Abstract
Objective
Although the prognostic value of positron emission tomography/computed tomography (PET/CT) using [18F]-fluorodeoxyglucose (18F-FDG) has been widely confirmed for diffuse large B cell lymphoma, its value for extranodal natural killer/T-cell lymphoma, nasal type (ENKTL), is still controversial. Therefore, we designed a prospective study to investigate the prognostic value of 18F-FDG PET/CT in patients with ENKTL.
Materials and methods
Thirty-three patients with newly diagnosed, untreated ENKTL, were enrolled in this study. Interim and post-therapy PET/CT scans were analyzed by visual evaluation, in accordance with the criteria set forth by the International Harmonization Project. Patients were classified as either positive or negative. Pretreatment maximum standardized uptake values (SUVmax) of 18F-FDG were recorded in the most 18F-FDG-intense lesions. The pretreatment 18F-FDG SUV as well as the interim and post-therapy PET/CT results were assessed for the ability to predict progression-free survival (PFS) and overall survival (OS).
Results
On the pretreatment scan, the SUVmax of the indicator lesion was >10.00 in 81.8 % of patients who were treatment-resistant and ≤10.00 in 86.4 % of patients who were treatment non-resistant (mean SUVmax, 12.93 and 8.10, respectively). Univariate analyses revealed that pretreatment SUVmax is a significant predictor (P < 0.01, P < 0.01) of PFS and OS, respectively. Multivariate analyses revealed that pretreatment SUVmax (P < 0.01, P = 0.01) and post-therapy PET/CT result (P < 0.01, P = 0.04) are independent predictors of PFS and OS, respectively.
Conclusions
18F-FDG uptakes prior to treatment and post-therapy PET/CT results can predict unfavorable outcomes following treatment in patients with ENKTL, but interim PET/CT results have little value in predicting survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extranodal natural killer/T-cell lymphoma, nasal type (ENKTL), as defined by the World Health Organization (WHO), is a relatively newly recognized distinct clinical and pathological entity [1]. It is a rare tumor with a higher relative incidence in Asia and South America [2]. Until recently, the optimal treatment strategy for ENKTL has not been well defined. An increased understanding of the disease has led to the development of several important management principles [3]. Staging and assessment of the efficacy of a given treatment are very important to the development of optimal treatment strategies.
Positron emission tomography (PET) using [18F]-fluorodeoxyglucose (18F-FDG) has emerged as a powerful functional imaging tool for the evaluation of diffuse large B cell lymphoma [4]. Because of the rarity of the disease, published data and recommendations concerning the role of 18F-FDG PET in ENKTL are limited. There is emerging evidence that semiquantitative PET parameters, such as SUVmax before treatment, may be predictors of survival independent of the International Prognostic Index (IPI) [5]. However, most studies are retrospective and the role of 18F-FDG PET in therapy response monitoring is relatively unexplored. Recently, a prospective study reported on the role of interim 18F-FDG PET in early response assessment [6], though there remains a dearth of prospectively designed studies that examine the role of pretreatment, interim, and post-therapy 18F-FDG PET/CT in the evaluation of patients with ENKTL. To address this issue, we conducted a prospective study of patients with newly diagnosed ENKTL who received a standardized treatment regimen. We investigated the prognostic value of pretreatment 18F-FDG uptake and interim and post-therapy PET/CT results.
Materials and methods
Patients
The study was approved by the West China Hospital, Sichuan University, Ethics Committee. All subjects signed a written consent form. Biopsy-proven new ENKTL patients were prospectively recruited to participate in the study between December 2011 and July 2013 in this single center study at West China Hospital. Forty-four patients were identified. Eleven patients were excluded (due to central nervous system involvement in three, a previous malignancy in three, and previous chemotherapy and/or radiotherapy in five). The remaining 33 patients were enrolled in the study.
