Abstract
Objective
The objective of this study is to determine whether 2-deoxy-2-[18F] fluoro-d-glucose with positron emission tomography (FDG-PET) imaging and quantitative PET parameters can predict outcome and differentiate patients with limited disease (LD) from extensive disease (ED) in patients with small cell lung cancer (SCLC).
Methods
We retrospectively evaluated data from 25 patients who underwent either initial staging (Group A, n 12) or restaging (Group B, n 13) by conventional imaging methods and FDG-PET according to the simplified staging scheme developed by the Veterans Administration Lung Cancer Study Group-2. FDG-PET images were both visually and quantitatively evaluated with SUVmax, SUVave, total metabolic tumor volume (with SUVmax > %50 and SUVmax > 2.5), total lesion glycolysis (TLG) (with SUVmax > %50 and SUVmax > 2.5). The correlation between quantitative PET parameters, disease stages and survival were analyzed.
Results
By conventional methods 14 of 25 (56%) patients were reported to have LD and 11 of 25 (44%) had ED. FDG-PET scan upstaged 9 out of 25 (36%) and downstaged 2 out of 25 (%8) patients. Among the quantitative PET parameters, TLGs were the only PET parameters that differentiated between Group A and Group B patients. FDG-PET staging (p = 0.019) could predict significant survival difference between stages on contrary to conventional staging (p = 0.055). Moreover, TLG [SUVmax > %50] was the only quantitative PET parameter that could predict survival (p = 0.027).
Conclusion
FDG-PET imaging is a valuable tool in the management of patients with SCLC for a more accurate staging. The use of quantitative PET parameters may have a role in prediction of stage and survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC) accounts for 16% of all lung cancers with an unfavorable prognosis, especially when the disease is extensive at presentation. SCLC may be lethal when untreated with a poor median survival of 2–4 months after diagnosis [1]. Most physicians use a simplified staging scheme developed by the Veterans Administration Lung Cancer Study Group-2 to classify the patients as having limited disease (LD) or extensive disease (ED). Limited-stage SCLC accounts for approximately 30% of patients at diagnosis, and includes those with tumor confined to the hemithorax of origin, the supraclavicular or the mediastinal lymph nodes. In extensive-stage SCLC, tumor has spread outside ipsilateral hemithorax with or without malign pleural/pericardial effusion or hematogenous metastases. While, contralateral mediastinal and ipsilateral supraclavicular lymph adenopathy are classified as limited stage disease, contralateral hilar and supraclavicular lymph adenopathy are classified as ED [2]. Although, chemotherapy and radiotherapy are mainly reserved for symptomatic sites in ED, the combination of chemotherapy and radiotherapy is used with a curative intent in the LD [3]. Therefore, it is crucial to differentiate LD from ED for appropriate therapy planning. In previous oncology practice, computerized tomography (CT), ultrasonography (US), whole body bone scintigraphy (WBBS) and magnetic resonance imaging (MRI) were the main diagnostic tools for staging. However, none of these morphological imaging techniques can reliably differentiate LD from ED.
The glucose analog 2-deoxy-2-[18F] fluoro-d-glucose (FDG) preferentially accumulates in most malignant tumors, reflecting increased glycolytic rate in these tumors [4]. With the increased availability, positron emission tomography (PET) or PET–CT with the use of 18F-FDG is now the modality of choice of staging of SCLC which was suggested by NCCN guidelines [5, 6]. Although, the value of FDG-PET is widely accepted for SCLC, the impact of quantitative FDG-PET parameters for either initial therapy planning or restaging needs to be confirmed by new studies.
The objective of this study is to determine whether quantitative evaluation of FDG-PET images can predict outcome and differentiate patients with LD from ED in patients with SCLC undergoing initial staging or restaging.
Materials and methods
Patients
This is a retrospective study using documents and data which belongs to patients referred to FDG-PET for initial staging or restaging. We evaluated data from 25 patients (male/female: 24/1, mean age: 61 ± 6 years; age range 50–71 years) with biopsy-proven SCLC who were diagnosed between October 2002 and June 2009. All patients underwent WBBS, bronchoscopy, brain MRI, abdominal US, CT, and FDG-PET to determine disease stage. Of the 25 patients, 12 had FDG-PET for initial staging and included in Group A. The remaining 13 patients had FDG-PET for restaging and included in Group B. The mean follow-up time for the patients in Group A and B was 11.7 ± 10.5 and 10.7 ± 8.3 months, respectively.
