable and the number of patients in whom the criteria can be performed.
Statistical analysis
Results are presented as mean and standard deviation. Continuous data were compared using the paired or unpaired Student t test when appropriate. A receiver-operating characteristic (ROC) curve was analyzed to determine the optimal cutoff values of the acute modifications of histogram bandwidth, phase SD and entropy to predict response to CRT. For all tests, a p value <0.05 was considered statistically significant. An intraclass correlation (ICC) was used for both intra-observer and inter-observer variability.
Results
Study population
Baseline characteristics of the 42 patients (36 men, mean age of 65 ± 10 years) are detailed in Table 1. Patients were mainly in class 2 NYHA (48%) with 50% of ischemic cardiomyopathy (ICM). Mean peak VO2 was 13 ± 4 mL/kg/m² and mean distance walked in 6 min was 383 ± 110 m. QRS duration was 155 ± 22 ms. Mean LVEF was 28 ± 6%, with a mean LVESV of 93 ± 34 mL/m². Four patients were excluded from the final analysis (two patients died, one patient was explanted for material infection, one patient was lost to view during follow-up).
Repeatability, reproducibility, feasibility of measurements
Variability of dyssynchrony parameters continues in Tables 2 and 3; ICC was around 0.9 (p < 0.0001) for all phase parameters, including intra-observer and inter-observer variability. TTE parameters showed a non-significant reproducibility for SPWMD and Ts-SD; SDI index showed significant ICC for intra-observer variability [0.75 (0.41–0.91); p < 0.0001], and for inter-observer variability [0.66 (0.25–0.87); p = 0.03]. For GMPS phase analysis, all cardiac segments were analyzable. For TTE, SPWMD was feasible in all patients, Ts-SD permitting to analyze 96% of myocardial segments, and SDI index was feasible in 90% of myocardial segments studied, permitting to assess SDI in 72% of the patients.
Dyssynchrony parameters and response to CRT (Table 4)
LV remodeling as response criteria
Twenty-four (63%) patients were considered as responders with a reduction of LVESV of at least 15%. Considering TTE parameters, only IVMD (p = 0.022) and LPEI (p = 0.048) were significantly associated with LV remodeling. For GMPS parameters, mean lateral wall perfusion was higher in responders (69 ± 10% vs. 62 ± 7%, p = 0.027). Before CRT activation, there was no difference in phase parameters between responders and non-responders. After CRT activation, β (p = 0.048) and SD (p = 0.007) were significantly lower in responders. Ә was not significantly lower (63 ± 9 vs. 70 ± 11%, p = 0.056). Decrease in β, SD and Ә was significantly associated with response to CRT (all p values <0.001).
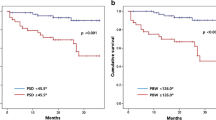
When ROC curve analysis was applied (Fig. 1), acute reduction cut-off values of 10% of phase β (sensitivity of 0.71 and specificity of 0.67), 1% of phase SD (sensitivity of 0.64 and specificity of 0.99), and 1% of phase ә (sensitivity of 0.71 and specificity of 0.80) were needed to determine responders to CRT.
Peak VO2 improvement as response criteria
There was no difference between the two groups considering TTE dyssynchrony parameters, except for septal flash, which was more present at baseline in non-responders (p = 0.01). For GMPS parameters, only Ә decreased significantly in responders (−8 ± 9 vs. 0 ± 8%, p = 0.013).
Influence of perfusion on GMPS phase analysis
Considering LV remodeling, LWMP was significantly higher in the responders’ group (69 ± 10 vs. 62 ± 7%, p = 0.027) and especially in the subgroup of ICM (64 ± 11 vs. 58 ± 3; p = 0.009). None of the phase parameters showed at baseline, before CRT activation, a significant improvement when adjusted to perfusion data (β corrected by LWMP 89° ± 29° vs. 77° ± 21°, p = 0.17; SD corrected by LWMP 23° ± 8° vs. 21° ± 5°, p = 0.36; Ә corrected by LWMP 96 ± 30 vs. 85 ± 15%, p = 0.21). Similar results were found when considering functional status improvement (β corrected by LWMP 89° ± 24° vs. 82° ± 30°, p = 0.93; SD corrected by LWMP 24° ± 6° vs. 22° ± 8°, p = 0.67; Ә corrected by LWMP 47 ± 8 vs. 42 ± 12%, p = 0.8).
Discussion
GMPS phase analysis
In this study, we confirmed that GMPS is a feasible and reproducible method to analyze LV mechanical dyssynchrony. Acute improvement in GMPS phase parameters just after CRT activation was associated with response to CRT, based on LV remodeling, after a short follow-up of 3 months.