The inclusion criteria were (1) patients with untreated ENKTL, histologically confirmed in accordance with the WHO classification [1]; (2) patients between 18 and 70 years of age and free of any concurrent disease precluding protocol treatment. Patients were excluded from the study if they were less than 18 years or greater than 70 years, or if they were diagnosed with central nervous system involvement, primary cutaneous T/NK-cell lymphomas, or anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Patients who had a previous malignancy, had previous chemo- or radiotherapy, were currently pregnant or lactating, or who had diabetes mellitus and an elevated fasting blood sugar level >130 mg/dL were also excluded from the study.
Patients underwent whole-body 18F-FDG PET/CT before initial treatment, after two cycles of chemotherapy plus radiation therapy for stage I–II ENKTL (or after two cycles of chemotherapy only for patients with stage III–IV ENKTL, and at the end of treatment (another two to four cycles of chemotherapy for patients with stage I–II ENKTL or four cycles of chemotherapy plus complementary radiation for patients with stage III–IV ENKTL). Interim and post-treatment PET/CT’s were performed three weeks after initial treatment and at the end of treatment, respectively.
PET/CT scan protocol
All patients underwent whole-body 18F-FDG PET/CT on a combined PET/CT scanner (Gemini GXL with a 16-slice CT component, Philips Corp., The Netherlands). After fasting for at least 6 h (blood glucose level <200 mg/dL), an intravenous injection of 185–370 MBq of 18F-FDG (5.18 MBq/kg) was administered. After resting for approximately 60 min, whole-body CT and PET scans were performed. The CT scan was performed first, with a voltage of 120 kV, a current intensity of 40 mA, a tube rotation of 0.8 s, and a section thickness of 4 mm, without oral or intravenous contrast agents. The CT acquisition data were used for attenuation correction and corrected PET images were reconstructed using the line-of-response (LOR) method. The acquired images from the PET and CT scans were sent for image registration and fusion with Syntegra software.
Image analysis
Positron emission tomography/computed tomography scans were read by two physicians specializing in nuclear medicine, who were unaware of any information about the patients or their clinical condition, and diagnoses were made by consensus. Simple, circular regions-of-interest (ROIs) were drawn by hand on axial, coronal, or sagittal co-registered PET/CT slices. Maximum standardized uptake values (SUVmax) were obtained and corrected for body weight using the standard formula: mean ROI activity (MBq/mL)/[injected dose (MBq)/body weight (kg)] [7]. ROIs were placed manually over all lesions, and the SUVmax was recorded for every lesion. Also, the highest SUVmax for every pretreatment scan was recorded, and these lesions were identified as indicator lesions.
In an effort to standardize the interpretation of the interim and post-treatment 18F-FDG PET scans, we adopted the method described by Cashen et al. [8]. and evaluated the 18F-FDG PET/CT scans on the basis of the criteria established by the International Harmonization Project for end-of-treatment PET [9]. The results were judged to be positive if they met one of the following consensus response criteria:
-
A.
Focal or diffuse 18F-FDG uptake above background.
-
B.
In a mass 2 cm or larger, mild and diffusely increased 18F-FDG uptake that is greater than uptake in mediastinal blood pool structures.
-
C.
Any increased uptake in a mass smaller than 2 cm.
-
D.
New lung nodules of 1.5 cm or larger, with uptake less than uptake in the mediastinal blood pool.
-
E.
Hepatic or splenic lesions of 1.5 cm or larger, if uptake is more than uptake in liver or spleen; or diffusely increased splenic uptake.
-
F.
Clearly increased focal or multifocal bone involvement.
