Abstract
Objectives
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) plays an important role in many oncological settings. In this study, we assessed the utility of 18F-FDG-PET for predicting the histological classification, stage and survival of thymic epithelial tumors.
Methods
We retrospectively analyzed 37 patients with thymic epithelial tumors who underwent PET before surgical resection and investigated the relationship between the maximum of standardized uptake value (SUVmax) of each tumor and the WHO classification, recurrence-free survival, and tumor-related gene expressions.
Results
The study included 15 males and 22 females, ranging in age from 22 to 81 years (mean 64 years). The tumor histology of 31 tumors was thymoma and that of the remaining tumors was thymic carcinoma. The Masaoka tumor stage was as follows: stage I in 18, II in 9, III in 5 and IV in 5 patients. The patients were divided into three groups according to a simplified histologic classification: low-risk thymoma (types A, AB and B1, n = 21), high-risk thymoma (types B2 and B3, n = 10) and thymic carcinoma (n = 6). The SUVmax of low-risk group (SUVmax ≤4.27) was significantly lower than that of high-risk group (p = 0.0114) and that of thymic carcinomas (SUVmax >4.27) was also significantly higher than that of thymomas (p < 0.0001). The group of high SUVmax (SUVmax >4.27) had significantly inferior recurrence-free survival to that of less value (SUVmax ≤4.27) (p = 0.0009). The SUVmax were not correlated with tumor-related gene expressions.
Conclusion
The SUVmax of 18F-FDG-PET reflects WHO classification of thymic epithelial tumors. High SUVmax predicts lower recurrence-free survival of the tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thymic epithelial tumors (thymoma and thymic cancer) commonly appear as primary neoplasms in the anterior mediastinum, though their occurrence is relatively rare. There are two types of classifications used for these thymic epithelial tumors; Masaoka tumor staging and TNM classification, which reflect the stage of progression, and WHO classification, which is determined by histological type. Both types are considered useful to predict prognosis [1–3]. Recently, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), which is generally used for diagnosing the malignancy and stage of a variety of tumors, has been reported useful for assessing thymic epithelial tumors [4–6]. In the present study, we retrospectively examined the utility of FDG-PET as a preoperative examination for cases of resectable thymic epithelial tumor.
Subjects and methods
Among patients who underwent a thymectomy for thymic epithelial tumors (thymoma, thymic carcinoma) in our department in the 7-year period from January 2006 to December 2012, we analyzed 37 underwent preoperative FDG-PET. They consisted of 15 men and 22 women, with a mean age of 64.0 ± 14.6 years (range 22–81 years). The maximum standardized uptake value (SUVmax) accumulation in the tumor was determined as a parameter. Masaoka tumor staging was utilized for diagnosing disease stage. For histological diagnosis of thymoma, the tumors were classified according to the WHO classification into 5 types; namely A, AB, B1, B2, and B3 [7]. As for histological diagnosis of thymomas, they were divided into 2 groups; low risk (A, AB, B1) and high risk (B2, B3).
For analyzing prognosis, recurrence-free survival period was determined by taking into consideration that the tumors were slow growing. Furthermore, the correlation between SUVmax and tumor-related gene expression was investigated in evaluable cases.
FDG-PET protocol
All patients fasted for at least 6 h before undergoing the FDG-PET examination, though they were allowed to ingest a sugar-free drink. The intravenous 18F-FDG dose was set according to body weight, with 4 MBq/kg given to those weighing <55 kg and 222 MBq/kg given to those weighing 55 kg or more. PET images were obtained at 1 h after 18F-FDG administration.
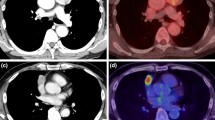
For PET imaging, a Biograph Sensation 16 or Biograph LSO (Siemens, Erlangen, Germany) device was used, and images were obtained at sites ranging from the meatus of the ear to the mid-thigh. Imaging was performed at 6–8 bed in accordance with the body height of the patient. To reduce radiation exposure, CT was performed at a low dose of 9 Eff. mAs. SUVmax was determined by setting the volume of interest (VOI) using Syngo.via (Siemens Healthcare, Forchheim, Germany).
Measurement of tumor-related gene expression
Excised specimens were thinly sliced and then tumor segments were collected by micro-dissection to extract mRNA. RT-PCR was performed according to Danenberg’s method [8] for semi-quantitation of mRNA of tumor-related genes (Response Genetic Inc., New York, USA), including excision repair cross-complementation group 1 (ERCC1), topoisomerase-1 (TOPO1), epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD).
