Abstract
Objective
In the era of targeted therapy for advanced renal cell carcinoma (RCC), appropriate prognosis prediction is necessary for optimal therapy with or without cytoreductive surgery. We evaluated prognostic implication of extrarenal metabolic tumor burden in nephrectomized patients with advanced RCC.
Methods
Forty-four patients with advanced RCC who underwent 18F-fluorodeoxyglucose PET/CT were retrospectively enrolled. The patients were treated with nephrectomy and targeted therapy. On PET/CT image of each patient, maximal standardized uptake value (SUVmax) of lesions were measured, and metabolic tumor burden was measured as total lesion glycolysis (TLG) by multiplying tumor volume and mean SUV. An overall TLG was calculated as the sum of those of all lesions. The prognostic value of PET parameters (SUVmax and TLG), and established major clinical factors (serum hemoglobin and corrected calcium, and number of metastatic sites) were tested with regard to overall survival.
Results
Among 44 patients, 8 died during mean follow-up time of 21.9 ± 17.7 months. On FDG PET/CT, a total of 250 lesions were analyzed. In univariate analyses, SUVmax, TLG, number of metastatic sites, serum hemoglobin and corrected calcium were significant prognostic factors. Among them, TLG remained as an independent prognostic factor in a multivariate analysis (P = 0.038). In subgroup analyses, TLG was still a significant prognostic factor in patients treated with sunitinib only and in patients on the first staging as well as restaging.
Conclusions
Extrarenal metabolic tumor burden is a significant prognostic factor in advanced RCC patients treated with targeted therapy. In selection of candidates for cytoreductive surgery, the measurement of metabolic tumor burden may be effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the US, renal cell carcinoma (RCC) is estimated to be 3.8 % of all cancers in 2011; approximately 61,000 individuals would be diagnosed with RCC and 13,000 would die from RCC [1]. In case of locally limited RCC, radical nephrectomy can be a curative treatment [2]; however, no radical treatment exists for advanced metastatic RCC at present. Recently, the prognosis of advanced RCC has been improved with development of molecular targeted therapy [3], and now there are several potential treatment strategies for advanced RCC, including targeted therapy with or without cytoreductive surgery such as nephrectomy and metastasectomy [4]. Although tumor burden reduction with cytoreductive surgery is supported by several studies in the era of targeted therapy [5–7], appropriate prediction of prognosis and selection of surgical candidates are of paramount importance.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) provides information on metabolic characteristics of cancers. FDG PET/CT has been used effectively for diagnosis, staging, treatment monitoring, and prognosis prediction in diverse cancers. In addition, it was suggested to use FDG PET for evaluation of tumor burden or therapeutic response monitoring [8, 9]. However, scarce reports exist with regard to the prognosis prediction of RCC with FDG PET/CT. In a recent preliminary study, the maximal standardized uptake value (SUVmax) on FDG PET was a significant prognostic factor for prediction of RCC patients’ survival [10], which was the first report on the impact of FDG PET for prognosis prediction in advanced RCC. Total lesion glycolysis (TLG) is another PET parameter for metabolic tumor burden, which reflects both the metabolic activity and tumor volume [8]. TLG has been utilized in several cancers as an effective marker of metabolic tumor burden.
In the present study, we evaluated extrarenal metabolic tumor burden of advanced RCC using FDG PET/CT, with regard to prediction of patients’ survival who was treated with targeted therapy after nephrectomy.
Materials and methods
Patients and follow-up
Forty-four patients who underwent FDG PET/CT for RCC were retrospectively enrolled in this study from our PET/CT database between September 2006 and October 2012. The inclusion criteria were as follows: (1) pathologically confirmed advanced RCC with stage IV (according to American Joint Committee on Cancer) which was treated with targeted therapy agents; (2) absence of other combined primary malignancy or previous systemic anti-cancer therapy; (3) PET/CT for initial staging of newly diagnosed RCC or restaging of recurred RCC after initial radical nephrectomy; (4) nephrectomy for primary renal lesion and presence of at least one measurable (≥1 cm) extrarenal metastatic lesion on chest and abdominopelvic contrast-enhanced CT studies. Survival time was defined as the time from PET/CT to the date of death or last follow-up. The date of death or last follow-up was surveyed by medical record review or telephone interview in case medical record was insufficient for determination of survival. The study design and exemption of informed consent were approved by the Institutional Review Board of our institution.