Treatment
Chemotherapy was initiated within 7 days after the pretreatment PET/CT scans. For patients with stage I–II ENKTL, the dosages and administration schedule for chemotherapy with l-asparaginase, vincristine, and prednisone (LVP) were as follows: l-asparaginase, 6000 IU/m2 administered intravenously on days 1 through 5; vincristine, 1.4 mg/m2 administered intravenously on day 1; and prednisone, 100 mg administered orally on days 1 through 5. Chemotherapy was repeated every 3 weeks. After two cycles of chemotherapy, radiation therapy (RT) was started, and then two to four cycles of chemotherapy were initiated 3 weeks after RT was completed. All patients were treated with a photon beam of 6 MV, and 3-dimensional conformal treatment planning was used. The clinical target volume (CTV) of limited stage IE disease included the nasal cavity, bilateral frontal and ethmoid sinuses, and ipsilateral maxillary sinus. For patients with extensive stage IE disease, the CTV was extended to the involved organs and/or tissues. For stage IIE disease, the CTV also included the affected cervical lymph node area. The planning target volume included the CTV with a 5.0-mm overlying margin. Supplementary doses of radiation in the ethmoid sinus and cervical lymph nodes were supplied by an electron beam. The RT prescription was 56 Gy in 28 fractions, once per day, and five fractions per week. If one or more of the following adverse events were observed—grade 4 leukopenia or neutropenia, platelet count <25,000/L, any grade 3 nonhematologic toxicity except for mucositis or dysphagia related to radiation, and/or Eastern Cooperative Oncology Group (ECOG) performance AQ7 status ≥3—then the RT was postponed until the toxicity was reduced to grade 2. For patients with stage III–IV ENKTL, RT was administered for residual lesions after six cycles of chemotherapy (LVP strategy). The total radiation dose ranged from 18.0 to 56.0 Gy (mean dose, 28 Gy).
Follow-up assessment
Follow-up examinations were performed every 3 months after the completion of treatment [10]. All patients underwent the following standard evaluations: a complete history and physical examination; CBC and serum chemistries, including lactate dehydrogenase (LDH) and other blood tests; bone marrow aspiration and biopsy; whole-body 18F-FDG PET/CT (not in all patients); CT or MR imaging of the head and neck (if necessary); and CT scans of the thorax, abdomen, and pelvis.
Treatment resistance was defined as progressive disease. Treatment non-resistance included patients who had stable disease and patients who experienced remission, complete, or partial.
Progression-free survival (PFS) was defined as the interval between the date of diagnosis and the date of first relapse, progression, or death from any cause. When patients were lost to follow-up, PFS was defined as the interval between the date of diagnosis and the date of the last disease-free follow-up visit. Overall survival (OS) was defined as the interval between the date of diagnosis and the time of death from any cause. When patients were lost to follow-up, OS was defined as the interval between the date of diagnosis and the date of the last follow-up visit.
Statistical analysis
The optimal SUVmax cutoff value was estimated from the receiver operating characteristic curve (ROC), and the value with the highest sum of sensitivity and specificity for distinguishing between the treatment-resistant and treatment non-resistant patients was used as the cutoff. The definitions of treatment resistance and treatment non-resistance are described above. The SUVmax cutoff value of the primary tumors was used to define two groups of patients. Fisher’s exact test was used to compare PET/CT indices [pretreatment SUVmax, interim, and post-therapy PET/CT results (positive or negative)] with other clinical variables [sex, age, B symptoms, ECOG performance status, International Prognostic Index (IPI) score, LDH level, Ann Arbor stage, and response to therapy]. The log-rank test and the Kaplan–Meier method were used for univariate survival analysis. Analysis with a Cox proportional hazards model was used to determine whether pretreatment SUVmax or a positive interim or post-therapy PET/CT was significantly associated with PFS and OS. The statistical software package SPSS (version 16.0; SPSS) was used for statistical calculations. A P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
Thirty-three patients underwent pretreatment and interim 18F-FDG PET/CT, and 28 patients underwent end-of-therapy 18F-FDG PET/CT. The five patients who did not undergo end-of-therapy 18F-FDG PET/CT did not do so because they withdrew their consent.
The clinical characteristics of the 33 enrolled patients (22 men and 11 women) are summarized in Table 1. The mean ± SD age of the patients was 42.6 ± 14.7 years, with 12.1 % of patients over the age of 60. With a mean follow-up of 19.4 months (range 9–34 months), eleven patients progressed, and four of the eleven died due to disease progression. No patients died in the absence of progression.
