Abstract
In clinical N0 early oral tongue carcinoma, treatment of occult lymph node metastasis is controversial. The purpose of this study was to assess the histopathological risk factors for predicting late lymph node metastasis in early oral tongue carcinoma. We retrospectively reviewed 48 patients with early oral tongue squamous cell carcinoma. Associations between the histopathological factors (depth of tumor, differentiation, blood vessel invasion, lymphatic invasion, and tumor budding) and late lymph metastasis were analyzed. Although the univariate analysis identified blood vessel invasion, lymphatic invasion, and high-grade tumor budding as predictive factors for neck recurrence (p < 0.001), the Cox proportional hazards model identified high-grade tumor budding as an independent predictive factor (p < 0.01). The combination of a tumor depth ≥ 3 mm and high-grade tumor budding yielded high diagnostic accuracy. Tumor depth and budding grade were identified as histopathological risk factors for late neck recurrence in clinical N0 early oral tongue carcinoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Late lymph node metastasis and local recurrence are the major cause of decreased survival in patients with clinical N0 early oral tongue carcinoma. Occult lymph node metastases are observed in 20–40% of cases and their successful control contributes to improved survival in patients with this disease [1–6]. The wait-and-see policy, sentinel node biopsy (SNB), or elective neck dissection (END) are selected for the management of clinical N0, however, the best option has not been determined. Recently, D’Cruz et al. gave priority to END and many authors have advocated the effectiveness of SNB [1, 3, 5, 7–9]. For more than half of the patients with pathological N0 disease, END is overtreatment and can cause unnecessary complications. On the other hand, some are of the opinion that END should only be recommended for patients with risk factors for late lymph node metastasis.
Although tumor depth, blood vessel invasion, and lymphatic invasion have been claimed to be histopathological risk factors, only tumor depth is used to guide decision making [6, 10] and it is still difficult to precisely identify high risk patients.
We speculated that some microscopically observable histopathological features could be related to the poor prognosis of early oral tongue carcinoma. Tumor budding is defined as the invasion of a single tumor cell or small clusters of tumor cells (<5 cells) in the tumor invasive front, and is indicative of one of the objective patterns of tumor invasion. A recent report claimed that tumor budding was a prognostic factor or risk factor for lymph node metastasis for colorectal cancer and other cancer types [11]. The budding number was counted at the highest view at 20 × 10 magnification and taken as the budding score. In Japanese guidelines of colorectal cancer, the budding score was classified into two groups, low (4 or less) or high grade (5 or more). Having high grade was known to be an important risk factor for lymph node metastasis in early colorectal cancer [12]. And budding score ≥ 5 was regarded as a cutoff point for prognostic factor in head and neck squamous cell carcinoma (SCC) [13–16]. Almangush et al. reported the combination of tumor budding (B) and a tumor depth (D) as a BD model for the prognostication of early oral tongue carcinoma [17]. The correlation between tumor budding and epithelial-mesenchymal transition has also been suggested [14, 18, 19]. As tumor budding was thought to be a microscopic indicator of tumor invasiveness, we focused on this feature for predicting late lymph node metastasis in this study.
In our institution, END is avoided for patients with early oral tongue carcinoma depending on the progress of diagnostic imaging examination and ultrasound imaging, in particular [20–22]. In this retrospective study, we analyzed the association between late lymph metastasis and histopathological factors including tumor budding, as well as the classical risk factors in patients with clinical N0 early oral tongue carcinoma treated by primary surgery without END.
Materials and Methods
A total of 154 patients were diagnosed with clinical early oral tongue SCC between September 2000 and March 2015 in the Kanagawa Cancer Center. Patients who were diagnosed as carcinoma in situ, recurrence of SCC, and who had received high dose rate brachytherapy or preoperative chemotherapy were excluded from the study. We retrospectively reviewed 48 patients with clinical early oral tongue SCC (cT1/2N0M0) who underwent primary partial glossectomy.
