Abstract
Objective
To determine prognostic value of bone marrow retention index (RI-bm) and bone marrow-to-liver ratio (BLR) measured on baseline dual-phase 18F-FDG PET/CT in a series of newly diagnosed patients with diffuse large B-cell lymphoma (DLBCL) treated homogeneously with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy.
Patients and methods
This prospective study enrolled 135 patients with newly diagnosed DLBCL. All patients underwent dual-phase 18F-FDG PET/CT. The following PET parameters were calculated for both tumor and bone marrow: maximum standardized uptake value (SUVmax) at both time points (SUVmax early and SUVmax delayed), SUVmax increment (SUVinc), RI, and BLR. Patients were treated with R-CHOP regimen and response at end of treatment was assessed.
Results
The final analysis included 98 patients with complete remission. At a median follow-up of 22 months, 57 patients showed no relapse, 74 survived, and 24 died. The 2-year relapse-free survival (RFS) values for patients with higher and lower RI-bm were 20% and 65.1%, respectively (p < 0.001), and for patients with higher and lower BLR were 30.2% and 69.6%, respectively (p < 0.001). The 2-year overall survival (OS) values for patients with higher and lower RI-bm were 60% and 76.3%, respectively (p = 0.023), and for patients with higher and lower BLR were 57.3% and 78.6%, respectively (p = 0.035). Univariate analysis revealed that RI-bm and BLR were independent significant prognostic factors for both RFS and OS (hazard ratio [HR] = 4.02, p < 0.001, and HR = 3.23, p < 0.001, respectively) and (HR = 2.83, p = 0.030 and HR = 2.38, p = 0.041, respectively).
Conclusion
Baseline RI-bm and BLR were strong independent prognostic factors in DLBCL patients.
Clinical relevance statement
Bone marrow retention index (RI-bm) and bone marrow-to-liver ratio (BLR) could represent suitable and noninvasive positron emission tomography/computed tomography (PET/CT) parameters for predicting pretreatment risk in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) who were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy.
Key Points
• Bone marrow retention index (RI-bm) and bone marrow-to-liver ratio (BLR) are powerful prognostic variables in diffuse large B-cell lymphoma (DLBCL) patients.
• High BLR and RI-bm are significantly associated with poor overall survival (OS) and relapse-free survival (RFS).
• RI-bm and BLR represent suitable and noninvasive risk indicators in DLBCL patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most prevalent subgroup of non-Hodgkin lymphoma (NHL) and accounts for approximately 25% of adult lymphoma worldwide [1]. It is an aggressive but potentially curable malignancy. In the last decade, cure rates have improved with the inclusion of the anti-CD-20 monoclonal antibody rituximab in the regimen of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [2, 3]. However, about 40% of patients relapse or experience refractory disease [4]. Moreover, although first-line treatment with upfront rituximab has become more successful, salvage therapy has become less effective [5]. Therefore, accurate pretreatment recognition of these poor-risk patients is essential for improving their prognosis using more risk-adapted therapies. In daily practice, DLBCL pretreatment prognosis is based on the International Prognostic Index (IPI) [6]. However, the discriminatory ability of IPI declines with rituximab [7]. In the rituximab period, a very high-risk category could not be recognized [8]. Therefore, novel prognostic factors are needed.
Fluorine-18 fluorodeoxyglucose positron emission tomography coupled with computed tomography (18F-FDG PET/CT) is the standard noninvasive method for pretreatment staging of DLBCL because of its ability to properly assess both nodal and extranodal involvement [9]. Nevertheless, the function of baseline PET/CT exceeds anatomic mapping, as it is also used to quantify FDG uptake by calculating the standardized uptake value (SUV) [10]. The uptake and elimination of 18F-FDG are time-dependent, with single-time-point (STP) imaging done 1 h after 18F-FDG administration. On the other hand, delayed time-point (DTP) imaging was conducted at least 2 h following injection [11]. As a result, benign and malignant lesions accumulate FDG differently on DTP images, thereby increasing the diagnostic accuracy of 18FDG PET/CT [12, 13]. DTP-PET can detect malignant tumors better than STP-PET.
