Abstract
Major pathology guidelines often mandate stating the histologic grade as a component of the pathology report for various types of cancer. However, the prognostic value of histologic grade in head and neck squamous cell carcinoma (HNSCC) is controversial at best, and there is a need for more reliable prognostic histologic factors to better stratify and manage patients with HNSCC. In this study, we compared three relevant histopathologic features (histologic grade, worst pattern of invasion (WPOI), and tumor budding) in a large single-center retrospective cohort of early oral tongue squamous cell carcinoma (OTSCC) with tumor greatest dimension ≤ 4 cm. Only histologic grade predicted distant metastasis free survival (DMFS) on univariate analysis. Tumor budding was associated with nodal metastasis, overall survival (OS), regional recurrence-free survival (RRFS), and DMFS and was a significant predictor for nodal metastasis on the multivariable logistic regression model. WPOI 5 was associated with high frequency of nodal metastasis and shortened OS and was an independent adverse prognostic factor for OS on multivariate analysis using the Cox proportional hazards model. WPOI and tumor budding were prognostically more relevant than histologic grade. Consideration should be given to include WPOI and tumor budding in the pathology reporting of OTSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histologic grading is a crucial component of pathologic reporting and a known prognostic factor in many types of human cancer, e.g. Nottingham grade of breast cancer [1] and grade group of prostatic carcinoma [2]. Major pathology guidelines, e.g., World Health Organization (WHO) classification [3, 4], the College of American Pathologists (CAP) protocol [5], and the International Collaboration on Cancer Reporting (ICCR) dataset [6], advocate for a three-tiered grading system for head and neck squamous cell carcinomas (HNSCC). This grading system was first proposed by Broders in 1920 [7] and was later adopted by the WHO classification of head and neck tumors [3, 4]. In brief, a tumor is classified into well, moderately, and poorly differentiated based on a combination of features, i.e., degree of keratinization, cytonuclear atypia, and infiltrative pattern [3]. However, the prognostic value of the grading system is controversial at best, with the majority of studies showing that the grading system correlates poorly with patients’ outcome [8,9,10,11,12,13]. Alternative grading schemes using a point system and/or other histologic features, especially when evaluated only at the invasive front have been advocated by several groups but failed to gain wide acceptance in the pathology community [8, 12].
In 1973, Jakobsson et al. [14] discussed the concept of pattern of invasion (POI) and included POI in a point-based grading system of HNSCC, which was later modified by Anneroth et al. in 1982 [15] and Bayne et al. in 1992 [8]. The four patterns of invasion described in their grading schemes were as follows: POI 1, pushing, well-delineated infiltrative borders; POI 2, infiltrative, solid cords, bands, and/or strands; POI 3, small groups or cords of > 15 cells; and POI 4, small group of < 15 tumor cells and/or single cells. In their cohorts, POI 1 and 2 were often associated with well-differentiated HNSCC while POI 3 and 4 were commonly seen in poorly differentiated tumors. In 2005, Brandwein-Gensler et al. modified and expanded the concept of POI, notably POI 2 was changed to “finger-like” pushing pattern, and a POI 5, defined as satellite tumor nodule(s) at least 1 mm away from the main tumor, was added [16]. These authors showed that the worst POI (WPOI) 5 was an independent adverse prognostic factor for recurrence and survival, whereas nuclear grade did not predict outcome in a large cohort of oral and oropharyngeal squamous cell carcinoma. Therefore, they advocated including WPOI (rather than grade) as a histologic component in the risk stratification model for HNSCC. Most but not all subsequent studies have confirmed the prognostic values of WPOI in HNSCC [11, 17,18,19,20,21,22,23,24,25,26,27]. In 2018, the ICCR dataset added WPOI as a mandatory pathology reporting element for oral cavity SCC [6].
Another relevant histologic concept is tumor budding. A tumor bud is defined as the presence of small clusters of < 5 tumor cells. Based on the guideline published by the International Tumor Budding Consensus Conference (ITBCC), tumor budding should be assessed using × 20 objective (with an adjusted standard field size of 0.786 mm2) within the hotspot at the invasive front, and graded as low (0–4 buds), intermediate (5–9 buds), and high (≥ 10 buds) for any given tumor [28]. In colorectal adenocarcinoma, high tumor budding is an important prognostic pathologic parameter to predict survival and recurrence, and is thought to reflect tumor dehiscence, motility and invasiveness and promote oncogenesis [28]. Similarly, in HNSCC, recent studies have shown that tumor budding is a novel prognostic factor, predicting risk of nodal metastasis at the time of the initial resection, overall survival (OS), disease free survival, and progression-free survival [29,30,31,32].