Examinations by computed tomography (CT), magnetic resonance imaging, ultrasonography, and positron emission tomography/CT (since 2004) were performed prior to surgery. All patients underwent excision of the primary tumor with adequate margins (≥10 mm). END and SLB were avoided. Patients were followed once every month for the first year, every 2 months for the second year, and every 3 months thereafter. Examination by ultrasonography or CT was performed once every 1–3 months for the first 2 years. For neck recurrence, minimum-area ND was performed and postoperative radiotherapy (RT) or concurrent chemoradiotherapy (CRT) were recommended for cases at high risk of extracapsular invasion, pN2b, and level IV or V. Overall survival (OS), local control (LC), and regional control (RC) were compared between the stages.
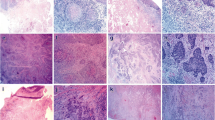
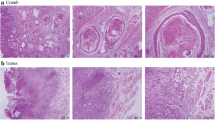
Histopathological risk factors of neck recurrence were analyzed using pathology specimens obtained at the time of the primary surgery. Histopathological review was performed by the author and an experienced pathologist blinded to the patients’ course (Y.H. and T.Y.). Tumor depth, differentiation, blood vessel invasion, lymphatic invasion, and tumor budding were analyzed. The resected tissues were fixed with 10% buffered formalin and embedded in paraffin. They were stained with hematoxylin and eosin (HE) and Elastica van Gieson (EvG) for elastic fibers (Fig. 1). Immunohistochemistry was performed on the formalin-fixed, paraffin-embedded tissues. Tissues were incubated with a prediluted antibody, D2-40 (Roche, Basel, Switzerland,) recognizing podoplanin on lymphatic endothelia (Fig. 1). BenchMark Ultra (Ventana Medical Systems, Tucson, USA) was used for D2-40 staining. Tumor depth was measured from the surface of the tumor to the deepest point of the invasive tumor in HE by automatic analyzer software, NIS Elements (Nikon, Tokyo, Japan) (Fig. 2). Differentiation was classified according to the WHO grade (Fig. 1). Blood vessel invasion was analyzed in EvG. Lymphatic invasion was immunohistochemically analyzed using D2-40 staining. Tumor budding is defined as the invasion of single tumor cell or small clusters of tumor cells (<5 cells) in the tumor invasive front (Fig. 3). First, the highest area of the budding number was found at 4 × 10 magnification, and then the budding number was counted at the highest view at 20 × 10 magnification. This number was taken as the budding score. The budding score was classified as low (4 or less) or high grade (5 or more) (Fig. 4).
Histopathological reviews with HE and Elastica van Gieson (EvG) staining and immunostaining for D2-40. A Blood vessel invasion was analyzed in EvG for staining elastic fibers. B Lymphatic invasion was immunohistochemically analyzed using D2-40 staining recognizing podoplanin on lymphatic endothelia. C Well differentiated squamous cell carcinoma (SCC) was analyzed with HE staining. D Moderately differentiated SCC was analyzed with HE staining
HE staining demonstrating tumor budding in the tumor invasive front. A Tumor budding is enclosed by the square (at 4 × 10 magnification). B Magnified view of the area enclosed in the square in A (at 20 × 10 magnification). The superior cluster consisting of 4 tumor cells was counted as budding (a) and the inferior cluster consisting of 5 tumor cells was not counted as budding (b), as described in the Materials and Methods.
OS, LC, and RC were calculated using the Kaplan–Meier method. Risk factors of neck recurrence were analyzed by univariate analysis using differentiation, blood vessel invasion, lymphatic invasion, and budding grade. The Cox proportional hazards model was used to test the risk factors detected by univariate analysis. p-values < 0.05 were considered to be significant. Correlations for each factor were analyzed using the Spearman’s rank correlation coefficient. All statistical analysis was performed using IBM SPSS version 23.