Several recent researches have evaluated the prognostic significance of maximum SUV (SUVmax) dynamic changes detected on DTP-PET imaging in certain tumors, such as squamous cell carcinoma of the head and neck [11] and non-small cell lung cancer [14]. In addition, potential quantitative PET metrics based on PET have recently been evaluated and proposed as predictive biomarkers for DLBCL [15]. Among the new promising dynamic PET indices obtained from DTP-PET are the increment in SUVmax (SUVinc) between the early and delayed phase images, the retention index (RI), which is defined as the proportional difference in SUVmax between the early and delayed phase images, and bone marrow-to-liver ratio (BLR) [16, 17]. However, most researches lack external validation of the described models and are retrospective, underpowered, and methodologically varied [18]. In order to better understand the prognostic value of RI and BLR on baseline dual-phase 18F-FDG PET/CT in terms of relapse-free survival (RFS) and overall survival (OS), we conducted this prospective study on a group of patients with newly diagnosed DLBCL who were all given the same R-CHOP chemotherapy.
Patients and methods
Study design and patient population
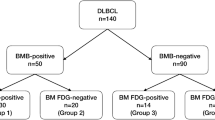
This study initially included 150 consecutive patients with newly diagnosed, pathologically proven CD20-positive DLBCL, who were treated at our institution between March 2019 and June 2022. All patients met the following inclusion criteria: (i) age > 18 years, (ii) normal echocardiography and ejection fraction, (iii) negative virology (HCV AB, HBsAg, HIV), and (iv) adequate liver and renal function. Exclusion criteria were (i) history of other malignancies (n = 9) and (ii) debilitating diseases (n = 6). The exclusion process resulted in a study cohort including 135 patients who were eligible to participate. After recruitment, all included patients were referred for performing pretreatment DTP 18F-FDG PET/CT examinations. A complete pretreatment workup was performed, including history, physical examination, and laboratory tests, including blood count, biochemical tests, hepatitis B and C serological markers, lactate dehydrogenase, and bone marrow (BM) examination. Baseline clinicopathological data were recorded, including age, sex, B symptoms, Eastern Cooperative Oncology Group (ECOG) performance status, number of involved extranodal sites, and bulky disease (tumor size ≥ 7.5 cm). The Ann Arbor classification was used for staging, and risk groups were established for all patients based on the IPI. Patients who did not achieve complete remission (CR) after initial treatment (n = 32) and those lost during the follow-up period (n = 5) were excluded from the final analysis. The final analysis included 98 patients with CR. Figure 1 illustrates a flow diagram of the study. This study was authorized by the institutional review board (approval no. ZU-IRB #9808; approved February 11, 2019), and all patients provided written informed consent.
18F-FDG PET/CT imaging protocol
All 18F-FDG PET/CT scans were performed using a SIEMENS Biograph True Point PET/64 MDCT scanner (Siemens Healthcare). All PET/CT scans were obtained within 2 weeks of the first cycle of chemotherapy. Prior to 18F-FDG injection, all patients were instructed to fast for 6 h, and adequate glucose-free hydration was recommended the day before. Blood glucose levels were measured in all patients before the examination. Serum glucose concentration was maintained at 150 mg/dL. An intravenous cannula was inserted, and 18F-FDG (7 MBq/kg) was injected 1 h before early 18F-FDG PET/CT scanning. After the injection, patients were asked to rest in a quiet room and minimize their movements and talk to diminish unnecessary muscular uptake. Patients were instructed to empty their bladders immediately before the scan. During image acquisition, patients were instructed to breathe shallowly. Patients were scanned in the supine position. A whole-body scan was performed from the base of the skull to the mid-thigh. PET scanning was achieved over numerous bed positions with an emission data acquisition time of 4 min per bed position. DTP PET/CT was conducted by imaging at two time points, 1 and 2 h after injection, and the SUVmax alteration between these time points was calculated. The selected bed positions in the delayed images were determined by nuclear medicine physicians according to the site of the lesions detected in the early images. FDG uptake of the BM was determined by measuring the SUVmax of the right posterior iliac crest in the early and delayed images. In all patients, SUVmax was measured in the early and delayed scans at the same location. A non-contrast-enhanced CT scan was performed prior to PET data acquisition and was utilized for attenuation correction during PET image reconstruction. The following parameters were used: 120 kV, 180 mA, 0.5-s tube rotation time, 5-mm slice thickness, 8-mm table feed, and 3-mm incremental reconstruction.