FDG PET/CT and image analysis
PET/CT images were acquired before start of targeted therapy. For FDG PET/CT, patients fasted for at least 6 h and blood glucose levels were confirmed <140 mg/dL. Patients were injected with FDG (5.18 MBq/kg) and images were acquired 1 h later. A CT scan was obtained first and an emission scan was consecutively obtained from skull base to proximal thigh, using PET/CT scanners (Gemini, Philips, or Biograph 40, Siemens). PET images were reconstructed using iterative algorithms. All images were displayed and analyzed using single analysis software (syngo.via, Siemens). Images were reviewed by two experienced nuclear medicine physicians, and all extrarenal metastatic lesions with visually discernible hypermetabolism were selected for analysis with assistance of CT images. Hypermetabolic focus that was regarded inflammatory lesion or physiologic activity was excluded from the analysis.
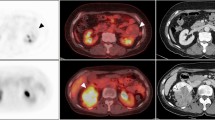
For each metastatic lesion, SUVmax and TLG values were measured. SUV was calculated in a pixel as (radioactivity)/(injected dose/body weight). TLG of a lesion was calculated as (mean SUV) × (metabolic tumor volume; MTV), in which MTV was measured with setting a margin threshold as 50 % of SUVmax. All the values of SUVmax, MTV, and mean SUV were automatically measured by the analysis software for each lesion. A patient’s SUVmax was defined as the highest SUVmax among those of all lesions, and TLG was defined as the sum of those of all lesions. An example case is shown in Fig. 1.
An example of PET image analysis with maximal intensity projection (a), PET axial (b), and fusion axial (c) images. This patient’s SUVmax was that of the subcarinal lymph node (11.76). By selecting a lesion, a volume margin was automatically drawn to measure MTV and TLG (b and c). The MTV and TLG of the subcarinal lymph nodes were 2.84 cm3 and 21.99, respectively. The sum of TLGs of the all lesions was 52.13 in this patient
Survival analysis and statistics
A patient’s survival time was determined from the date of PET/CT scan to the date of death or the last follow-up. Optimal cutoff of SUVmax and TLG for survival analysis was derived from receiver-operating characteristic (ROC) curve analysis. The survival curves were evaluated by Kaplan–Meier methods according to clinical and metabolic parameters; serum hemoglobin, serum calcium corrected as previously reported [11], number of metastatic sites, SUVmax, and TLG. Hazard ratios were calculated and tested by log-rank tests. Multivariate analyses were performed using Cox proportional hazard regression models in stepwise manner, for independent significant factors. Data were demonstrated as mean ± SD, and P values less than 0.05 were regarded significant. All statistical analyses were performed using a software package (MedCalc ver. 9.5, MedCalc Software, Belgium).
Results
Clinical features and outcomes
Forty-four patients (M:F = 32:12) were included in the analysis; the age was 62.0 ± 13.0 (range 28–89) years. In 21 patients, FDG PET/CT was performed for initial staging; before nephrectomy in 8 patients, and after nephrectomy in 13 patients. The time interval between nephrectomy and PET/CT was 11.0 ± 27.5 (range 5–65) days. In the other 23 patients, PET/CT was performed for restaging of recurred disease. As targeted therapy agents, sunitinib was used in 29 patients, sorafenib in 5, everolimus in 5, temsirolimus in 4, and pazopanib in 1. There was no severe adverse effect of targeted therapy such as hepatic failure, heart failure, high blood pressure, serious bleeding, osteonecrosis and tumor lysis syndrome. The characteristics of the 44 patients are summarized in Table 1. Three patients received local treatment (cyber-knife for the cervical spine metastases in 2, and liver segmentectomy in 1).
During follow-up period of 21.9 ± 17.7 (range 2.9–78.7) months, 8 (18.2 %) patients died from RCC.
Prognostic factors in survival analysis
A total of 250 metastatic lesions were discernible and included in the analysis (5.7 ± 3.9, range 1–19). Eighteen patients had 1 site of organ metastasis and 26 patients had ≥2 sites of organ metastasis. Most common metastatic site was the lung, which had metastases in 26 patients. Other metastatic sites included the liver, spleen, bone, muscle, pancreas, adrenal gland, lymph node, mesentery and peritoneum. There was no brain metastasis in the study population.