PET imaging protocol
FDG-PET imaging studies were performed 60 min after intravenous injection of 370–555 MBq (10–15 mCi) of F-18 FDG with an ECAT EXACT scanner (Siemens-CTI, Knoxville, TN). A series of three to five overlapping 47-slice emission and transmission images were obtained to include the region from the neck to the upper thighs using a 68Ge/68Ga rod source for the transmission measurement. Images were reconstructed using an ordered-subset expectation–maximization iterative algorithm. Emission images were corrected for measured attenuation using a segmented attenuation-correction algorithm. Images were displayed as whole-body reprojection images and as slices in three orthogonal planes.
FDG-PET analysis
Visual assessment
All PET images were evaluated qualitatively by two experienced nuclear medicine specialists in routine clinical fashion including correlation with other imaging studies, principally CT. Foci of FDG uptake were identified as representing tumor if the accumulation of FDG was moderately to markedly increased relative to comparable normal contralateral or surrounding soft tissues, mainly exceeding blood pool activity in mediastinum and liver activity in abdomen. All images were evaluated for primary tumor as well as metastatic regional lymph nodes, bone and other distant metastases.
Quantitative assessment
All of the PET studies were quantitatively analyzed using similar methods. We used absolute and relative threshold method for contouring each tumor site having visually increased FDG uptake. For absolute threshold method, we created a 3D contour within a spherical region by manually drawing ROI around voxels that are equal or greater than absolute value of 2.5 standardized uptake values (SUV) on contiguous axial slices. For relative threshold method, we created a 3D contour around voxels that are equal to or greater than 50% of the maximum voxel value inside a spherical region [7, 8]. Tumor volumes obtained with absolute and relative threshold method were used for total lesion glycolysis (TLG) and total metabolic tumor volume (TMTV) computation.
-
1.
TMTV: The total volume of the all visually positive FDG uptake sites for malignant tissue was computed from the contoured and threshold region by counting the number of voxels in the three-dimensional region and automatically multiplying by the known volume of a voxel (0.43 × 0.43 × 0.43 cm3) [7].
-
2.
SUVmax: The SUVmax was computed by standard methods from the activity in the most intense voxel in the three-dimensional tumor region from the transaxial whole body images.
-
3.
SUVave: The SUVave was determined from the average voxel counts within the tumor region.
-
4.
TLG: TLG represents the absolute glycolysis of tumor tissue and depends on both tumor volume and FDG uptake expressed as SUVave [9].
Comparison between PET and conventional imaging methods
Patients were staged according to the simplified staging scheme developed by the Veterans Administration Lung Cancer Study Group-2. The staging was done separately by conventional imaging methods (WBBS, computed tomography, brain MRI) and FDG-PET. All primary and recurrent lesions had biopsy confirmation. The suspicious tumor sites were not biopsied but accepted positive for metastatic disease if (1) showed progression on follow-up, or (2) meet the metastatic criteria by both metabolic and anatomic imaging techniques (e.g.,: lytic destructive bone lesion with high FDG uptake or lymph node >1 cm with FDG uptake above the activity of liver or mediastinal blood pool). Those tumor sites were accepted as negative if (1) a site with a negative biopsy, or (2) no progression detected by appropriate evaluation for at least 6 months in the absence of further treatment, or (3) FDG uptake lower than background and negative conventional imaging.
The differences between quantitative PET parameters (SUVmax, SUVave, TMTV and TLG) in different disease stages (as determined by FDG-PET imaging vs. conventional methods) for the patients in Group A and B were also compared.
Statistical analysis
The Mann–Whitney U test was used to test for differences between the quantitative PET data and disease stage (limited vs. extensive) besides patient groups (Group A vs. B). A statistically significant difference was defined as a p value < 0.05 (two-tailed). The correlations between quantitative PET parameters and disease stage were calculated with spearman correlation coefficient (SCC). The correlation coefficient values were evaluated as; 0–0.25 none or very weak correlation, 0.25–0.5 weak to moderate correlation, 0.50–0.75 good correlation, 0.75–1 very good correlation. Kaplan–Meier and Cox regression analysis tests were used to test the hypothesis that whether quantifiable PET data of the patients with small lung cancer can predict patient survival. All quantitative values are given as mean ± standard deviation (SD).
Results
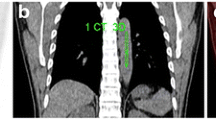
By conventional methods 14 of 25 (56%) patients were reported to have LD and 11 of 25 (44%) had ED according to the staging scheme developed by the Veterans Administration Lung Cancer Study Group-2. After FDG PET scan; 7 of 25 (28%) patients were reported to have LD and 18 of 25 (72%) had ED. FDG-PET scan upstaged 9 out of 25 (36%) (Fig. 1) and downstaged 2 out of 25 (%8) patients. Of the 9 upstaged patients; 5 were referred for primary staging, 2 were referred for therapy response evaluation and 2 were referred for restaging. Of the 2 downstaged patients; one was referred for therapy response, and one was referred for primary staging (Table 1).