Friehling et al. [23] have shown, in a monocentric trial with 44 patients and a follow-up of 20 months, a significant link between worsening GMPS phase parameters after CRT activation and adverse patient outcome (composite of HF hospitalizations, death, appropriate defibrillator discharges and device deactivation for worsening HF symptoms). Actually, LV remodeling is an objective parameter widely used in CRT trials to define responders and non-responders to CRT; it is well known that patients without LV remodeling have a worse prognosis. Acute changes in GMPS phase analysis could be used to define non-responders immediately after CRT implantation, and therefore might be a potential tool to anticipate alternative therapeutics such as left ventricular assistance or cardiac transplantation with less delay.
In this trial, GMPS phase parameters obtained before CRT activation did not permit to predict response to CRT, and according to our results, GMPS phase analysis should not be used in routine as a selection criteria for CRT implantation. These results are discordant with previous trials. Boogers et al. [17] (QGS software) found a larger bandwidth and standard deviation at baseline in the responders’ group, even if the patients’ characteristics were similar, the response criteria were NYHA class variation, and follow-up of 6 months, while Azizian et al. [22] showed similar results using a cut-off of 15% improvement in LVESV. Another trial based on Emory Cardiac Toolbox software [13] showed larger bandwidth and SD in the responders’ group before CRT activation, based on NYHA class improvement. This shows the importance of achieving a larger clinical trial based on various response criteria. Because of the difference between GMPS phase values we did not use cut-off values as defined by Boogers.
In this study, LWMP was significantly higher in the responders’ group and especially in the subgroup of patients with ICM. Adelstein et al. [20] previously showed in over 620 patients that myocardial perfusion, representing the scar burden, is an important aspect to consider before CRT implantation. However, even after adjusting to perfusion data of the LV lateral wall, baseline phase parameters did not influence response to CRT either.
This trial showed better reproducibility and feasibility of GMPS phase analysis comparatively to all TTE dyssynchrony index. This result is attributable to automated processing and weight-adjusted dose of the radiotracer, which allow usable data for all patients, whereas TTE is dependent on the acoustic characteristics of the patients and experience of the operator.
TTE and dyssynchrony
Considering LV remodeling, SPWMD, LVFT, mitral and aortic overlap, Ts-SD did not permit predicting responders. These results are consistent with the previous, non-conclusive PROSPECT trial. An SDI index >25% was more present in the responders’ group, but not significantly (18 vs. 7, p = 0.058), and was realizable in only 72% of the patients. Septal flash was not associated with response to CRT, and was significantly more present in the non-responders’ group when peak VO2 improvement was used as response criteria.
Limits
This study has all the limitations associated with single-site and small effective studies. The delay of 3 months to evaluate echocardiographic and clinical response to CRT may be too short as LV remodeling may occur up to 6 months after device implantation.
GMPS does not analyze the right ventricle and cannot be used for interventricular dyssynchrony assessment. Gated blood pool SPECT angiography is able to assess VG and VD functions and phase, and remain to be evaluated in the prediction of response to CRT.
Using clinical evaluation to define response to CRT, TTE or phase analysis may not predict accurate response to CRT, even considering acute modifications of phase parameters after CRT activation. First, physical performances used as response criteria require many patients to obtain a significant difference (in CONTAK [24] trial, 490 patients were included to show an improvement of only 0.8 mL/kg/min of the peak VO2). Then, disruption of physical test in patients requiring CRT, as the consequence of malnutrition, asthenia, osteo-articular disease, leads to missing data (statistical analysis on 29 patients who performed physical tests over 38).
Multivariate analysis comparing TTE and GMPS parameters was not performed as TTE considered parameters only before CRT activation and GMPS before and after.
New knowledge gained
We showed that GMPS phase analysis derived by QGS software is feasible with IQ SPECT collimators technology, but did not permit, before CRT activation, to predict response to CRT. Phase modifications after CRT activation permit to predict LV remodeling but not physical improvement, which confirm that clinical response and echocardiographic response are not always linked. Phase parameters are not more accurate when adjusted to perfusion data at baseline.
Conclusion
GMPS phase analysis obtained by QGS software showed excellent feasibility and reproducibility for the evaluation of cardiac dyssynchrony, higher than those obtained with TTE. Acute modifications of phase parameters immediately after CRT activation may predict response to CRT, but not at baseline, even when adjusted to perfusion data.