Clearly, all three parameters (histologic grade, WPOI, and tumor budding) take into account invasive patterns and tumor cell dehiscence (i.e., small clusters of tumor cells) and show a certain degree of overlapping. For example, a tumor with high tumor budding (i.e., ≥ 10 tumor buds of < 5 cells) by definition has a WPOI of 4 (small tumor clusters of < 15 cells) or above and are often poorly differentiated by the Broders’ grading scheme. Conversely, tumors with a WPOI 1 to 3 contain no tumor bud and are frequently graded as well differentiated. The major differences among the three parameters are as follows: grade is a multifactorial subjective qualitative measurement, WPOI is objective and qualitative, whereas tumor budding is objective and semi-quantitative. The current CAP checklist for HNSCC [5] includes grade as a mandatory reporting element, WPOI as an optional element, whereas tumor budding is excluded.
In this study, we aimed to investigate the prognostic role of histologic grade, WPOI and tumor budding in predicting nodal metastasis, survival, and recurrence in a large single-center retrospective cohort of 329 patients with early oral tongue squamous cell carcinoma (OTSCC).
Materials and methods
Study cohort
After obtaining Institutional Review Board approval, the surgical departmental database was searched for patients who fulfilled the following inclusion criteria: (1) patients who underwent primary resection at Memorial Sloan Kettering Cancer Center (New York, NY, USA) from 2000 to 2012, (2) a diagnosis of OTSCC with a tumor greatest dimension of 4 cm or less, and (3) the slides of primary resection were available for review. Exclusion criteria were (1) synchronous HNSCC, (2) prior treatment of the reference carcinoma, (3) distant metastasis at presentation, and (4) prior history of non-endocrine head and neck cancer. A total of 329 patients were included in this retrospective study.
Pathologic review
A detailed pathologic review was conducted by 3 head and neck attending pathologists (RG, NK, or BX).
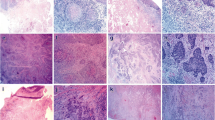
The worst pattern of invasion (WPOI) was defined as follows: WPOI 1, broad pushing tumor front; WPOI 2, finger-like pushing invasion; WPOI 3, large tumor islands > 15 cells; WPOI 4, small tumor island ≤ 15 cells or tumor strands; and POI 5, satellite tumor nodule at least 1 mm away from the main tumor (Fig. 1 and Supplementary Fig. 1) [16, 33].
Worst pattern of invasion (POI), tumor budding, and histologic grade in oral tongue squamous cell carcinoma (OTSCC). a WPOI 1: broad pushing invasion. b WPOI 2: finger-like tumor front. c WPOI 3: large tumor islands > 15 cells. d–f WPOI 4: small tumor islands ≤ 15 cells. g POI 5: satellite nodule(s) at least 1 mm away from the main tumor. A tumor bud (black arrows) is defined as a tumor cluster of < 5 tumor cells or single tumor cells. Tumor budding is assessed at × 20 objective (field size 0.785 mm2) and is classified as low (0–4 buds, d), intermediate (5–9 buds, e), and high (≥ 10 buds, f). By definition, a tumor with WPOI 1–3 lacks tumor buds (i.e., low tumor budding), whereas a tumor with WPOI 4–5 may show any degree of budding. Histologic grade is classified using a combination of keratinization, cytonuclear atypia, and infiltration pattern. Well-differentiated OTSCCs (a–c) show minimal cytologic atypia and abundant keratinization. Moderately differentiated tumors (d and g) are infiltrative, have notable cytologic atypia, and show a certain degree of differentiation/keratinization. Poorly differentiated OTSCCs (e and f) frequently lack clear evidence of differentiation/keratinization and demonstrate marked nuclear pleomorphism and a growth pattern of single cells/small tumor clusters
The number of tumor buds (defined as a tumor cluster of < 5 tumor cells) was counted using × 20 objective with an adjusted standard field size of 0.785 mm2 at the site with the highest number of buds within the tumor (i.e., hotspot). Tumor budding was then classified as low (0–4 buds), intermediate (5–9 buds), and high (≥ 10 buds) [28].