On patient basis, overall SUVmax was 9.4 ± 6.9 (range 1.4–29.4) and overall TLG was 261.6 ± 585.7 (range 1.3–3189.2). Serum hemoglobin level at the time of PET imaging was 13.1 ± 1.7 g/dL for male and 11.6 ± 1.3 g/dL for female. Corrected serum calcium level was 8.9 ± 0.6 mg/dL. Cutoff values were used as; 13.0 and 11.5 g/dL for male and female serum hemoglobin, respectively, and 9.0 mg/dL for corrected serum calcium according to previous studies [12, 13], 2 or more sites of organ metastasis [14], 7.0 for SUVmax, and 160 for TLG according to the results of ROC curve analysis.
On univariate survival analyses, serum hemoglobin, corrected calcium, number of metastatic sites, SUVmax and TLG were selected as significant prognostic factors for survival (Table 2). Survival curves for these parameters are shown in Fig. 2. When the significant parameters of serum hemoglobin, corrected calcium, number of metastatic sites, SUVmax, and TLG were tested on a multivariate analysis, TLG remained as the only independent significant factor with eliminating other parameters (P = 0.038, regression coefficient; 2.984). Median survival of high TLG group was 45.4 months, while it was not available to estimate that of low TLG group because the cumulative survival did not fall below 50 %.
Subgroup analyses
A subgroup survival analysis was performed in 21 patients in whom PET was performed for initial staging. In this subgroup, 4 patients died from the progress of RCC. In Kaplan–Meier survival analysis, only TLG (P = 0.008) was a significant predictor, whereas SUVmax (P = 0.13), number of metastatic sites (P = 0.19), serum hemoglobin (P = 0.72), and corrected calcium (P = 0.82) were not.
In the subgroup of 23 patients in whom PET was performed for restaging, 4 patients died from progress of RCC. In Kaplan–Meier survival analysis, TLG (P = 0.0009), SUVmax (P = 0.02), number of metastatic sites (P = 0.04), serum hemoglobin (P = 0.01), and corrected calcium (P = 0.0006) were significant prognostic factors. However, TLG remained as the only independent significant factor on multivariate analysis (P = 0.04).
Another subgroup analysis was performed in the patients who were treated with sunitinib only, which is the most commonly used targeted therapy agent for RCC. Twenty-nine patients were included in this subgroup, of which 6 died. In Kaplan–Meier analysis, SUVmax (P = 0.04), and TLG (P = 0.004) were selected as significant prognostic factors, whereas serum hemoglobin (P = 0.43), corrected calcium (P = 0.12) and number of metastatic sites (P = 0.11) were not, in spite of some borderline significance. In addition, only TLG remained an independent prognostic factor with eliminating other factors, on a multivariate analysis (P = 0.02, regression coefficient; 2.523). Survival curves according to serum hemoglobin, SUVmax and TLG in this subgroup are demonstrated in Fig. 3.
Survival curves in a subgroup analysis of patients treated with sunitinib, according to serum hemoglobin (a), corrected calcium (b), number of metastatic sites (c), SUVmax (d), and TLG (e). SUVmax and TLG were significant prognostic factor while other parameters were not probably due to small sample size (P values were obtained from Kaplan–Meier analyses). Hb serum hemoglobin
Discussion
The present study demonstrated that extrarenal metabolic tumor burden has significant prognostic implication in predicting survival of advanced RCC patients who were treated with targeted therapy agents after nephrectomy.
In advanced RCC that is usually resistant to conventional chemotherapy [2], nephrectomy or metastasectomy was performed only for palliation because it was regarded to have minimal effect on the survival of patients [15]. With the emergence of immunotherapy, it was reported that nephrectomy reduces cancer-specific death and delays disease progression [16, 17]. Recently, molecular targeted therapy has improved prognosis of patients, and the effect of nephrectomy was reevaluated. In a recent study, overall survival was longer in combined cytoreductive surgery and targeted therapy group than targeted therapy-only group [5]. Nephrectomy is commonly performed in advanced RCC patients currently, and all the enrolled patients underwent nephrectomy in the present study. Regarding metastasectomy, there are limited data on its prognostic role although recent studies demonstrated benefit of metastasectomy for survival in patients with advanced RCC [6, 7].