Pretreatment PET/CT analysis
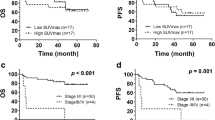
The SUVmax of each patient’s indicator lesion, the lesion with the highest SUVmax, was measured on pretreatment 18F-FDG PET imaging. The optimal SUVmax cutoff value on the ROC curve that maximized both sensitivity and specificity was 10.00 (Table 1). Patients were divided on the basis of their SUVmax cutoff value into high SUVmax (SUVmax >10.00, n = 12) and low SUVmax (SUVmax ≤10.00, n = 21) groups. The presence of B symptoms was found to be significantly associated with SUVmax (P < 0.05) (Table 2). In addition, univariate analysis demonstrated that SUVmax was significantly associated with shorter PFS (P < 0.01, Fig. 1a) and OS (P < 0.01, Fig. 1b). Nine patients who were resistant to therapy had an SUVmax of greater than 10.00, whereas only two patients who were treatment-resistant had an SUVmax of less than 10.00 (these two patients had SUVmax values of 9.00 and 9.70, respectively, both quite near the mean). Nineteen out of 22 treatment non-resistant patients (86.4 %) had an SUVmax less than or equal to 10.00. The mean SUVmax of the high SUVmax and low SUVmax groups was 12.93 and 8.10, respectively.
a Pretreatment PET/CT prognostic factors for PFS. SUVmax was the only independent determinant of patient PFS following multivariate analysis (P < 0.01). b Pretreatment PET/CT prognostic factors for OS. SUVmax was the only independent determinant of patient OS following multivariate analysis (P < 0.01)
Interim PET/CT analysis
Nineteen (57.6 %) of the 33 patients had negative interim PET/CT scans, while 14 patients (42.4 %) had positive PET/CT scans. There was no significant association between any of the clinical variables and interim PET/CT scan result (Table 2). Further, univariate analysis did not demonstrate any association between interim PET/CT result and both PFS (P = 0.07, Fig. 2a) and OS (P = 0.09, Fig. 2b). The 2-year PFS rates in the interim PET/CT-negative and -positive groups were 68.4 and 48.2 %, respectively, and the 2-year OS rates were 83.9 and 56.7 %, respectively. The PPV, NPV, and accuracy of interim PET/CT in predicting PFS and OS in the 33 patients who underwent this analysis are listed in Table 3.
Post-therapy PET/CT analysis
Of the 28 patients who underwent post-therapy PET/CT, 19 patients (67.9 %) had negative scans and nine patients (32.1 %) had positive scans. There was a significant association between B symptoms and post-therapy PET/CT results (P = 0.01) (Table 2). Further, patients with positive post-therapy PET/CT scans had both significantly shorter PFS (P < 0.01, Fig. 3a) and OS (P = 0.01, Fig. 3b). The 2-year PFS rates in the post-therapy PET/CT-negative and -positive groups were 92.3 and 13.3 %, respectively, and the 2-year OS rates were 90.9 and 44.4 %, respectively. The PPV, NPV, and accuracy of post-therapy PET/CT in predicting PFS and OS in the 28 patients who underwent this analysis are listed in Table 3.
a Post-treatment PET/CT prognostic factors for PFS. SUV was the only independent determinant of patient PFS following multivariate analysis (P < 0.01). b Post-treatment PET/CT prognostic factors for OS. SUV was the only independent determinant of patient OS following multivariate analysis (P = 0.01)
Multivariate analysis for PFS and OS
Univariate analysis determined that sex, Ann Arbor stage, B symptoms, LDH level, SUVmax of the primary tumor, and post-therapy PET/CT result were significant predictors of PFS (P < 0.05, Table 4), and that sex, Ann Arbor stage, B symptoms, SUVmax of the primary tumor, and post-therapy PET/CT result were significant predictors of OS (P < 0.05, Table 5). After multivariate analysis, SUVmax of the primary tumor remained a statistically significant predictor of PFS (P < 0.01, hazard ratio (HR) = 6.2, 95 % confidence interval (CI) 4.3–10.9) and OS (P = 0.01, HR = 6.6, 95 % CI 1.9–14.0) (Table 6). Following multivariate analysis post-therapy PET/CT result also remained a statistically significant predictor of PFS (P < 0.01, HR = 2.8, 95 % CI 1.3–10.2) and OS (P = 0.04, HR = 2.6, 95 % CI 1.0–6.3) (Table 6).