The histologic grade of the tumor was classified using the Broders’ definition [7] into three tiers, being well, moderately, or poorly differentiated based on a combination of cytonuclear atypia, keratinization, and infiltrative pattern [3, 4]. In brief, a well-differentiated OTSCC had no to minimal cytonuclear atypia, abundant keratinization, and keratin pearl, and often with a WPOI of 1 to 3. A moderately differentiated tumor showed notable cytonuclear atypia, less numerous areas of keratinization, and infiltrative growth. A poorly differentiated OTSCC showed marked cytonuclear atypia, no or minimal keratinization, and often an infiltrating pattern of single cells and small tumor clusters.
Other histologic features collected included tumor greatest dimension, depth of invasion, perineural invasion, lymphovascular invasion, margin status (classified as positive—tumor present at the inked margin or negative), AJCC pT, and pN stage.
Outcomes and statistical analysis
All statistics were performed using SPSS software v25.0 (IBM Corporation, Armonk, NY, USA) or SAS software (SAS Institute, Cary, NC, USA). The endpoints studied included OS, LRFS, regional recurrence-free survival (RRFS), distant metastasis free survival (DMFS), and the risk of nodal metastasis at the time of initial resection.
Univariable logistic regression model was performed to calculate the risk of nodal metastasis in the primary resection according to the grade, tumor budding status, and WPOI. Parameters that were significant on univariable analysis were subjected to the multivariable logistic regression model adjusted with AJCC 8th pT stage, perineural invasion, lymphovascular invasion, and margin status. The odds ratio (OR) and its 95% confidence interval (CI) were calculated.
The prognostic values of grading, tumor budding, and POI were determined using the univariate Cox proportional hazards model. Kaplan–Meier curves were plotted. Hazard ratio (HR) and its 95% CI were calculated. Subsequent multivariate analysis using Cox proportional hazards model was performed for OS to adjust with other known prognostic factors, e.g., AJCC 8th pT stage, pN stage, extranodal extension, perineural invasion, lymphovascular invasion, and margin status. Insufficient events were observed in our cohort to conduct multivariate analysis for local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), and distant metastasis-free survival (DMFS). p Values less than 0.05 were considered significant.
Results
Clinicopathologic characteristics of the study cohort
The clinicopathologic features of the study cohort are shown in Table 1.
The majority of the OTSCC (n = 262, 79.6%) were classified as moderately differentiated. The histologic grade of the remaining tumors was well differentiated in 43 (13.1%) and poorly differentiated in 24 (7.2%).
Tumor budding was graded as low (0–4 buds per × 20 objective, field size 0.785 mm2) in 200 (60.8%), intermediate (5–9 buds) in 64 (19.5%), and high (≥ 10 buds) in 65 (19.8%).
The distribution of WPOI among our cohort was as follows: WPOI I in 4 (1.2%), WPOI 2 in 25 (7.6%), WPOI 3 in 76 (23.1%), WPOI 4 in 190 (57.8%), and WPOI 5 in 34 patients (10.3%).
A significant association was detected among any two of these three histologic parameters (Supplementary Table 1, Fisher’s exact test, p < 0.001). All four OTSCCs with WPOI 1 were classified as well-differentiated and low tumor budding. Conversely, WPOI 5 was not seen in well-differentiated OTSCC. Tumor classified as poorly differentiated or high tumor budding only showed WPOI 4 and 5. There was also an association of poorly differentiated histologic grade with high tumor budding. The frequency of high tumor budding in well, moderately, and poorly differentiated OTSCC was 4.7% (2/43), 18.3% (48/262), and 62.5% (15/24), respectively.
The pT stage defined by AJCC 8th edition was pT1 in 179 (54.4%), pT2 in 114 (34.7%), and pT3 in 36 (10.9%). The greatest dimension of the tumor was 2 cm or less in 259 patients (78.7%) and 2.1 to 4 cm in the remaining 70 (21.3%). Perineural invasion, lymphovascular invasion, and positive resection margin were identified in 76 (23.1%), 31 (9.4%), and 7 (2.3%) patients, respectively.
Risk of nodal metastasis in the primary resection according to histologic grade, tumor budding, and WPOI
At the time of primary resection, 54 patients (16.4%) harbored pathology confirmed lymph node metastasis (pN1/N2/N3). Univariable logistic regression analysis shows that nodal metastasis was significantly associated with tumor budding and WPOI, but not histologic grading. The OR, its 95% CI, and p values are shown in Table 2. When substratified for histologic grade, the association between nodal metastasis and tumor budding/WPOI was only significant in moderately differentiated OTSCC (Supplementary Table 2).