For optimal application of cytoreductive surgery, prognosis prediction is of paramount importance for selection of surgery candidates who can get benefit. Several prognosis prediction systems have been developed, and one of the most commonly used systems was suggested by the Memorial Sloan-Kettering Cancer Center group, including Karnofsky performance status (KPS), lactate dehydrogenase (LDH), serum hemoglobin, corrected serum calcium, and prior nephrectomy [12]. Among these 5 factors, 3 factors including KPS, serum hemoglobin, and corrected calcium were selected again as significant prognostic factors in previously treated RCC patients [13]. Another prediction system suggested by National Comprehensive Cancer Network (NCCN) Guideline included 6 factors; KPS, LDH, serum hemoglobin, corrected serum calcium, interval from original diagnosis to the start of systemic therapy, and number of metastatic sites [14]. The present study also demonstrated significant prognostic values of serum hemoglobin, corrected serum calcium and number of metastatic sites, although KPS was not evaluated due to lack of complete clinical data. The analyses in some subgroup demonstrated those factors were not significant, which might be caused by low statistical power of small case numbers and different cutoff values.
Metabolic characteristics of cancer cells are used for prognosis prediction in many cancers. SUVmax on FDG PET, which reflects most aggressive component of cancer cells, has prognostic values in various solid tumors such as hepatocellular carcinoma, osteosarcoma, and lymphoma [18–20]. Also for RCC, SUVmax was tested as a prognostic factor, in which patients with high SUVmax (≥8.8) had 64 % of death rate and median survival of 156 days, while patients with low SUVmax (<8.8) had only 13 % of death rate [10]. The present study also demonstrated that high SUVmax is significantly related with poor prognosis.
Tumor burden is another important prognostic factor in advanced RCC. Motzer et al. [21] included the number of metastatic sites in their nomogram for prognosis prediction of RCC patients who received sunitinib therapy. Furthermore, Barbastefano et al. [7] reported that the percentage of tumor burden reduction had significant correlation with progression-free survival of patients, in which the method of Response Evaluation Criteria in Solid Tumors (RECIST) [22] was used for evaluation of tumor burden. However, metabolic tumor burden that is measured as TLG on FDG PET/CT can provide comprehensive information on metabolic activity and tumor volume. TLG was suggested as a complementary parameter to RECIST system in evaluation of treatment response [9]. TLG has been used for treatment monitoring after chemotherapy in several cancers [23–25] including RCC [26]. Regarding prognosis prediction, TLG proved a significant prognostic factor in several cancers such as osteosarcoma, small cell lung cancer, and pleural mesothelioma [19, 27, 28]. Thus, we measured TLG for tumor burden of extrarenal metastatic lesions and tested its prognostic significance in advanced RCC.
In the present study, metabolic tumor burden was a significant prognostic factor comparable to conventional clinical factors. In patients with high metabolic tumor burden (TLG ≥ 160), median survival was shorter than patients with low metabolic tumor burden. However, it is still unclear which group can get benefit from cytoreductive surgery. In the present study, most of the patients received targeted therapy only without local therapy, except 3 patients who received palliative radiotherapy or surgery. Thus, it may be speculated that aggressive tumor burden reduction would be beneficial for the patients with high metabolic tumor burden if available, because they showed shorter survival despite use of targeted therapy. In addition, changes in metabolic tumor burden after targeted therapy would be effective for selection of the surgical candidates. Further studies are warranted whether and how metabolic tumor burden can be used for selection of appropriate candidates for cytoreductive surgery.