Results
Thirty-three (69%) of the 48 study patients were male and 15 were female, with a median age of 61 years (range 34–87 years). The median follow-up was 71 months (range 7–179 months). Thirty-nine (81%) and 9 (19%) of patients were classified with stage I and II disease, respectively. The 5-year OS rate was 93% (Stage I/II 91%/100%), the 5-year LC rate was 87% (Stage I/II 84%/100%) and the 5-year RC was 80% (Stage I/II 78%/89%). There were no significant differences of OS, LC, and RC between Stage I and II. No distant metastasis was found in any of the patients (Fig. 5).
Kaplan–Meier curves of A overall survival (OS), B local control (LC), and C regional control (RC). The 5-year OS rate was 93% (Stage I/II 91%/100%), the 5-year LC rate 87% (Stage I/II 84%/100%) and the 5-year RC was 80% (Stage I/II 78%/89%). There were no significant differences of OS, LC, and RC between Stage I and Stage II
Neck recurrence was observed in 9 patients within 2 years after the primary surgery. Eight of these patients (89%) had stage I disease. Single and multiple lymph node recurrence were observed in 5 (56%) and 4 (44%) patients, respectively. All lymph node recurrence was observed on the ipsilateral side and unilateral selective ND was performed in 8 patients (89%). Extracapsular spread was observed in half of these patients. Postoperative RT/CRT was performed for 3 patients (33%). Of the 4 patients with multi-lymph recurrence, 1 patient did not undergo postoperative RT/CRT because of previous radiation history (malignant lymphoma) and 1 patient refused postoperative RT. One patient (11%) who underwent definitive CRT without ND later died of the disease (Table 1).
All neck incidences of recurrence developed in patients with a tumor depth ≥ 3 mm, as shown in Fig. 6. The log-rank test identified blood vessel invasion (p < 0.001), lymphatic invasion (p < 0.001), and high-grade tumor budding (p < 0.001) as histopathological risk factors for neck recurrence (Fig. 7; Table 2). The Cox proportional hazards model identified high-grade tumor budding as an independent histopathological risk factor (Table 3). An intermediate correlation (r = 0.399) was observed between blood vessel invasion and budding grade, whereas little correlation (r = 0.181) was found between blood vessel invasion and lymphatic invasion.
The combination of a tumor depth ≥ 3 mm and high-grade tumor budding as a risk factor for neck recurrence had a sensitivity of 89%, a specificity of 95%, a positive predictive value (PPV) of 80%, and a negative predictive value (NPV) of 97%, indicating that the high diagnostic value of this histopathological criterion (Fig. 8; Table 4).
Discussion
Reported treatment outcomes of neck recurrence in patients with clinical N0 early oral tongue carcinoma vary. Yuen et al. reported that among 63 patients with early oral tongue cancer, the 33 patients treated with END showed a superior salvage rate of neck recurrence than the 30 patients undergoing a wait-and-see approach (67% vs. 50%) in a retrospective setting, and END treatment increased the disease-free actuarial survival rate (86% vs. 55%) [2, 20]. In contrast, in a 2009 prospective study, Yuen’s group reported that there was no significant difference in the salvage rate of neck recurrence or disease-specific survival rate between END and observation among 71 patients with early oral tongue carcinoma as long as RT for high risk patients and routine follow-ups by ultrasonography once every 3 months were performed [20]. A recent randomized controlled study conducted by D’Cruz et al. showed that the END group had higher rate of OS and disease-free survival than did the observation group [1]. In this report, the NPV for the preoperative diagnosis of occult neck disease in the observation group was 55%. This low preoperative NPV might explain the reason for the unfavorable prognosis of the observation group. Thus, the management of occult lymph node metastasis in clinical N0 early oral tongue carcinoma remains controversial.