Statistics
Values are shown as the mean ± SD. For comparisons between groups, an unpaired t test was used, while analysis of variance (ANOVA) was used for comparing among 3 or more groups. Survival rate was calculated by the Kaplan–Meier method. A log-rank test was used for comparisons among groups. Receiver operating characteristic (ROC) curve analysis was done to estimate the optimal sensitivity and specificity in the prediction of recurrence according to different cutoff levels of SUV levels, using MedCalc ver.12.7.7 (Ostend, Belgium). A risk rate of 5 % or below was considered to be significant.
Results
Patient backgrounds are shown in Table 1. Eighteen were classified as clinical stage 1, 9 as stage 2, 5 as stage 3, and 5 as stage 4. Histological type was type A in 4 patients, type AB in 14, type B1 in 3, type B2 in 7, type B3 in 3, and thymic carcinoma in 6. Masaoka stage did not correlate with SUVmax.
Mean SUVmax was 3.04 ± 0.79 in the low-risk and 4.42 ± 2.06 in the high-risk thymoma groups (Fig. 1), which were significantly different (p = 0.0114). Furthermore, SUVmax in the thymic carcinoma group (9.50 ± 6.76) was significantly greater than in the thymoma groups as a whole (3.48 ± 1.46) (p < 0.0001). ROC curve of relationship between SUVmax and recurrence-free survival is shown in Fig. 2. Area under ROC curve was 0.881 (p < 0.0001). Cutoff value was 4.27. SUVmax of thymic carcinoma was not <4.27 and low-risk thymoma was not higher than 4.27.
Five-year recurrence-free survival for the low-risk thymoma group was 100 %, whereas that for the high-risk group was 63 %. Also, the 4-year survival rate for thymic carcinoma was 54 %. Recurrence-free survival was significantly lower in the high-risk thymoma and thymic carcinoma groups as compared to the low-risk thymoma group (p = 0.0011).
Recurrence-free survival rates for patients with thymic epithelial tumor (thymoma + thymic carcinoma) using an SUVmax cutoff level of 4.27 are shown in Fig. 3. Five-year survival for those with SUVmax more than 4.27 was 40.9 %, while that for those with a level of 4.27 or less was 95.5 %, which showed a significantly poor prognosis than those with SUVmax of 4.27 or less (p = 0.0009).
Tumor-related gene expression was simultaneously examined in 9 of the 37 patients. Histologic types of the nine patients were 4 of thymoma (type A: 3, type B3: 1) and 5 of thymic carcinoma. The relationships between the relative expression levels of various mRNAs and SUVmax are shown in Fig. 4. No correlation was observed among ERCC1, TOPO-1, EGFR, VEGF, TS, and DPD.
Discussion
Our findings indicate that SUVmax of FDG-PET reflects not only the WHO classification, but also serves as a parameter for predicting prognosis prior to treatment. Previous reports have shown that SUVmax obtained in FDG-PET examinations for thymoma and thymic carcinoma were useful for predicting low- and high-grade tumors, as defined in the WHO classification [5, 6, 9–11]. Benveniste et al. [5] found that SUVmax levels were different between thymoma and thymic carcinoma, and reported a significant difference between Type A-B2 and B3, even in thymoma cases. Similarly, Sung et al. [6] noted significant differences regarding SUVmax among type A-B1, B2-3, and thymic carcinoma groups. Our results were similar to those. Furthermore, the SUVmax may be useful to distinguish low-risk thymoma (SUVmax ≤4.27) from thymic carcinoma (SUVmax >4.27). Accordingly, we consider that FDG-PET findings reflect the WHO classification for thymic epithelial tumors. Meanwhile, Benveniste et al. [5] did not find a correlation between SUVmax and Masaoka tumor stage. As our results were also similar to theirs, we propose that FDG-PET results have a stronger relationship with histological type than Masaoka progression stage.
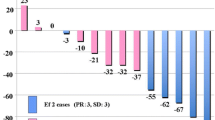
Previous studies have suggested that WHO classification and Masaoka tumor stage might reflect the prognosis of thymic tumors [1–3]. In the present study of subjects divided into 3 groups by WHO classification, we found significant differences regarding prognosis among those groups. Meanwhile, no previous studies have investigated whether SUVmax obtained in FDG-PET examinations at the time of diagnosis have a direct relationship with the prognosis of patients with thymic tumors. Kaira et al. [12] compared FDG-PET findings before and after chemotherapy or radiotherapy in 12 patients with unresectable thymic epithelial tumors, including 10 with thymic carcinoma, and analyzed the ratio (T/M ratio) of maximum and mean SUV in the mediastinum for comparing therapeutic effect and survival. According to their report, when analyzing only patients with thymic carcinoma, those with better post-therapeutic partial metabolic response had better survival. However, they found no significant difference when all 12 patients were analyzed.