On multivariable logistic regression when adjusted for AJCC pT stage, margin status, perineural invasion, and lymphovascular invasion, only high tumor budding was independently associated with a high risk of nodal metastasis (compared with low tumor budding, OR = 5.203, 95% CI 2.249–12.037, p < 0.001).
The prognostic values of histologic grade, tumor budding, and WPOI
The median follow-up of our cohort was 73 months (range 0.03–224 months). At the time of last follow-up, there were 100 deaths, 54 local recurrences, 43 regional recurrences, and 12 distant recurrences. The number of events for LRFS, RRFS, and DMFS was insufficient for multivariate survival analysis. Therefore, multivariate analysis was only conducted for OS.
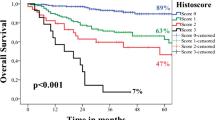
The results of univariate and multivariate survival analysis using the Cox proportional hazards model are shown in Table 3, and the Kaplan–Meier curves are provided in Fig. 2. On univariate survival analysis, the histologic grade was a significant prognostic factor for DMFS (HR = 4.388, 95% CI 1.176–16.369, p = 0.001), but not OS, LRFS, or RRFS. High tumor budding was associated with decreased OS (HR = 2.235, 95% CI 1.433–3.488, p < 0.001), RRFS (HR = 4.651, 95% CI 2.388–9.058, p < 0.001), and DMFS (HR = 6.121, 95% CI 1.785–20.986, p = 0.004). WPOI 5 was an adverse prognostic factor for OS (HR = 2.774, 95% CI 1.659–4.638, p < 0.001), but not LRFS, RRFS, or DMFS.
Kaplan–Meier curves for overall survival (top rows), regional recurrence free survival (RRFS, bottom left), and distant metastasis-free survival (DMFS, bottom middle and right). On univariate survival analysis, high tumor budding was associated with decreased overall survival, RRFS, and DMFS. Pattern of invasion 5 was associated with adverse overall survival. Poorly differentiated carcinoma predicted decreased DMFS but not overall survival
When substratified for histologic grade, tumor budding, and WPOI predicted OS only in moderately differentiated OTSCC on univariate survival analysis (Supplementary Table 2).
On multivariate analysis using the Cox proportional hazards model for OS, WPOI remained an independent prognostic factor for OS (HR = 1.994, 95% CI 1.142–3.481, p = 0.015). The 3-year, 5-year, and 10-year OS for patients with WPOI 5 was 55%, 55%, and 37%, respectively, compared with 88%, 81%, and 67% for patients with WPOI of 1 to 4. Tumor budding failed to reach significance on multivariate analyses.
Discussion
The prognostic value of the histologic grade in HNSCC has not been clearly established in the literature. In the 3rd edition of WHO classification [3], it is stated that “histologic grade correlates poorly with patient outcome. The value of grading improves when only the deeply invasive margins of the tumor are evaluated.” Indeed, numerous studies have shown that histologic grade did not correlate with the risk of nodal metastasis [10], disease-specific survival [8, 12, 13, 20], disease-free survival [16, 20], LRFS [11], or RRFS [11, 24] in HNSCC from various subsites, e.g., floor of mouth [8], oral cavity [10, 12, 20], oral cavity and oropharynx [16], and oral tongue [11, 13, 24]. The only study which found a significant correlation between grade and outcome is by Lin et al. [34]. The authors reported that poorly differentiated tumors were associated with higher risk of nodal metastasis at the initial resection (being 45.9% compared with 29.3% in moderately differentiated and 6.1% in well-differentiated tumors) and decreased recurrence free survival (hazard ratio = 1.973, 95% CI 1.167–3.336) compared with well-differentiated tumor in a large cohort of 2535 patients with oral squamous cell carcinoma from a Taiwanese medical center. There was no significant difference between well-differentiated and moderately differentiated tumors.
Similar to the majority of previous studies, in our cohort of early OTSCC, we found that grade only predicted DMFS on univariate analysis but correlated poorly with the risk of nodal metastasis, OS, LRFS, and RRFS. The frequency of well-differentiated, moderately differentiated, and poorly differentiated OTSCC in the current study was 13.1%, 79.6%, and 7.3%, respectively, similar to what was reported by Lin et al. (17%, 78%, and 5%, respectively). The observed prognostic difference between our cohort and Lin et al. might have been a result of the different cohort size (329 vs. 2535), different stage of the analyzed tumors (early OTSCC vs. oral cavity squamous cell carcinoma of any stage), and the ethnic composition of the study population (single US center vs. single Taiwanese center) [34].