The importance of tumor depth as a predictor of occult lymph metastasis in oral cavity SCC is specified in the NCCN Clinical Practice Guidelines [10]. The guideline recommends that the appropriateness of END for a tumor depth of 2–4 mm has to be determined by clinical judgment whereas END should be considered for a depth of ≥4 mm. However, D’Cruz et al. reported neck metastases were observed in 5.6 and 16.9% of cases with a tumor depth of 3 and 4 mm, respectively [1]. This finding suggests that indications for END based only on the depth of the primary tumor could result in overtreatment for many of these patients. Huang et al. also reported that 4 mm was the optimal cutoff point for tumor thickness in a meta-analysis. In this study, the combination of a tumor depth ≥ 4 mm and high-grade tumor budding as a risk factor for neck recurrence had a sensitivity of 67%, a specificity of 95%, a PPV of 75%, and a NPV of 93%. The combination of a tumor depth ≥ 3 mm and high-grade tumor budding showed a higher diagnostic value than this result. Therefore, a tumor depth ≥ 3 mm was considered as a suitable cutoff point to evaluate the histopathological tumor depth and budding grade.
As the rate of occult metastasis in early oral tongue carcinoma is 20–40%, routine END can be regarded as overtreatment for many patients and the morbidity of ND cannot be overlooked. There have been some reports that SNB, which is a less invasive method than END, has a high NPV for occult metastasis [5, 7–9]. The NCCN Clinical Practice Guidelines describe SLB as an alternative to END [10]. As sentinel nodes are identified by lymphoscintigraphy and evaluated intraoperatively, cases with occult disease can undergo additional ND. The shortcomings of SLB include the need for a surgical procedure involving the neck and limits to the number of institutions where SNB is available.
In contrast, the evaluation of histopathological tumor depth and budding grade affords a noninvasive and superior method that can be readily undertaken in many institutions. As for the patterns of invasion of oral cancer, the Jakobsson criteria, Anneroth criteria, Yamamoto-Kohama mode of invasion, and Bryne criteria are all well-known [23–26]. Diffuse invasion, dissociation of a small number of single cells, and unclear infiltration borders are considered to be prognostic features in those criteria. However, these histopathological characteristics are not numerical, but categorical parameters. On the other hand, budding score is a simple numerical parameter that could be used as an objective indicator representing those categorical parameters and applied smoothly to statistical analysis. Brandwein-Gensler et al. proposed 5 types of patterns of tumor invasion (POI) and a histologic risk assessment score of LC and OS in early and advanced oral SCC [27]. POI type 4, which includes marked and widespread cellular dissociation in small groups of cells (n < 15) and/or in single cells, and POI type 5, which includes tumor satellites of any size ≥ 1 mm away from main tumor or next closest satellite with intervening normal tissue, were taken as the high risk scores. Heerma et al. also reported POI was an independent prognostic factor in early oral SCC [28]. The specimen that has at least one tumor budding is naturally classified into POI type 4 or 5. Therefore, high-grade tumor budding may be the worst prognostic factor in these high risk types.
In this study, we speculate that no poorly differentiated SCC, according to the WHO grade, was seen due to the small number of enrolled patients. There was no significant difference of regional recurrence between moderately and well differentiated SCC.
In colorectal cancer, tumor budding has already been accepted as an additional prognostic factor [29] and it is recognized as an important risk factor for lymph node metastases in the endoscopic treatment of early colorectal cancer [30]. Tumor budding was also reported as the prognostic factor for lymph node metastasis in other cancers such as esophageal, laryngeal, nasopharyngeal, and skin cancers [31–34]. In oral cancer, few studies have evaluated tumor budding as a prognostic factor or risk factor for lymph node metastasis, with only three studies reviewing the evaluation of both histopathological depth and tumor budding. Based on a study of 91 patients with oral cancer, Seki et al. suggested that a histopathological tumor depth ≥ 3 mm and a budding score ≥ 3 were associated with lymphatic metastasis, and this cutoff point offered a NPV and sensitivity of 100% with high PPV and specificity [35]. Their study included patients with floor of mouth and advanced cancer, such as T3/4, who received preoperative chemotherapy. Based on a study of 233 patients with early tongue cancer, Almangush et al. indicated that a histopathological tumor depth ≥ 4 mm and a budding score ≥ 5 were associated with poor prognosis [15]. They used the cutoff point of budding score ≥ 5 and a tumor depth ≥ 4 mm for scoring. The high risk score was correlated with loco-regional recurrence and death. The score was introduced as the BD model [17]. Our current study is the first to evaluate histopathological tumor depth and tumor budding as risk factors of late neck metastasis in patients with untreated early oral tongue carcinoma and validated for the BD model.