In the present study, we examined 37 patients with resectable thymoma and thymic carcinoma, and found a significant difference for recurrence-free survival between patients divided by an SUVmax cutoff value of 4.27. SUVmax obtained prior to treatment were significant predictors of recurrence-free survival. Thus, our findings indicate that FDG-PET is an important examination for predicting the prognosis of thymic epithelial tumors at the time of diagnosis.
Since the SUVmax values were high in the high-risk thymoma and thymic carcinoma groups, demonstrating high malignancy, we investigated the correlation with tumor-related genes considered to be possibly important in thoracic tumors. However, we found no significant correlation with any of the tumor-related genes examined. A previous study found that VGEF expression was specifically increased in thymic carcinoma [13]. On the other hand, we observed no correlation between expression of the VGEF gene and SUVmax. Among the tumor-related genes examined, it is known that TS and DP are involved in nucleic acid metabolism, while ERCC1 is involved in DNA repair and TOPO-1 in cell division. We think that the genes examined in the present study might not have showed any correlation because they are not directly involved in glucose metabolism. Meanwhile, another study suggested the involvement of EGFR in glucose metabolism [14], while that was not seen in our results, though that result might have been affected by the small number of cases. Because the number of examined cases for each subtype of thymic epithelial tumors and examined tumor-related gene expression were limited, additional studies and case accumulation are necessary.
Conclusion
We found that FDG-PET findings are related with WHO classification in regard to thymic epithelial tumors. Furthermore, recurrence-free survival after surgical resection was significantly low in cases with an SUVmax of more than 4.27. Accumulation of additional cases as well as further detailed studies is important, as thymic epithelial tumors are rare.
References
Okumura M, Miyoshi S, Fujii Y, et al. Clinical and functional significance of WHO classification on human thymic epithelial neoplasms: a study of 146 consecutive tumors. Am J Surg Pathol. 2001;25:103–10.
Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94:624–32.
Fang W, Chen W, Chen G, et al. Surgical management of thymic epithelial tumors: a retrospective review of 204 cases. Ann Thorac Surg. 2005;80:2002–7.
Kumar A, Regmi SK, Dutta R, et al. Characterization of thymic masses using 18F-FDG PET-CT. Ann Nucl Med. 2009;23:569–77.
Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol. 2013;40:1595–601.
Sung Y, Lee K, Kim B, et al. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med. 2006;47:1628–34.
Müller-Hermelink HK, Möller P, Engel P, et al. Tumours of the thymus. In: Travis WD, Bramblla E, Müller-Hermelink HK, et al. editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart, World Health Organization classification of tumours. 7th ed. Lyon: IARC Press; 2004. p. 148–151.
Salonga D, Danenberg KD, Johnson M, Metzger R, Groshen S, Tsao-Wei DD, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–7.
Inoue A, Tomiyama N, Tatsumi M, et al. 18F-FDG PET for the evaluation of thymic epithelial tumors: correlation with the World Health Organization classification in addition to dual-time-point imaging. Eur Nucl Med Mol Imaging. 2009;36:1219–25.
Eguchi T, Yoshida K, Hamanaka K, et al. Utility of 18F-fluorodeoxyglucose positron emission tomography for distinguishing between the histological types of early stage thymic epithelial tumours. Eur J Cardiovasc Surg. 2012;41:1059–62.
Fukumoto K, Taniguchi T, Ishikawa Y, et al. The utility of 18F-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur J Cardiovasc Surg. 2012;42:e152–6.
Kaira K, Murakami H, Miura S, Kaira R, Akamatsu H, Kimura M, Ono A, Tsuya A, Nakamura Y, Naito T, Takahashi T, Endo M, Yamamoto N. 18F-FDG uptake on PET helps predict outcome and response after treatment in unresectable thymic epithelial tumors. Ann Nucl Med. 2011;25:247–53.
Toba H, Kondo K, Sadohara Y, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and the relationship between fluorodeoxyglucose uptake and the expression of hypoxia-inducible factor-1a, glucose transporter-1 and vascular endothelial growth factor in thymic epithelial tumours. Eur J Cardiovasc Surg. 2013;44:e105–12.
Nose A, Mori Y, Uchiyama-Tanaka Y, et al. Regulation of glucose transporter (GLUT1) gene expression by angiotensin II in mesangial cells: involvement of HB-EGF and EGF receptor transactivation. Hypertens Res. 2003;26:67–73.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seki, N., Sakamoto, S., Karube, Y. et al. 18F-fluorodeoxyglucose positron emission tomography for evaluation of thymic epithelial tumors: utility for World Health Organization classification and predicting recurrence-free survival. Ann Nucl Med 28, 257–262 (2014). https://doi.org/10.1007/s12149-014-0804-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-014-0804-2