It is noteworthy that alternative grading schemes exist for HNSCC. For example, a point-based grading system (Bayan’s grading) evaluated only at the invasive tumor front seemed to provide improved prognostication information [8]. Such grading method has been shown to be an independent prognostic factor for survival in 79 patients with floor of mouth squamous cell carcinoma [8] and an independent predictor for disease specific survival in 85 patients with oral cavity squamous cell carcinoma [12].
Compared with histologic grade, the literature on WPOI and tumor budding in HNSCC appeared to be more consistent. Most studies performed univariate analysis and showed that WPOI 4 and 5 were associated with the risk of bone invasion [13] and nodal metastasis [20, 21, 23], worse disease-specific survival [20, 21, 35], and RRFS [25], whereas WPOI 5 was linked with high frequency of locoregional recurrence [26] and decreased disease-free survival [16, 22]. Only three studies did not report a significant association between WPOI and nodal metastasis (in 49 cases of oral squamous cell carcinoma [10] and 88 cases of OTSCC [17]), or progression free survival in 49 cases of OTSCC [19]), possibly due to small sample size.
The reported results of multivariate survival analysis were more variable. While some studies showed that WPOI was an independent prognostic factor for RRFS [25], nodal metastasis [20, 36], and recurrence-free survival [18], others did not report WPOI to be significant on multivariate analysis for risk of nodal metastasis [10], locoregional recurrence [26], progression-free survival [19], disease-free survival [22], LRFS [11], and RRFS [11]. The discrepant results in regard to multivariate analysis may be in part explained by the cohort size. Those studies which reported a significant result tended to have a larger cohort size, two of the four studies had a cohort size of 336 to 340 patients [18, 20], whereas those studies with no significant result on multivariate analysis had a sample size of 39 to 126 patients [10, 11, 19, 22, 26]. In the current study with a large sample of 329 patients, we reported that WPOI 5 was the only independent prognostic factor for OS on multivariate analysis among the other three parameters studied, further supporting the prognostic role of WPOI 5 in HNSCC. Meta-analysis to pool prognostic data from multiple studies or large-scale multicenter studies may be useful to confirm WPOI as an important histologic factor to risk stratify patients with HNSCC. The current ICCR dataset includes WPOI as a mandatory reporting element [6], whereas WPOI is an optional component in the CAP checklist [5].
A limitation of WPOI 5 in the clinical setting is that the frequency of WPOI 5 is quite low in HNSCC, being 10.3% (34 patients) in the current cohort. Therefore, WPOI5 alone will not be able to capture all HNSCCs with adverse outcomes (i.e., 43 patients with nodal metastases at the initial resection, 100 deaths, 54 local recurrences, and 43 regional recurrences in the current study). A multifactorial histologic stratification is needed to stratify patients with HNSCC.
In two recent meta-analyses by Almangush et al. in 2018 (which included 9 studies) and Zhu et al. in 2019 (which included 15 studies), high tumor budding was found to be associated with lymph node metastasis at the initial resection, OS, disease-free survival, and progression-free survival in HNSCC [29, 30]. Additionally, the authors have shown that high tumor budding was associated with OS in OTSCC [30] and clinical T1/T2 oral squamous cell carcinoma [29]. Several individual studies performed multivariate analysis, confirming an independent prognostic role of tumor budding in predicting nodal metastasis [20, 36], OS [37], disease-specific survival [18], progressive-free survival [37], and RRFS [24].
Most of the published individual studies adopted a two-tiered system (high budding vs. low budding) and the cutoff used varied from 3, 5, to 10 buds [18, 20, 22, 24, 29, 30, 36, 37]. The only study that used the three-tiered system proposed by the ITBCC consensus showed that tumor budding was an independent prognostic factor for disease free survival in a cohort of 91 patients with clinical T1/T2N0 oral squamous cell carcinoma (21). In contrast, WPOI failed to reach a significant level in the same study [22]. Data from our current study further confirm the role of tumor budding in early OTSCC. Among the three factors studied, high tumor budding was the only independent predictor for increased risk of nodal metastasis on multivariable logistic regression model and was associated with poor OS, RRFS and DMFS on univariate survival analysis. Together, these data support the prognostic relevance of tumor budding in HNSCC, including OTSCC. Furthermore, several authors have suggested including tumor budding in the risk stratification model for HNSCC, e.g., budding-depth of invasion (BD) model [25, 38]. Therefore, it is relevant to score tumor budding in a standardized fashion as defined by ITBCC consensus and to include it as an element of pathology reporting for HNSCC to provide additional prognostication information.