18F-FDG> PET/CT image analysis
Images were reconstructed and displayed on a workstation (Syngo Multimodality Workplace; Siemens Medical Solutions) that offered multiplanar reformatted PET, CT, and fused PET/CT images. Two experienced nuclear medicine physicians (A.E. and H.A.) with over 10 years of experience in lymphoma imaging consensually interpreted all PET/CT images. In case of disagreement, a third opinion was requested (B.A.). All physicians were blinded to management outcomes and other clinical data. All images were qualitatively and quantitatively interpreted. A qualitative assessment of hypermetabolic lesions was performed on both the corrected and uncorrected PET images. Quantitative evaluation of SUVmax was performed using the following formula: SUVmax = (μCi/g in tissue) / (total μCi injected/body weight). The following PET parameters were calculated for the tumor (the most active lesion) and the BM: SUVmax at both time points (SUVmax early and SUVmax delayed), SUVinc, RI, and BLR. SUVinc was calculated by subtracting SUVmax delayed from SUVmax early. RI was calculated as follows: RI = 100 × [SUVmax delayed − SUVmax early / SUVmax early]. BLR was calculated as follows: BLR = SUVmax of BM (SUVbm) / SUVmax of liver, assuming that the liver had no pathological FDG uptake. A quantitative evaluation is shown in Fig. 2.
Dual-time point 18FDG-PET/CT images and the selected ROI for tumor, BM, and liver FDG uptake in a 65-year-old male patient with histologically proven stage III DLBCL. The patient received the R-CHOP regimen and achieved CR, but relapsed at the end of the study. A Early and B delayed coronal fused 18F FDG-PET/CT images reveal multiple FDG-avid LNs with increased FDG accumulation in the delayed images. C Early and D delayed axial fused 18F FDG-PET/CT images show the ROI drawn around the lesion with the highest FDG uptake (retroaortic LN) (blue arrow) and the left iliac crest (black arrow) to calculate SUVmax (early and delayed), SUVinc, RI-bm, and RI-t. E Axial fused 18F FDG-PET/CT image shows the ROI (black arrow) was drawn to calculate the SUVmax of the liver and BLR. The RI-bm and BLR values were 46 and 0.7, respectively. ROI, region of interest; PET/CT, positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value; SUVinc, increment in SUVmax between early and delayed phase images; BM, bone marrow; RI, retention index; RI-t, retention index of SUVmax of tumor; RI-bm, retention index of SUVmax of bone marrow; BLR, bone marrow-to-liver ratio
Treatment strategies and outcomes
All patients were treated with the R-CHOP-21 regimen (rituximab: 375 mg/m2 intravenously on day 1, cyclophosphamide: 750 mg/m2 intravenously on day 1, doxorubicin 50 mg/m2 intravenously on day 1, vincristine: 1.4 mg/m2 maximum 2 mg intravenously on day 1, and prednisone 100 mg/day orally on days 1 to 5). R-CHOP was administered for 4–6 cycles depending on the stage. The response to the treatment was categorized according to the Lugano classification [19] as CR, partial remission, no response, or progressive disease. Patients were evaluated after 2 to 3 cycles, and responding patients continued the protocol while progressing patients shifted to salvage therapy and were excluded from the final analysis. All patients with CR were followed up every 2 months in the first year and every 3 months in the second year.
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD), median, and range. Categorical variables were expressed as numbers and percentages. When appropriate, percentages of categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. RFS was calculated as the time from the date of initiation of treatment to the date a relapse was detected or the last follow-up at which no relapse was detected (censored). The OS was defined as the time from the date of diagnosis to the date of death from any cause or the last patient follow-up (censored). The optimal cutoff values of the PET/CT parameters for RFS and OS were established using receiver operating characteristic (ROC) curve analysis. Stratification of RFS and OS was determined based on clinical and pathological characteristics. The Kaplan–Meier survival curve was used to estimate these time‐to‐event distributions, and a two-sided exact log‐rank test was used for comparison. A univariate Cox regression analysis was used to examine the relationships between various study parameters and RFS and OS. All tests were two-sided. p value < 0.05 was considered statistically significant. MedCalc (MedCalc Software bvba 13) and SPSS 22.0 (SPSS Inc.) were used for all statistical analyses.