Measurement of the deepest point of tumor invasion is required to evaluate pathological tumor depth and identification of the highest area of budding is required to evaluate budding grade. Accurate evaluation of these features is difficult in preoperative biopsy specimens or intraoperative frozen sections. Seki et al. compared tumor depth and budding score in biopsies with those in resected specimens in 44 patients with untreated oral cancer [35]. A good correlation was observed for budding score, but not for tumor depth. Therefore, it appears that it is difficult to determine preoperatively the need for additional treatment by measuring tumor depth in biopsy specimens.
Tumor budding can be evaluated by routine HE staining and can also be easily detected by keratin immunohistochemistry. However, Okamura et al. claimed that keratin immunohistochemistry was not superior to HE for predicting lymph node metastasis in early colorectal cancer [36]. The optimal method for the evaluation of tumor budding, including the superiority of keratin immunohistochemistry to HE, in early oral tongue carcinoma should be explored in future studies.
In our institution, END was avoided by emphasizing follow-up involving imaging studies. We tried to identify neck recurrence as early as possible by meticulous follow-up imaging examinations and treatment with preservative surgery. Many reports on SNB showed its high NPV of more than 95% for occult neck metastasis [5, 8, 9, 37, 38], which is higher than the NPV of our preoperative imaging diagnosis (79%). Kovács et al. reported that, the 5-year OS was 83% in 103 patients with T1-2 oral/oropharyngeal cancer who received SNB [7]. The 5-year OS in the current study was not inferior to the Kovács study, suggesting that the follow-up strategy in our institution could have an impact to that of SNB-guided ND strategy on the prognosis of patients. A prospective study on the prognostic significance of more frequent follow-ups by imaging examinations of the neck in patients with histopathological risk factors associated with the primary tumor should be conducted.
Major limitations of this study were that it was a single institution setting and the number of patients was small, so the reproducibility was lacking. Furthermore the associations between the grade of tumor budding and poorly differentiated SCC or distant metastasis should have been evaluated, however, none of the patients had poorly differentiated SCC or distant metastasis in this study. A multicenter large-scale clinical study should be performed to establish the predictive significance of tumor depth and budding for late lymph node metastases in patients with clinical N0 early oral tongue carcinoma. In addition, a prospective study to clarify whether secondary END based on such assessment improves treatment outcomes is also warranted.
Conclusions
Tumor depth and budding grade, as well as a combination of the two, were identified as histopathological risk factors for late neck recurrence in patients with clinical N0 early oral tongue carcinoma treated by primary surgery without END.
References
D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373:521–9.
Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19(7):583–8.
Lim YC, Lee JS, Koo BS, Kim SH, Kim YH, Choi EC. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: elective neck dissection versus observation. Laryngoscope. 2006;116(3):461–5.
Kelner N, Vartanian JG, Pinto CA, Coutinho-Camillo CM, Kowalski LP. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg. 2014;52(7):590–7.
Broglie MA, Haerle SK, Huber GF, Haile SR, Stoeckli SJ. Occult metastases detected by sentinel node biopsy in patients with early oral and oropharyngeal squamous cell carcinomas: impact on survival. Head Neck. 2013;35(5):660–6.
Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115(7):1489–97.
Kovacs AF, Stefenelli U, Seitz O, et al. Positive sentinel lymph nodes are a negative prognostic factor for survival in T1-2 oral/oropharyngeal cancer-a long-term study on 103 patients. Ann Surg Oncol. 2009;16(2):233–9.
Govers TM, Hannink G, Merkx MA, Takes RP, Rovers MM. Sentinel node biopsy for squamous cell carcinoma of the oral cavity and oropharynx: a diagnostic meta-analysis. Oral Oncol. 2013;49(8):726–32.
Alkureishi LW, Ross GL, Shoaib T, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol. 2010;17(9):2459–64.
Bosman FT CF, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. pp. 134–46.