When we substratified the study cohort by histologic grade, the impacts of WPOI and tumor budding on risk of nodal metastasis of OS appeared to be significant only in the moderately differentiated OTSCC. However, such results should be interpreted with caution as the number of cases in well-differentiated and poorly differentiated OTSCC was very small, being 43 and 24, respectively. Larger studies or meta-analyses are needed to determine the prognostic significance of WPOI and tumor budding in each histologic grade.
The current study is the only large-scale study that evaluated tumor budding using the standardized ITBCC consensus approach and also included a multivariate analysis. It is also the only study which correlated all three histologic parameters (histologic grade, tumor budding using ITBCC consensus guideline, and WPOI). The limitation of the study was that it only included OTSCC ≤ 4 cm in size. Therefore, the results might not be generalized to larger tumors or HNSCC originated from other sites.
In conclusion, we compared the performance of three related histologic parameters, the multifactorial descriptive histologic grade, the qualitative WPOI, and the semi-quantitative tumor budding, and found that WPOI and tumor budding were superior to histologic grade in OTSCC. While WPOI 5 was an independent adverse prognostic factor for OS, high tumor budding was associated with a high risk of nodal metastasis on multivariable logistic regression analysis. Therefore, we propose to include tumor budding and WPOI in the routine pathologic reports of patients with OTSCC.
References
Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse GM, Badve S, Ellis IO (2010) Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 12(4):207. https://doi.org/10.1186/bcr2607
Kench JG, Judge M, Delahunt B, Humphrey PA, Kristiansen G, Oxley J, Rasiah K, Takahashi H, Trpkov K, Varma M, Wheeler TM, Zhou M, Srigley JR, Egevad L (2019) Dataset for the reporting of prostate carcinoma in radical prostatectomy specimens: updated recommendations from the International Collaboration on Cancer Reporting. Virchows Arch 475(3):263–277. https://doi.org/10.1007/s00428-019-02574-0
Barnes EL, Eveson JW, Reichart P, Sidransky D (2005) World Health Organization Classification of Tumours: pathology and genetics of head and neck tumours. International Agency for Research on Cancer (IARC), Lyon
El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ (2017) World Health Organization Classification of Tumours: pathology and genetics of head and neck tumours, 4th edn. International Agency for Research on Cancer (IARC), Lyon
Seethala RR, Weinreb I, JB McHugh, Calson DL, Ferris RL, Harrison LB, McHugh JB, Pettus J, Richardson MS, Shah JP, Thompson LDR, Wenig BM (2017) College of American Pathologist (CAP) protocol for the examination of specimens for patients with cancers of the lip and oral cavity. https://documents.cap.org/protocols/cp-headandneck-lip-oralcavity-17protocol-4001.pdf. Accessed 6/27/2020 2020
Müller S, Boy SC, Day TA, Magliocca KR, Richardson MS, Sloan P, Tilakaratne WM, Zain RB, Thompson LDR (2019) Data set for the reporting of oral cavity carcinomas: explanations and recommendations of the guidelines from the International Collaboration of Cancer Reporting. Arch Pathol Lab Med 143(4):439–446. https://doi.org/10.5858/arpa.2018-0411-SA
Broders AC (1920) Squamous-Cell Epithelioma Of The Lip: A Study Of Five Hundred And Thirty-Seven Cases. J Am Med Assoc 74(10):656–664. https://doi.org/10.1001/jama.1920.02620100016007
Bryne M, Koppang HS, Lilleng R, Kjaerheim A (1992) Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol 166(4):375–381. https://doi.org/10.1002/path.1711660409
Roland NJ, Caslin AW, Nash J, Stell PM (1992) Value of grading squamous cell carcinoma of the head and neck. Head Neck 14(3):224–229. https://doi.org/10.1002/hed.2880140310
Kane SV, Gupta M, Kakade AC, D'Cruz A (2006) Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol 32(7):795–803. https://doi.org/10.1016/j.ejso.2006.05.004