Results
Patient characteristics
The final analysis included 98 patients with CR. Table 1 provides an overview of the patients’ demographic, clinical, and pathological baseline characteristics. The mean age was 49.3 ± 11.4 years (range = 22–67 years), with 31.6% of patients being above the age of 60 years. The male sex was slightly predominant (52%). Thirty-one patients (31.6%) had B symptoms and 72 (73.5%) had an ECOG performance status > 0 at diagnosis. Forty-nine percent of patients presented at an advanced stage (III or IV), whereas the remaining 51% were diagnosed at an early stage (stage I or II). Based on the IPI scores, 39.8% of patients had a low risk (0–1), 26.5% had a low-to-intermediate risk (2), 20.4% had a high-intermediate risk (3), and 13.3% had a high risk (4–5). At the time of diagnosis, 19 patients (19.4%) had pathologically proven BM involvement. Extranodal involvement was found in 39.8% of patients, with 15.3% having more than one site. In 14.3% of patients, bulky disease was present, while LDH levels were elevated in 46.9%. All patients were treated with the R-CHOP-based regimen. Following the completion of chemotherapy, 31% of patients underwent consolidative radiotherapy. Fifty-seven patients had no evidence of relapse after a median follow-up of 22 months (range = 5–24 months), whereas 74 were still living and 24 had passed away.
Dual-phase18F-FDG PET/CT metabolic parameters
Table 2 summarizes the metabolic parameters of 18F-FDG PET/CT. The tumor’s mean SUVinc (SUVinc-t) was 3.1 ± 1.2, and its mean RI (RI-t) was 20.5 ± 8.7. The mean RI of BM (RI-bm) was 25.9 ± 16.9, and the mean SUVinc of BM (SUVinc-bm) was 0.3 ± 0.2. The mean BLR was 0.8 ± 0.7.
Relationship between metabolic parameters and treatment outcomes
As shown in Table 3, the relapse rates differed significantly between patients with high and low RI-bm (p = 0 0.001) and high and low BLR (p < 0 0.001). However, no statistically significant differences were found between patients with high and low SUVinc-t (p = 0 0.059), high and low RI-t (p = 0.658), or high and low SUVinc-bm (p = 0.481). Regarding the mortality rate, there were statistically significant differences between patients with high and low RI-bm (p = 0 0.020) and high and low BLR (p = 0 0.040). However, no statistically significant differences were found between patients with high and low SUVinc-t (p = 0 0.513), high and low RI-t (p = 0.559), or high and low SUVinc-bm (p = 0.560). As shown in Table 4, regarding 2-year RFS, highly statistically significant differences were found between patients with high and low RI-bm, high and low BLR, and high and low IPI (p < 0 0.001). Regarding the 2-year OS, statistically significant differences were found between patients with high and low RI-bm (p = 0.023), high and low BLR (p = 0.035), and high and low IPI (p < 0 0.001). Patients with higher RI-bm and BLR had significantly poor RFS (Fig. 3A–C) and OS (Fig. 3D–F).
Kaplan–Meier plots of the studied patients. The left panel represents RFS, and the right panel represents OS. A, D Images stratified by RI-bm. B, E Images stratified by BLR. C, F Images stratified by the IPI. RFS, relapse-free survival; OS, overall survival; RI-bm, retention index of SUVmax of bone marrow; BLR, bone marrow-to-liver ratio; IPI, International Prognostic Index
Survival analysis and prognostic impact of RI-bm and BLR
RI-bm and BLR were found to be significant prognostic factors for both RFS and OS in univariate analysis using the Cox proportional hazards model (HR = 4.02, 95% CI = 2.03–7.94, p < 0.001, HR = 3.23, 95% CI = 1.74–5.99, p < 0.001, respectively); (HR = 2.83, 95% CI = 1.10–7.23, p = 0.030, HR = 2.38, 95% CI = 1.03–5.46, p = 0.041, respectively) (Table 5). Additionally, older age, stage I disease, lower ECOG performance status, IPI risk score, BM involvement, extranodal involvement, and LDH level were significant predictors of OS and RFS. Higher ECOG status, bulky disease, higher stage, RI-t, SUVinc-bm, and SUVinc-t had poor OS, but the findings were not statistically significant.