Abbreviations
- β:
-
Bandwidth
- Ә:
-
Entropy
- ε :
-
Strain
- CRT:
-
Cardiac resynchronization therapy
- GMPS:
-
Gated myocardial perfusion SPECT
- HF:
-
Heart failure
- ICC:
-
Intraclass correlation
- ICM:
-
Ischemic cardiomyopathy
- IVMD:
-
Interventricular mechanical delay
- LLWC:
-
Left lateral wall contraction
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- LVEDV:
-
Left ventricular end-diastolic volume
- LVESV:
-
Left ventricular end-systolic volume
- LVFT:
-
Left ventricular filling time
- LWMP:
-
Lateral wall myocardial perfusion
- NYHA:
-
New York Heart Association
- TTE:
-
Trans-thoracic echocardiography
- SD:
-
Standard deviation
- SPECT:
-
Single-photon emission computed tomography
- SPWMD:
-
Septal-posterior wall motion delay
References
Abraham WT, Fisher W, Smith A, Delurgio DB, Leon A, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53.
Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39(12):2026–33.
Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49.
Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–329.
Auricchio A, Prinzen FW. Non-responders to cardiac resynchronization therapy—the magnitude of the problem and the issues. Circ J. 2011;75:521–7.
Yu CM, Sanderson JE, Gorcsan J III. Echocardiography, dyssynchrony, and the response to cardiac resynchronization therapy. Eur Heart J. 2010:2326–37.
Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005;45:677–84.
Lim P BA, Popovi ZB, Greenberg NL, Patel D, Thomas JD, et al. Longitudinal strain delay index by speckle tracking imaging: a new marker of response to cardiac resynchronization therapy. Circulation. 2008;118:1130–7.
Seo Y, Ito H, Nakatani S, Takami M, Naito S, Shiga T, et al. The role of echocardiography in predicting responders to cardiac resynchronization therapy—results from the Japan Cardiac Resynchronization Therapy Registry Trial (J-CRT). Circ J. 2011;75:1156–63.
Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to CRT (PROSPECT) Trial. Circulation. 2008;117(20):2608–16.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369(10):1395–405.
Doltra A, Bijnens B, Tolosana JM, Borràs R, Khatib M, Penela D, et al. Mechanical abnormalities detected with conventional echocardiography are associated with response and midterm survival in CRT. JACC Cardiovasc Imaging. 2014;7(10):969–79.
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–11.
Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49.
Boogers MJ, Chen J, Veltman CE, van Bommel R J, Mooyaart EAQ, Al Younis I, et al. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: a comparison with tissue Doppler imaging. Eur J Nucl Med Mol Imaging. 2011;38:2031–9.
Van Kriekinge SD, Nishina H, Ohba M, Berman DS, Germano G. Automatic global and regional phase analysis from gated myocardial perfusion SPECT Imaging: application to the characterization of ventricular contraction in patients with left bundle branch block. J Nucl Med. 2008;49.
Boogers MM, Van Kriekinge SD, Henneman MM, Ypenburg C, Van Bommel RJ, Boersma E, et al. Quantitative gated SPECT derived phase analysis on gated myocardial perfusion SPECT detects left ventricular dyssynchrony and predicts response to cardiac resynchronization therapy. J Nucl Med. 2009;50:718–25.
Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:623–56.
Gasparini M, Regoli F, Ceriotti C, Galimberti P, Bragato R, De Vita S, et al. Remission of left ventricular systolic dysfunction and of heart failure symptoms after cardiac resynchronization therapy: temporal pattern and clinical predictors. Am Heart J. 2008;155:507–14.
Adelstein EC, Tanaka H, Soman P, Miske G, Haberman SC, Saba SF, et al. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J. 2011(32):93–103.
Ypenburg C, Van Bommel R J, Willem Borleffs CJ, Bleeker GB, Boersma E, Schalij MJ, et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53(6):483–90.
Azizian N RF, Ghaedian T, Golabchi A, Bahadorian B, Khanlarzadeh V, et al. LV dyssynchrony assessed with phase analysis on gated myocardial perfusion SPECT can predict response to CRT in patients with end-stage heart failure. Res Cardiovasc Med. 2014;3(4):e20720.
Friehling M, Chen J, Saba S, Bazaz R, Schwartzman D, Adelstein EC, et al. A prospective pilot study to evaluate the relationship between acute change in left ventricular synchrony after cardiac resynchronization therapy and patient outcome using a single-injection gated SPECT protocol. Circ Cardiovasc Imaging. 2011;4:532–9.
Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42(8):1454–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or disclosure statement.
Rights and permissions
About this article
Cite this article
Gendre, R., Lairez, O., Mondoly, P. et al. Research of predictive factors for cardiac resynchronization therapy: a prospective study comparing data from phase-analysis of gated myocardial perfusion single-photon computed tomography and echocardiography. Ann Nucl Med 31, 218–226 (2017). https://doi.org/10.1007/s12149-017-1148-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-017-1148-5