Yanamoto S, Yamada S, Takahashi H, Kawasaki G, Ikeda H, Shiraishi T, Fujita S, Ikeda T, Asahina I, Umeda M (2013) Predictors of locoregional recurrence in T1-2N0 tongue cancer patients. Pathol Oncol Res 19(4):795–803. https://doi.org/10.1007/s12253-013-9646-9
Wagner VP, Webber LP, Curra M, Klein IP, Meurer L, Carrad VC, Martins MD (2017) Bryne’s grading system predicts poor disease-specific survival of oral squamous cell carcinoma: a comparative study among different histologic grading systems. Oral Surg Oral Med Oral Pathol Oral Radiol 123(6):688–696. https://doi.org/10.1016/j.oooo.2017.02.012
Barbosa da Silva LA, Diniz de Sousa Lopes ML, Sá MC, de Almeida Freitas R, Coletta RD, da Silveira EJD, Miguel M (2020) Histopathological grading and its relationship with outcome in oral tongue squamous cell carcinoma. J Oral Pathol Med 50:183–190. https://doi.org/10.1111/jop.13118
Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B (1973) Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol 12(1):1–8. https://doi.org/10.3109/02841867309131085
Anneroth G, Batsakis J, Luna M (1987) Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res 95(3):229–249. https://doi.org/10.1111/j.1600-0722.1987.tb01836.x
Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, Wang BY (2005) Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 29(2):167–178
de Matos FR, Lima E, Queiroz LM, da Silveira EJ (2012) Analysis of inflammatory infiltrate, perineural invasion, and risk score can indicate concurrent metastasis in squamous cell carcinoma of the tongue. J Oral Maxillofac Surg 70(7):1703–1710. https://doi.org/10.1016/j.joms.2011.08.023
Almangush A, Bello IO, Keski-Santti H, Makinen LK, Kauppila JH, Pukkila M, Hagstrom J, Laranne J, Tommola S, Nieminen O, Soini Y, Kosma VM, Koivunen P, Grenman R, Leivo I, Salo T (2014) Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck 36(6):811–818. https://doi.org/10.1002/hed.23380
Kolokythas A, Park S, Schlieve T, Pytynia K, Cox D (2015) Squamous cell carcinoma of the oral tongue: histopathological parameters associated with outcome. Int J Oral Maxillofac Surg 44(9):1069–1074. https://doi.org/10.1016/j.ijom.2015.01.027
Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, Chaturvedi A, Anand N, Malhotra K, Shukla S (2017) Development of a new outcome prediction model in early-stage squamous cell carcinoma of the oral cavity based on histopathologic parameters with multivariate analysis: the Aditi-Nuzhat Lymph-node Prediction Score (ANLPS) system. Am J Surg Pathol 41(7):950–960. https://doi.org/10.1097/pas.0000000000000843
Sakata J, Yamana K, Yoshida R, Matsuoka Y, Kawahara K, Arita H, Nakashima H, Nagata M, Hirosue A, Kawaguchi S, Gohara S, Nagao Y, Hiraki A, Shinohara M, Toya R, Murakami R, Nakayama H (2018) Tumor budding as a novel predictor of occult metastasis in cT2N0 tongue squamous cell carcinoma. Hum Pathol 76:1–8. https://doi.org/10.1016/j.humpath.2017.12.021
Shimizu S, Miyazaki A, Sonoda T, Koike K, Ogi K, Kobayashi JI, Kaneko T, Igarashi T, Ueda M, Dehari H, Miyakawa A, Hasegawa T, Hiratsuka H (2018) Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma: With special reference to the mode of invasion and worst pattern of invasion. PLoS One 13(4):e0195451. https://doi.org/10.1371/journal.pone.0195451
Chatterjee D, Bansal V, Malik V, Bhagat R, Punia RS, Handa U, Gupta A, Dass A (2019) Tumor budding and worse pattern of invasion can predict nodal metastasis in oral cancers and associated with poor survival in early-stage tumors. Ear Nose Throat J 98(7):E112–e119. https://doi.org/10.1177/0145561319848669
Yamakawa N, Kirita T, Umeda M, Yanamoto S, Ota Y, Otsuru M, Okura M, Kurita H, Yamada SI, Hasegawa T, Aikawa T, Komori T, Ueda M (2019) Tumor budding and adjacent tissue at the invasive front correlate with delayed neck metastasis in clinical early-stage tongue squamous cell carcinoma. J Surg Oncol 119(3):370–378. https://doi.org/10.1002/jso.25334