All SCLC cancers showed moderate to the marked uptake of FDG in the primary and metastatic tumor sites on the FDG-PET scan. Among the PET indices, only SUVmax and SUVave were correlated with each other (p = 0.001). Mean and standard deviations of all quantitative FDG-PET data indices and the correlation between Group A and Group B patients are given in Table 2. Among the quantitative PET parameters, TLG [SUVmax > %50] (p = 0.03) and TLG [SUVmax > 2.5] (p = 0.03) were the only parameters that differentiated between Group A and Group B patients. No statistically significant difference was found between the SUVmax, SUVave, TMTV (cm3) [SUVmax > %50] and TMTV (cm3) [SUVmax > 2.5] for the patients in Group A and B (p > 0.05) (Table 2).
Although, SUVmax and SUVave values were not statistically different in patients with LD and ED, patients who had ED tend to have higher TMTV and TLG values than those with LD as determined by two methods (Table 3).
On the other hand, while TLG [SUVmax > %50] and TLG [SUVmax > 2.5] (SCC:0.915) showed very good correlation with each other, the correlation between TMTV (cm3) [SUVmax > %50] and TMTV (cm3) [SUVmax > 2.5] showed slight decrease, but still very good (SCC = 0.787) (Table 4). In comparing FDG-PET based quantitative indices and conventional imaging methods for determining disease stage, there was a weak- to- moderate correlation (SCC = 0.408) (Table 4).
The follow up data were available for 20 of 25 patients. Of these 20 patients, 12 (Group A/B: 6/6) were dead during the follow-up. The mean follow-up time was 11.8 ± 9.3 months. Of the 20 patients, 2 patients (Group A) were in remission and 6 patients (Group A/B: 2/4) were alive with disease.
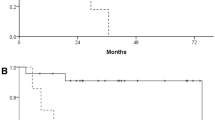
Kaplan–Meier analysis showed that only FDG-PET staging (p = 0.019) could predict significant survival difference between stages on contrary to conventional staging (p = 0.055) (Fig. 2). Cox regression analysis showed that only TLG [SUVmax > %50] could predict survival with a statistically significant value (p = 0.027) while SUVmax (p = 0.3); SUVave (p = 0.29), TMTV [SUVmax > %50] (p = 0.23), TMTV [SUVmax > 2.5] (p = 0.25) and TLG [SUVmax > 2.5] (p = 0.06) were not able to predict survival.
When all the parameters (patient groups (A and B), TLG [SUVmax > %50], TLG [SUVmax > 2.5], SUVmax, SUVave, TMTV [SUVmax > %50], TMTV [SUVmax > 2.5]) were included in multivariate Cox regression analysis, TLG [SUVmax > %50] was still the only significant parameter that could predict survival (p = 0.046). Neither Group A nor Group B was found as a prognostic factor.
Discussion
SCLC is one of the most lethal cancers with a rapid progressive course [3]. Accurate staging and therapy response evaluation are crucial for the management of patients and further drug research. Due to the changes in patients’ stage, currently PET–CT has started to become a routine imaging modality for staging and therapy response evaluation [5]. In our patient group PET-staging changed the stage of 11 of 25 (44%) patients. Of the 25 patients, 9 (36%) was upstaged and 2 (8%) was downstaged (Table 1). The change in the stage was also reported by several groups, mostly increase in stage. In a primary staging setting, Brink et al. [10] reported a change in the stage of 14 of 120 (12%) patients (10 upstaged and 4 downstaged). In a group of SCLC patients with various indications (37% staging and 63% restaging), Blum et al. [11] had reported that 5 of 15 (33%) patients who had PET for staging were upstaged from LD to ED and treated without thoracic radiotherapy. In a recent study, Azad et al. [12] reported alteration in stage classification in 12 of 46 (26%) patients with FDG-PET. The results in our study showed higher change in the stage of the patients. The variation between the results of these studies is mainly due to the differences in patient population, variation in indications and describing disease stage (LD vs. ED). For example, on contrary to us, Azad et al. [12] defined the LD as disease confined to the ipsilateral hemithorax that can be encompassed by a single radiotherapy port, and ED as disease beyond the ipsilateral hemithorax.