Discussion
In the present study, we address the issue of the value of 18FDG–PET/CT results prior to treatment, during (interim) treatment, and after completion of therapy in patients with ENKTL. The results indicate that pretreatment uptake of 18F-FDG in patients with ENKTL can predict treatment resistance and survival outcomes. In addition, SUVmax of the primary tumor is a statistically significant predictor of both PFS and OS. These findings suggest that patients with higher 18F-FDG uptake prior to therapy have poorer outcomes and that post-therapy PET/CT may be an accurate predictor of survival.
Part of the presumed value of interim 18F-FDG PET is its potential for prognostic and predictive capabilities. However, our results illustrate the limitations of interim PET/CT in this population. In the present study, interim PET/CT could not significantly predict PFS or OS. This finding is inconsistent with prior research that demonstrated a positive association between the variables [6, 11]. Discrepancies between the studies may be due to many reasons. The type of therapy patients receive varies from center to center, and therefore, subjects in one study likely do not receive the same treatment regimen as subjects in another study. For example, all of the patients in our study received the LVP chemotherapy regimen, whereas the patients assessed by Li et al. were treated with a variety of regimens including CHOP, EPOCH, alternating triple therapy (CHOP-B, IMVP-16, and DHAP), or GEMOX1L-asp. Further, most of our patients received radiotherapy prior to their interim PET/CT scans, while patients in the study conducted by Li and colleagues received no radiotherapy. Studies have shown that therapy-related changes including inflammation can be detected in lymphoma patients up to 2–3 months following radiation therapy or chemoradiotherapy [12]. Given that the interim scans in our study were performed during the fourth week following initial treatment, inflammatory changes may have been responsible for a number of false-positive results. Additional studies with homogenous treatment regimens lacking radiotherapy may be required to determine why our results differed from those of Li et al. and Khong et al.
In this study, post-therapy 18F-FDG PET/CT results were significantly predictive of PFS and OS. This finding is contrary to the study performed by Cahu et al. [13], who found no significant association between PFS or OS and post-therapy 18F-FDG PET results. The reason for the discrepancy is unclear but may be explained by several differences between the studies. The patients in the study performed by Cahu et al. were assessed with PET only, whereas the patients in our study were assessed with PET/CT. Several studies have shown that the diagnostic performance of PET/CT is superior to that of PET alone [14]. Therefore, it is not surprising that the PET/CT protocol that our study employed had a relatively higher post-therapy PPV, NPV, and accuracy than the PET only protocol used in Cahu’s study. Moreover, Cahu’s patients had both variable treatment strategies and tumor histology. This difference is important because PFS, OS, and relapse rates vary depending on the type of therapy [15]. Finally, Cahu’s patients were recruited from five independent institutions with different PET systems and protocols, which likely introduced further heterogeneity into the study’s methods. Because of our study’s contradictory findings, it is a valuable contribution to the literature, and it strengthens our belief that additional prospective studies must be conducted before guidelines on the use of PET/CT in the assessment of treatment response in patients with ENKTL are developed. Our results also support the idea that patients with positive post-therapy results should be considered as candidates for more aggressive treatments.