Hori Y, Kubota A, Yokose T, Furukawa M, Matsushita T, Oridate N (2020) Association between pathological invasion patterns and late lymph node metastases in patients with surgically treated clinical No early oral tongue carcinoma. Head Neck 42(2):238–243. https://doi.org/10.1002/hed.25994
Larson AR, Kemmer J, Formeister E, El-Sayed I, Ha P, George J, Ryan W, Chan E, Heaton C (2020) Beyond depth of invasion: adverse pathologic tumor features in early oral tongue squamous cell carcinoma. Laryngoscope 130(7):1715–1720. https://doi.org/10.1002/lary.28241
Yue LE, Sharif KF, Sims JR, Sandler ML, Baik FM, Sobotka S, Everest S, Brandwein-Weber M, Khorsandi AS, Likhterov I, Urken ML (2020) Oral squamous carcinoma: aggressive tumor pattern of invasion predicts direct mandible invasion. Head Neck 42:3171–3178. https://doi.org/10.1002/hed.26360
Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P (2017) Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Modern Pathol 30(9):1299–1311. https://doi.org/10.1038/modpathol.2017.46
Zhu Y, Liu H, Xie N, Liu X, Huang H, Wang C, Hou J (2019) Impact of tumor budding in head and neck squamous cell carcinoma: a meta-analysis. Head Neck 41(2):542–550. https://doi.org/10.1002/hed.25462
Almangush A, Pirinen M, Heikkinen I, Mäkitie AA, Salo T, Leivo I (2018) Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br J Cancer 118(4):577–586. https://doi.org/10.1038/bjc.2017.425
Mäkitie AA, Almangush A, Rodrigo JP, Ferlito A, Leivo I (2019) Hallmarks of cancer: tumor budding as a sign of invasion and metastasis in head and neck cancer. Head Neck 41(10):3712–3718. https://doi.org/10.1002/hed.25872
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (eds) (2017) AJCC cancer staging manual, 8th edn. Springer Nature, New York
Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, Schiff B, Owen RP, Smith J, Sarta C, Hebert T, Nason R, Ramer M, DeLacure M, Hirsch D, Myssiorek D, Heller K, Prystowsky M, Schlecht NF, Negassa A (2010) Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol 34(5):676–688. https://doi.org/10.1097/PAS.0b013e3181d95c37
Lin NC, Hsu JT, Tsai KY (2020) Survival and clinicopathological characteristics of different histological grades of oral cavity squamous cell carcinoma: a single-center retrospective study. PLoS One 15(8):e0238103
Almangush A, Bello IO, Coletta RD, Mäkitie AA, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Soini Y, Kosma VM, Koivunen P, Kelner N, Kowalski LP, Grénman R, Leivo I, Läärä E, Salo T (2015) For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch 467(1):39–46. https://doi.org/10.1007/s00428-015-1758-z
Manjula BV, Augustine S, Selvam S, Mohan AM (2015) Prognostic and predictive factors in gingivo buccal complex squamous cell carcinoma: role of tumor budding and pattern of invasion. Ind J Otolaryngol Head and Neck Surg 67(Suppl 1):98–104. https://doi.org/10.1007/s12070-014-0787-2
Karpathiou G, Vieville M, Gavid M, Camy F, Dumollard JM, Magné N, Froudarakis M, Prades JM, Peoc'h M (2019) Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck 41(6):1918–1927. https://doi.org/10.1002/hed.25629
Almangush A, Coletta RD, Bello IO, Bitu C, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Tommola S, Soini Y, Kosma VM, Koivunen P, Kowalski LP, Nieminen P, Grénman R, Leivo I, Salo T (2015) A simple novel prognostic model for early stage oral tongue cancer. Int J Oral Maxillofac Surg 44(2):143–150. https://doi.org/10.1016/j.ijom.2014.10.004
Funding
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additionally, CV reports receiving a research grant from Fundación Alfonso Martín Escudero.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Xu, B., Salama, A.M., Valero, C. et al. The prognostic role of histologic grade, worst pattern of invasion, and tumor budding in early oral tongue squamous cell carcinoma: a comparative study. Virchows Arch 479, 597–606 (2021). https://doi.org/10.1007/s00428-021-03063-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03063-z