Another issue that has to be pointed out for staging is the frequency of brain metastases in different study groups. Because of high physiological FDG uptake, brain metastases can be easily missed by FDG-PET imaging which had significant impact on patients’ mortality. Of the 25 patients in our study group, 3 had brain metastases, one of whom was missed by FDG-PET imaging (Table 1). Although, as in this case, patients with brain metastases usually have additional distant metastasis with no change in disease stage, we all agree with the well-accepted suggestion that FDG-PET must be completed by cranial MRI [6].
Although SUVmax values in ED were higher than they were in LD, they did not show a statistically significant difference between ED and LD (Table 2). In addition, SUVmax, SUVave, absolute and thresholded TMTVs were higher at initial presentation than restaging and there was no statistical significant difference between disease stages. There are reports in the literature showing that higher SUV values had increased the risk of nodal metastases and higher stage in non-SCLC. However, there are no reports with SCLC [13].
Visual interpretation of the FDG-PET images is primarily based on differences in contrast between normal and tumor tissue. The most commonly used semi-quantitative parameter to assess tumor FDG uptake is the standardized uptake value (SUV) defined as the tissue concentration of FDG, as measured by PET, divided by the injected dose per kilogram of body weight as a normalization factor. SUV can be reported as either the average measured value (SUVave) within a ROI or as the maximal pixel value (SUVmax) within the ROI. TLG is another quantitative index used to assess FDG uptake which depends on both tracer uptake and tumor volume. TLG has been introduced as a potentially more reliable method which is less influenced by partial volume effects related to camera performance and motion-induced blurring, overestimation of tumor volume and underestimation of SUV [14]. However, it is not clear whether these quantitative PET methods have similar sensitivity, and there is not a consensus concerning which method (absolute or threshold) should be used to define tumor borders for lung cancers.
TLG has gained popularity in last decade and used in different tumor groups. Lee et al. [15] reported the performance of TLG in malignant pleural mesothelioma. The author stated that according to multivariate analysis adjusted for treatment modality TLG was found as an independent factor associated with tumor progression (HR 1.001, p = 0.031). Time to tumor progression was shorter in patients with a high volume-based parameter of PET than in those with a low value. As taking into account not only the metabolic uptake but also the extend volume of the disease, TLG provides more detailed status of the disease. Our study was the only study that used TLG as quantitative parameter in patients with SCLC and the quantitative technique employed here differs from other published methods by use of the three-dimensional image data rather than one or several two-dimensional slices in obtaining quantitative PET data. This approach, in conjunction with the technique based on the maximum or thresholded FDG activity in the tumor region, permitted calculation of the volume of the primary tumor, SUVave and TLG of the tumor sites [7, 9]. In this study, despite the SUVmax, SUVave and tumor volumes, both absolute and thresholded TLG showed statistically significant difference among patients referred for initial staging. Moreover, on contrary to SUVmax and SUVave, both TMTV and TLG indices were higher in patients with ED than LD. However, in a recent study by Costelloe et al. [16] showed the prognostic value of not only TLG but also SUVmax. This may be due to tumor heterogenity, differences in staging description and therapy protocols.
TLG and tumor volumes were also used for therapy response evaluation. Roedl et al. [17] found that the decrease of tumor volume between the pre- and post-treatment PET–CT scans was a better predictor of histopathologic response and survival than the decrease of the SUV and of the clinical response evaluation based on RECIST and WHO criteria in patients with adenocarcinoma of esophagus. On the other hand, Arslan et al. [7] reported that change in three-dimensionally determined metabolically active tumor volumes may enable the assessment of response to neoadjuvant therapy in patients with esophageal cancer. On the contrary, TLG was found less accurate in predicting tumor response than were measurements of the intratumoral (18)F-FDG concentration (SUVmax, SUVave) in patients with sarcoma [18].
In routine clinical practice, different methods are used to calculate the TLG values such as SUVmax > %50 and SUVmax > 2.5 [16–19]. According to results of this study, TLG values determined by absolute (SUVmax > 2.5) and thresholded (SUVmax > 50%) methods could be used interchangeably with no statistical significant difference with a very good correlation (SCC:0.915) (Tables 2, 3, 4).
Kaplan–Meier and cox regression analysis showed that only FDG-PET staging (p = 0.019) could predict significant survival difference between stages on contrary to conventional staging (p = 0.055) (Fig. 2). This finding shows the superiority of FDG-PET imaging not only in the staging but also in the prognostication. TLG [SUVmax > %50] was the only quantitative PET index that could predict survival with a statistically significant value (p = 0.027). Costelloe et al. [16] found that in addition to increase in TLG, high SUVmax after chemotherapy was associated with poor overall survival (p = 0.035). However, we could not predict the survival in our patient group with SUVmax (p = 0.3) as well as with other quantitative PET parameters.