Discussion
Recently, a number of studies have been conducted to assess the predictive efficacy of quantitative FDG PET/CT parameters in the pretreatment evaluation of DLBCL [20,21,22,23,24,25]. Most studies, however, focused on SUVmax, total lesion glycolysis (TLG), and metabolic tumor volume (MTV). Some authors have reported that MTV improves the predictive power of FDG PET/CT, helps identify patients with poor prognosis, and accurately selects patients for efficient therapy [26, 27]. However, inconsistent results have been reported in the literature. According to certain studies, TLG on FDG PET/CT imaging was a more accurate predictor and linked highly to patient outcomes than MTV [20, 28, 29]. In other studies, neither total MTV nor TLG was a predictive factor [30,31,32]. In this research, we assessed the prognostic significance of RI and BLR on baseline DTP 18F-FDG PET/CT in a group of patients with newly diagnosed DLBCL who were treated with R-CHOP chemotherapy. We found that RI-bm and BLR were independent prognostic variables for both RFS and OS. Our results support those already published by other researchers [17, 34]. Lee et al [17] reported that BLR was an independent predictive factor for PFS in patients with lymphoma without BM involvement. Chang et al [33] evaluated the predictive value of RI-bm in DLBCL patients. They concluded that the RI of the right posterior iliac crest was a reliable predictor of 3-year OS in these patients. Lim et al [34] investigated the relationship between metabolic parameters derived from DTP 18F-FDG PET/CT and clinical tools that predict outcomes in high-grade lymphoma patients. They found that RI-t was significantly correlated with staging, extranodal involvement, IPI, and revised IPI.
The findings of this study also revealed that high RI-bm and BLR were substantially associated with poor OS and RFS, although remission was achieved with R-CHOP therapy. In agreement with Chang et al [33], this finding is important in clinical practice. Hence, more care should be given to treatment strategies and follow-up in patients with high RI-bm and BLR.
In our study, we found that the BM parameters on dual-phase PET/CT showed prognostic significance, while we failed to prove the predictive value of tumor-related parameters (RI-t and SUVinc-t). These findings are comparable to those of Chang et al [33], who demonstrated that the SUVinc of BM has a strong correlation with BM involvement by tumor cells. Moreover, although there was a significant difference between early and late SUVt, the dual-phase SUVt showed no significant values for predicting OS. Previous studies [33, 35,36,37] have suggested possible explanations for the strong relationship between SUV-bm and survival. These explanations were mainly related to host BM conditions, tumor-associated macrophages, metabolic status of BM cells, and the presence of hidden metastatic cancer cells in the BM.
The rationale for using BLR is to reduce the interindividual variation in SUV-bm with correction by normal 18F-FDG uptake of the liver [38]. According to our findings, patients with high BLR on pretreatment FDG PET/CT scans had a poor prognosis. This is concordant with the findings of Lee et al [17], who investigated the significance of FDG uptake by BM for predicting RFS in lymphoma patients without BM involvement. The researchers reported that FDG uptake by BM was considerably related to poor RFS. The prognosis of patients with high FDG uptake of BM was comparable to that of patients with BM involvement.
Although our study was prospective, it had some limitations. First, the sample size is small. Second, this study was carried out at a single center. Third, the duration of the follow-up period was relatively short. Fourth, the inability to perform multivariate analysis due to statistical issues. Fifth, the patients with or without BM involvement were mixed up in the analysis. Finally, we did not correlate the BLR and RI-bm to BM involvement as we focused on the prognostication assessment of these biomarkers.
In conclusion, this study revealed that RI-bm and BLR are powerful prognostic variables in DLBCL patients independent of IPI. High BLR and RI-bm were significantly associated with poor OS and RFS. This could represent a suitable and noninvasive method for predicting pretreatment risk in these patients. Large prospective studies with long-term follow-up are recommended to assist in the specification of risk evaluation and treatment strategies.