Regarding FDG PET in urological cancers, FDG activity in urine may be a limitation, because it may mask true lesions. However, most of primary renal lesions can be delineated with use of hybrid PET/CT images, and we evaluated only extrarenal lesions in the nephrectomized patients. Thus, FDG activity in urine was not a significant limitation in this study. Another limitation of the present study is small number and relative heterogeneity of patients. To reduce possible confounding from drugs, a subgroup analysis was performed in the patients who were treated with sunitinib, the most commonly used targeted therapy agent [29]. In this subgroup, similar result was obtained that TLG has a significant and independent prognostic value, while other factors were eliminated in a multivariate analysis. Likewise, both of initial staging and restaging subgroups also showed that TLG was a significant prognostic factor. However, low number of events per independent variable is still a limitation of this study. The results should be cautiously interpreted because the regression coefficients may be biased in case of low number of events. Thus, more patient number and prospective design are required in further studies to confirm the prognostic role of metabolic tumor burden.
In conclusion, extrarenal metabolic tumor burden is a significant prognostic factor in advanced RCC patients who were treated with targeted therapy. For selection of candidates for cytoreductive surgery, measurement of metabolic tumor burden would be effective. Further studies are warranted on whether and how metabolic tumor burden may be used for selection of appropriate candidates for cytoreductive surgery.
References
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36.
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32.
Pirrotta MT, Bernardeschi P, Fiorentini G. Targeted-therapy in advanced renal cell carcinoma. Curr Med Chem. 2011;18:1651–7.
Crispen PL, Blute ML. Role of cytoreductive nephrectomy in the era of targeted therapy for renal cell carcinoma. Curr Urol Rep. 2012;13:38–46.
Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–6.
Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011;117:2873–82.
Barbastefano J, Garcia JA, Elson P, Wood LS, Lane BR, Dreicer R, et al. Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU Int. 2010;106:1266–9.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Namura K, Minamimoto R, Yao M, Makiyama K, Murakami T, Sano F, et al. Impact of maximum standardized uptake value (SUVmax) evaluated by 18-Fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (18F-FDG-PET/CT) on survival for patients with advanced renal cell carcinoma: a preliminary report. BMC Cancer. 2010;10:667.
Orrell DH. Albumin as an aid to the interpretation of serum calcium. Clin Chim Acta. 1971;35:483–9.
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40.
Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer Version 1. 2013. http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed 15 May 2013.
Montie JE, Stewart BH, Straffon RA, Banowsky LH, Hewitt CB, Montague DK. The role of adjunctive nephrectomy in patients with metastatic renal cell carcinoma. J Urol. 1977;117:272–5.
Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–6.
Lara PN Jr, Tangen CM, Conlon SJ, Flanigan RC, Crawford ED. Predictors of survival of advanced renal cell carcinoma: long-term results from Southwest Oncology Group Trial S8949. J Urol. 2009;181:512–6.
Kim BK, Kang WJ, Kim JK, Seong J, Park JY, Kim DY, et al. (18) F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma. Cancer. 2011;117:4779–87.
Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–7.
Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol. 2011;93:502–8.
Motzer RJ, Bukowski RM, Figlin RA, Hutson TE, Michaelson MD, Kim ST, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–8.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–96.
Benz MR, Allen-Auerbach MS, Eilber FC, Chen HJ, Dry S, Phelps ME, et al. Combined assessment of metabolic and volumetric changes for assessment of tumor response in patients with soft-tissue sarcomas. J Nucl Med. 2008;49:1579–84.
Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–41.
[26] Gilles R, de Geus-Oei LF, Mulders PF, Oyen WJ. Immunotherapy response evaluation with (18)F-FDG-PET in patients with advanced stage renal cell carcinoma. World J Urol 2011.
Arslan N, Tuncel M, Kuzhan O, Alagoz E, Budakoglu B, Ozet A, et al. Evaluation of outcome prediction and disease extension by quantitative 2-deoxy-2-[18F] fluoro-D-glucose with positron emission tomography in patients with small cell lung cancer. Ann Nucl Med. 2011;25:406–13.
Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of [18]F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010;17:2787–94.
Powles T, Chowdhury S, Jones R, Mantle M, Nathan P, Bex A, et al. Sunitinib and other targeted therapies for renal cell carcinoma. Br J Cancer. 2011;104:741–5.
Acknowledgments
This study was supported by the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (A070001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yoon, HJ., Paeng, J.C., Kwak, C. et al. Prognostic implication of extrarenal metabolic tumor burden in advanced renal cell carcinoma treated with targeted therapy after nephrectomy. Ann Nucl Med 27, 748–755 (2013). https://doi.org/10.1007/s12149-013-0742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-013-0742-4