There are limitations of this work based on study design and computational factors. For example, the treatment selection may be a prognostic factor after initial staging or restaging and have an effect on the results in a retrospective study like this. Besides that, a large number of quantitative parameters are compared between Groups A and B (Table 2) and between patients with LD and ED (Table 3) in this study. The use of multiple measurement parameters in such a small number of patients perhaps over-complicates the study and over-analyses the available data. Thus, an important limitation of this study that no correction for multiple comparisons was applied. These results should be considered preliminary and exploratory in nature in this study, and statistically significant differences should be viewed as a preliminary result requiring confirmation in a prospective study involving a larger group of patients and different patient populations.
In conclusion, FDG-PET imaging is a valuable tool in the management of patients with SCLC for a more accurate staging and can successfully differentiate patients with limited from extensive disease. In addition, FDG-PET staging with quantitative PET parameters; especially TLG [SUVmax > %50] may have a role in the prediction of outcome and survival. Further prospective studies are needed in a larger number of patients where all calculated PET parameters could be compared with disease stage, clinical outcome and disease-free survival rates.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;3:271–6.
Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;9494:1385–96.
Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996;12:1641–74.
Johnson BE, Crawford J, Downey RJ, Ettinger DS, Fossella F, Grecula JC, et al. Small cell lung cancer clinical practice guidelines in oncology. National Comprehensive Cancer Network (NCCN). J Natl Compr Canc Netw. 2006;6:602–22.
Podoloff DA, Ball DW, Ben-Josef E, Benson AB 3rd, Cohen SJ, Coleman RE, et al. NCCN task force: clinical utility of PET in a variety of tumor types. J Natl Compr Canc Netw. 2009;7(Supp 2):S1–26.
Arslan N, Miller TR, Dehdashti F, Battafarano RJ, Siegel BA. Evaluation of response to neoadjuvant therapy by quantitative 2-deoxy-2-[18F] fluoro-d-glucose with positron emission tomography in patients with esophageal cancer. Mol Imaging Biol. 2002;4:301–10.
Lin LL, Yang Z, Mutic S, Miller TR, Grigsby PW. FDG-PET imaging for the assessment of physiologic volume response during radiotherapy in cervix cancer. Int J Radiat Oncol Biol Phys. 2006;1:177–81.
Hoekstra CJ, Paglianiti I, Hoekstra OS, Smit EF, Postmus PE, Teule GJ, et al. Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-d-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med. 2000;6:731–43.
Brink I, Schumacher T, Mix M, Ruhland S, Stoelben E, Digel W, et al. Impact of [18F]FDG-PET on the primary staging of small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2004;12:1614–20.
Blum R, MacManus MP, Rischin D, Michael M, Ball D, Hicks RJ. Impact of positron emission tomography on the management of patients with small-cell lung cancer: preliminary experience. Am J Clin Oncol. 2004;2:164–71.
Azad A, Chionh F, Scott AM, Lee ST, Berlangieri SU, White S, et al. High impact of (18)F-FDG-PET on management and prognostic stratification of newly diagnosed small cell lung cancer. Mol Imaging Biol. 2010;4:443–51.
Park HK, Jeon K, Koh WJ, Suh GY, Kim H, Kwon OJ, et al. Occult nodal metastasis in patients with non-small cell lung cancer at clinical stage IA by PET/CT. Respirology. 2010;8:1179–84.
Akhurst T, Ng VV, Larson SM, O’Donoghue JA, O’Neel J, Erdi Y, et al. Tumor burden assessment with positron emission tomography. Clin Positron Imaging. 2000;2:57–65.
Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of (18) F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010;10:2787–94.
Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;3:340–7.
Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET–CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008;3:278–86.
Benz MR, Allen-Auerbach MS, Eilber FC, Chen HJ, Dry S, Phelps ME, et al. Combined assessment of metabolic and volumetric changes for assessment of tumor response in patients with soft-tissue sarcomas. J Nucl Med. 2008;10:1579–84.
Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;2:328–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arslan, N., Tuncel, M., Kuzhan, O. et al. Evaluation of outcome prediction and disease extension by quantitative 2-deoxy-2-[18F] fluoro-d-glucose with positron emission tomography in patients with small cell lung cancer. Ann Nucl Med 25, 406–413 (2011). https://doi.org/10.1007/s12149-011-0478-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-011-0478-y