Abbreviations
- 18F-FDG:
-
F-18 fluorodeoxyglucose
- B symptoms:
-
Are night sweats, fever, drenching, and a weight loss of more than 10% over 6 months
- BLR:
-
Bone marrow-to-liver ratio
- BM:
-
Bone marrow
- CR:
-
Complete remission
- DLBCL:
-
Diffuse large B-cell lymphoma
- DTP:
-
Dual-time-point
- ECOG:
-
Eastern Cooperative Oncology Group
- IPI:
-
International Prognostic Index
- NHL:
-
Non-Hodgkin lymphoma
- OS:
-
Overall survival
- PET/CT:
-
Positron emission tomography/computed tomography
- RFS:
-
Relapse-free survival
- RI:
-
Retention index
- RI-bm:
-
Retention index of SUVmax of bone marrow
- RI-t:
-
Retention index of SUVmax of tumor
- STP:
-
Single-time-point
- SUVinc:
-
Increment in SUVmax between initial and delayed phase images
- SUVmax:
-
Maximum standardized uptake value
- SUVt:
-
SUVmax of tumor
References
Sehn LH, Salles G (2021) Diffuse large B-cell lymphoma. N Engl J Med 384:842–858
Sehn LH, Donaldson J, Chhanabhai M et al (2005) Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 23:5027–5033
Candelaria M, Dueñas-Gonzalez A (2021) Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in diffuse large B-cell lymphoma. Ther Adv Hematol 12:2040620721989579
Pfreundschuh M, Kuhnt E, Trümper L et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera international trial [MInT] group. Lancet Oncol 12:1013–1022
Gisselbrecht C, Glass B, Mounier N et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28:4184–4190
The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 329:987–994
Bari A, Marcheselli L, Sacchi S et al (2010) Prognostic models for diffuse large B-cell lymphoma in the rituximab era: a never-ending story. Ann Oncol 21:1486–1491
Gutierrez A, Bento L, Diaz-Lopez A et al (2020) Evaluation of the MD Anderson tumor score for diffuse large B-cell lymphoma in the rituximab era. Eur J Haematol 104:400–408
Tilly H, Gomes da Silva M, Vitolo U et al (2015) Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v116–v125
Huang H, Xiao F, Han X et al (2016) Correlation of pretreatment 18F-FDG uptake with clinicopathological factors and prognosis in patients with newly diagnosed diffuse large B-cell lymphoma. Nucl Med Commun 37:689–698
Yonezawa N, Minamikawa T, Kitajima K et al (2017) The maximum standardized uptake value increment calculated by dual-time-point 18F-fluorodeoxyglucose positron emission tomography predicts survival in patients with oral tongue squamous cell carcinoma. Nagoya J Med Sci 79:189–198
Nakajo M, Jinguji M, Aoki M, Tani A, Sato M, Yoshiura T (2020) The clinical value of texture analysis of dual-time-point 18F-FDG-PET/CT imaging to differentiate between 18F-FDG-avid benign and malignant pulmonary lesions. Eur Radiol 30:1759–1769
Grisanti F, Zulueta J, Rosales JJ et al (2021) Diagnostic accuracy of visual analysis versus dual time-point imaging with 18F-FDG PET/CT for the characterization of indeterminate pulmonary nodules with low uptake. Rev Esp Med Nucl Imagen Mol (Engl Ed) 40:155–160
Shimizu K, Okita R, Saisho S et al (2015) Clinical significance of dual-time-point 18F-FDG PET imaging in resectable non-small cell lung cancer. Ann Nucl Med 29:854–860
Gormsen LC, Vendelbo MH, Pedersen MA et al (2019) A comparative study of standardized quantitative and visual assessment for predicting tumor volume and outcome in newly diagnosed diffuse large B-cell lymphoma staged with 18F-FDG PET/CT. EJNMMI Res 9:36
Lee JK, Min K, So KA et al (2014) The effectiveness of dual-phase 18F-FDG PET/CT in the detection of epithelial ovarian carcinoma: a pilot study. J Ovarian Res 7:1–6
Lee SC, Kim HJ, Lee SM, Lee JW (2017) Prognostic value of bone marrow 18F-FDG uptake on PET/CT in lymphoma patients with negative bone marrow involvement. Hell J Nucl Med 20:17–25
Frood R, Burton C, Tsoumpas C et al (2021) Baseline PET/CT imaging parameters for prediction of treatment outcome in Hodgkin and diffuse large B cell lymphoma: a systematic review. Eur J Nucl Med Mol Imaging 48:3198–3220
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Zhou M, Chen Y, Huang H, Zhou X, Liu J, Huang G (2016) Prognostic value of total lesion glycolysis of baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B-cell lymphoma. Oncotarget 7:83544