Watanabe T, Itabashi M, Shimada Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–39.
Marangon Junior H, Rocha VN, Leite CF, de Aguiar MC, Souza PE, Horta MC. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43(3):199–204.
Xie N, Wang C, Liu X, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J Oral Pathol Med. 2015;44(4):266–72.
Almangush A, Bello IO, Keski-Santti H, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36(6):811–8.
Almangush A, Salo T, Hagstrom J, Leivo I. Tumour budding in head and neck squamous cell carcinoma - a systematic review. Histopathology. 2014;65(5):587–94.
Almangush A, Coletta RD, Bello IO, et al. A simple novel prognostic model for early stage oral tongue cancer. Int J Oral Maxillofac Surg. 2015;44(2):143–50.
Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M. Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res. 2015;35(11):6111–20.
Jensen DH, Dabelsteen E, Specht L, et al. Molecular profiling of tumour budding implicates TGFbeta-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236(4):505–16.
Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31(6):765–72.
Yuasa K, Kawazu T, Kunitake N, et al. Sonography for the detection of cervical lymph node metastases among patients with tongue cancer: criteria for early detection and assessment of follow-up examination intervals. AJNR Am J Neuroradiol. 2000;21(6):1127–32.
Flach GB, Tenhagen M, de Bree R, et al. Outcome of patients with early stage oral cancer managed by an observation strategy towards the N0 neck using ultrasound guided fine needle aspiration cytology: No survival difference as compared to elective neck dissection. Oral Oncol. 2013;49(2):157–64.
Jakobsson PA, Eneroth CM, Killander D, Moberger G, Martensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol. 1973;12(1):1–8.
Anneroth G, Hansen LS. A methodologic study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dental Res. 1984;92(5):448–68.
Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984;6(5):938–47.
Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18(8):432–7.
Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–78.
Heerema MG, Melchers LJ, Roodenburg JL, Schuuring E, de Bock GH, van der Vegt B. Reproducibility and prognostic value of pattern of invasion scoring in low-stage oral squamous cell carcinoma. Histopathology. 2016;68(3):388–97.
Wada H, Shiozawa M, Katayama K, et al. Systematic review and meta-analysis of histopathological predictive factors for lymph node metastasis in T1 colorectal cancer. J Gastroenterol. 2015;50(7):727–34.
Kawachi H, Eishi Y, Ueno H, et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Modern Pathol. 2015;28(6):872–9.
Almangush A, Karhunen M, Hautaniemi S, Salo T, Leivo I. Prognostic value of tumour budding in oesophageal cancer: a meta-analysis. Histopathology. 2016;68(2):173–82.
Luo WR, Gao F, Li SY, Yao KT. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology. 2012;61(6):1072–81.
Sarioglu S, Acara C, Akman FC, et al. Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract. 2010;206(2):88–92.
Fujimoto M, Yamamoto Y, Matsuzaki I, et al. Tumor budding is an independent risk factor for lymph node metastasis in cutaneous squamous cell carcinoma: a single center retrospective study. Journal of cutaneous pathology. 2016;43:766–71
Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E1582–90.
Okamura T, Shimada Y, Nogami H, et al. Tumor budding detection by immunohistochemical staining is not superior to hematoxylin and eosin staining for predicting lymph node metastasis in pt1 colorectal cancer. Dis Col Rect. 2016;59(5):396–402.
Pezier T, Nixon IJ, Gurney B, et al. Sentinel lymph node biopsy for T1/T2 oral cavity squamous cell carcinoma–a prospective case series. Ann Surg Oncol. 2012;19(11):3528–33.
Civantos FJ, Zitsch RP, Schuller DE, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol. 2010;28(8):1395–400.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hori, Y., Kubota, A., Yokose, T. et al. Predictive Significance of Tumor Depth and Budding for Late Lymph Node Metastases in Patients with Clinical N0 Early Oral Tongue Carcinoma. Head and Neck Pathol 11, 477–486 (2017). https://doi.org/10.1007/s12105-017-0814-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-017-0814-1