Our study has several limitations. First, the time between the cessation of therapy and the post-therapy PET/CT scan was relatively short. Transient inflammatory changes in response to therapy can lead to false-positive uptake of 18F-FDG in a residual mass, and these types of changes are more frequent after radiation therapy or chemoradiation than after chemotherapy alone [16]. As a result, the relatively low PPV and accuracy for survival outcomes in our study might be explained by the difficulty in distinguishing residual tumor from mere inflammatory change. A second limitation of our study is that all interim PET/CT images were interpreted in accordance with the IHP criteria. The use of this method was intended to standardize the interpretations. However, because 18F-FDG uptake is a continuous variable, the use of a binary scoring system may not be appropriate. That is to say, examining the change in SUVmax between the pretreatment scan and the interim and post-treatment scans may have yielded different results. Further research could look at change in SUVmax and its relationship with PFS and OS. Our study is also limited by the relatively short follow-up time after treatment and by the small number of total patients included. Finally, the SUVmax cutoff value as estimated by the receiver operating curve had the highest summed sensitivity and specificity for distinguishing between treatment-resistant and treatment non-resistant patients. However, selection of different cutoff values for the SUVmax of the pretreatment indicator lesion may have yielded different relationships with progression and survival.
Our results demonstrate the value of 18F-FDG PET/CT, when performed at diagnosis and at the end of therapy, for the prediction of disease progression in patients with ENKTL. However, we have shown that interim PET/CT does not predict PFS or OS. Therefore, patients with high 18F-FDG uptake prior to the initiation of therapy or with positive results on post-therapy PET/CT should be considered as candidates for an intensive therapeutic strategy to improve their survival.
References
Swerdlow SH. International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008.
Peh SC. Host ethnicity influences non-Hodgkin’s lymphoma subtype frequency and Epstein–Barr virus association rate: the experience of a multi-ethnic patient population in Malaysia. Histopathology. 2001;38:458–65.
Kwong YL, Anderson BO, Advani R, Kim WS, Levine AM, Lim ST, et al. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1093–101.
Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood. 2007;110:3507–16.
Kim CY, Hong CM, Kim DH, Son SH, Jeong SY, Lee SW, et al. Prognostic value of whole-body metabolic tumour volume and total lesion glycolysis measured on (1)(8)F-FDG PET/CT in patients with extranodal NK/T-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013;40:1321–9.
Khong PL, Huang B, PhinLee EY, SumChan WK, Kwong YL. Midtreatment 18F-FDG PET/CT scan for early response assessment of SMILE therapy in natural killer/T-cell lymphoma: a prospective study from a single center. J Nucl Med. 2014;55:911–6.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11S–20S.
Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011;52:386–92.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in lymphoma. J Clin Oncol. 2007;25:571–8.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244.
Li YJ, Li ZM, Xia XY, Huang HQ, Xia ZJ, Lin TY, et al. Prognostic value of interim and posttherapy 18F-FDG PET/CT in patients with mature T-cell and natural killer cell lymphomas. J Nucl Med. 2013;54:507–15.
Naumann R, Vaic A, Beuthien-Baumann B, Bredow J, Kropp J, Kittner T, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Br J Haematol. 2001;115:793–800.
Cahu X, Bodet-Milin C, Brissot E, Maisonneuve H, Houot R, Morineau N, et al. 18F-fluorodeoxyglucose-positron emission tomography before, during and after treatment in mature T/NK lymphomas: a study from the GOELAMS group. Ann Oncol. 2011;22:705–11.
Reske SN. PET and restaging of malignant lymphoma including residual masses and relapse. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S89–96.
Kim SJ, Kim WS. Treatment of localized extranodal NK/T cell lymphoma, nasal type. Int J Hematol. 2010;92:690–6.
Brepoels L, Stroobants S, Verhoef G. PET and PET/CT for response evaluation in lymphoma: current practice and developments. Leuk Lymphoma. 2007;48:270–82.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81471693) and the Sichuan Provincial Department of science and technology support program. (2012SZ0005).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Jiang and X. Zhang are co-first authors. They contributed equally to the work.
Rights and permissions
About this article
Cite this article
Jiang, C., Zhang, X., Jiang, M. et al. Assessment of the prognostic capacity of pretreatment, interim, and post-therapy 18F-FDG PET/CT in extranodal natural killer/T-cell lymphoma, nasal type. Ann Nucl Med 29, 442–451 (2015). https://doi.org/10.1007/s12149-015-0964-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-0964-8