Shagera QA, Cheon GJ, Koh Y et al (2019) Prognostic value of metabolic tumour volume on baseline 18F-FDG PET/CT in addition to NCCN-IPI in patients with diffuse large B-cell lymphoma: further stratification of the group with a high-risk NCCN-IPI. Eur J Nucl Med Mol Imaging 46:1417–1427
Zhou Y, Zhao Z, Li J et al (2019) Prognostic values of baseline, interim and end-of therapy 18F-FDG PET/CT in patients with follicular lymphoma. Cancer Manag Res 11:6871
Chang CC, Cho SF, Chuang YW (2017) Prognostic significance of total metabolic tumor volume on 18F-fluorodeoxyglucose positron emission tomography/ computed tomography in patients with diffuse large B-cell lymphoma receiving rituximab-containing chemotherapy. Oncotarget 8:99587–99600
Chen S, He K, Feng F et al (2019) Metabolic tumor burden on baseline 18F-FDG PET/CT improves risk stratification in pediatric patients with mature B-cell lymphoma. Eur J Nucl Med Mol Imaging 46:1830–1839
Xie M, Zhai W, Cheng S, Zhang H, Xie Y, He W (2016) Predictive value of F-18 FDG PET/CT quantization parameters for progression-free survival in patients with diffuse large B-cell lymphoma. Hematology 21:99–105
Mikhaeel NG, Smith D, Dunn JT et al (2016) Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging 43:1209–1219
Cottereau AS, Lanic H, Mareschal S et al (2016) Molecular profile and FDGPET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res 22:3801–3809
Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U (2013) Baseline total lesion glycolysis measured with (18)F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging 3:272–281
Kim TM, Paeng JC, Chun IK et al (2013) Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer 119:1195–1202
Adams HJ, de Klerk JM, Fijnheer R et al (2015) Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol 94:532–539
Gallicchio R, Mansueto G, Simeon V et al (2014) F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol 92:382–389
Schoder H, Zelenetz AD, Hamlin P et al (2016) Prospective study of 3’-deoxy-3’-18F-fluorothymidine PET for early interim response assessment in advanced-stage B-cell lymphoma. J Nucl Med 57:728–734
Chang CC, Cho SF, Chuang YW, Lin CY, Huang YF, Tyan YC (2018) Prognostic significance of retention index of bone marrow on dual-phase 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with diffuse large B-cell lymphoma. Medicine (Baltimore) 97:e9513
Lim DH, Lee JH (2017) Relationship between dual time point FDG PET/CT and clinical prognostic indexes in patients with high grade lymphoma: a pilot study. Nucl Med Mol Imaging 51:323–330
Lin S, Zhang X, Huang G et al (2021) Myeloid-derived suppressor cells promote lung cancer metastasis by CCL11 to activate ERK and AKT signaling and induce epithelial-mesenchymal transition in tumor cells. Oncogene 40:1476–1489
Zhang S, Che D, Yang F et al (2020) Correction: Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget 11:4845
Baldassarri M, Fallerini C, Cetta F et al (2018) Omic approach in non-smoker female with lung squamous cell carcinoma pinpoints to germline susceptibility and personalized medicine. Cancer Res Treat 50:356–365
Inoue K, Goto R, Okada K, Kinomura S, Fukuda H (2009) A bone marrow F-FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann Nucl Med 23:643–649
Acknowledgements
The authors thank all staff members and colleagues in Radiology, Clinical Oncology and Nuclear Medicine Departments-Zagazig University and Nasser Institute for their helpful cooperation and all the study participants for their patience and support.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mohammad Abd Alkhalik Basha.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
The institutional review board of the Faculty of Human Medicine, Zagazig University, approved the study (approval number, ZU-IRB #9808; date, February 11, 2019).
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Azony, A., Basha, M.A.A., Almalki, Y.E. et al. The prognostic value of bone marrow retention index and bone marrow-to-liver ratio of baseline 18F-FDG PET/CT in diffuse large B-cell lymphoma. Eur Radiol 34, 2500–2511 (2024). https://doi.org/10.1007/s00330-023-10150-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10150-z