Abstract
The role of human papillomavirus (HPV) as an etiologic and transformational agent in inverted Schneiderian papilloma (ISP) is unclear. Indeed, reported detection rates of HPV in ISPs range from 0 to 100%. The true incidence has been confounded by a tendency to conflate high- and low-risk HPV types and by the inability to discern biologically relevant from irrelevant HPV infections. The recent development of RNA in situ hybridization for high-risk HPV E6/E7 mRNA now allows the direct visualization of transcriptionally active high-risk HPV in ISP, providing an opportunity to more definitively assess its role in the development and progression of ISPs. We performed p16 immunohistochemistry and high-risk HPV RNA in situ hybridization on 30 benign ISPs, 7 ISPs with dysplasia, 16 ISPs with carcinomatous transformation, and 7 non-keratinizing squamous cell carcinomas (SCCs) with inverted growth that were unassociated with ISP. Transcriptionally active HPV was not detected in any of the 52 ISPs including those that had undergone carcinomatous transformation, but it was detected in two of seven (29%) non-keratinizing SCCs that showed inverted growth. There was a strong correlation between high-risk HPV RNA in situ hybridization and p16 immunohistochemistry (97%; p < 0.01). These results indicate that transcriptionally active high-risk HPV does not play a common role in either the development of ISP or in its transformation into carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inverted Schneiderian papillomas (ISPs) are uncommon neoplasms that comprise 0.5 to 4.0% of all sinonasal tumors [1–3]. While ISPs are fundamentally benign, they can demonstrate locally destructive growth, and they have a high rate of local recurrence [4–6]. Moreover, 5–15% of ISPs undergo malignant transformation into squamous cell carcinoma (SCC) and, less commonly, other carcinoma types such as mucoepidermoid carcinoma [7–9]. In cases with associated malignancy, the precise relationship of ISP to SCC is unclear. Although studies have confirmed the neoplastic (rather than reactive) nature of ISPs, these lesions do not fit the profile of the prototypic precursor lesion of head and neck SCC. ISPs are known to be clonal cellular proliferations, but they do not harbor the key genetic alterations that occur commonly and early during the initiation and progression of head and neck SCC [10]. A recent study identified identical EGFR mutations in a large proportion of ISPs and associated SCCs, strongly supporting a common origin for both tumor components and further underscoring a biology that is distinct from conventional SCCs of the head and neck where EGFR mutations are uncommon [11]. However, the factors that drive malignant transformation within this pathway have not yet been fully elucidated.
The notable absence of conventional genetic alterations in ISPs has suggested the possibility of high-risk human papillomavirus (HPV) as an alternative driver of malignant transformation. To date, however, the role of HPV in carcinomatous transformation of ISP is unresolved. Reported detection rates of HPV in ISPs have ranged from 0 to 100% [1, 3, 4, 7, 10, 12–34]. As for its role in biological progression, some studies have implicated HPV as a driving force behind tumor recurrence and carcinomatous transformation [9, 17, 22, 29, 34] while others have not [20, 28, 33]. The exasperating inability to discern the true role of HPV in ISP tumorigenesis largely reflects flaws in study design. First, some historical studies have overstated the role of HPV in carcinomatous transformation of ISPs by wholesale HPV testing without discriminating between low-risk and high-risk types [22, 35]. Second, many historical studies have employed assays that have not been able to couple the presence of HPV with evidence of its biologic activity, and thus have been unable to discern clinically relevant from irrelevant (e.g. viral contaminant or passenger virus) HPV infections [12]. The recent development of RNA in situ hybridization probes complementary to E6/E7 mRNA now permits direct visualization of viral transcripts in routinely processed tissues. In formalin-fixed and paraffin-embedded samples, RNA in situ hybridization permits direct visualization of transcriptionally active HPV RNA with a sensitivity and specificity that exceeds other methods of HPV detection [36–42]. Using a high-risk RNA ISH approach, we analyzed a series of ISPs along a morphologic and biologic continuum culminating in squamous cell carcinoma-ex ISP to elucidate the role of high-risk HPV in the process of carcinomatous transformation.
Materials and Methods
Case Selection
Study approval was obtained from the Johns Hopkins Medical Institutions Internal Review Board. Specimens were retrieved from the surgical pathology archives at the Johns Hopkins Hospital and included all consecutive cases of benign ISPs diagnosed from 2014 to 2015, all consecutive cases of ISPs with dysplasia diagnosed from 1999 to 2016, all consecutive cases of SCC arising from an ISP diagnosed from 1999 to 2016, and all consecutive cases of non-keratinizing SCCs that demonstrated a prominent inverted growth pattern diagnosed from 1999 to 2016. Pertinent clinical and demographic information was obtained from The Johns Hopkins Hospital’s electronic medical records. The histologic slides were reviewed and the diagnosis was confirmed for all cases.
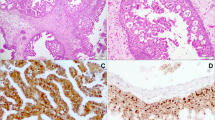
Histologically, ISPs were defined by the presence of thickened epithelium with inverted growth and variable amounts of squamous, columnar, and mucinous cells (Fig. 1). All cases demonstrated intraepithelial neutrophils with scattered intraepithelial microabscesses. Cases diagnosed as dysplasia and SCC ex-ISP demonstrated clearly identifiable areas of pure benign ISP, but also showed areas with increased nuclear pleomorphism, high nuclear-cytoplasmic ratios, cellular dyspolarity, numerous apoptotic bodies, and prominent mitoses (Fig. 2a). In the cases of SCC ex-ISP, the atypia was full-thickness and more severe (Fig. 2b). The presence of overt stromal invasion was taken as definite evidence of carcinomatous transformation. The diagnosis of SCC ex-ISP required histologic or clinical documentation of a synchronous or metachronous ISP. For the non-keratinizing sinonasal SCCs, the tumors infiltrated the stroma as lobules of tumor cells with smooth rounded boarders simulating inverted growth (Fig. 3). Unlike conventional SCC, these carcinomas were characterized by the absence of keratinization and the lack of irregular stromal infiltration by angulated cords and nests of tumor cells. The non-keratinizing sinonasal SCCs were not associated with a synchronous or metachronous ISP.
Benign ISP is architecturally characterized by thickened surface epithelium showing deep downward growth as inverted rounded lobules (a). The epithelial cells are heavily permeated by neutrophils. Although the inverted nests are highly cellular with mitotic activity and loss of polarity, they lack significant cellular pleomorphism (b)
Inverted Schneiderian papilloma with carcinomatous progression. Benign inverted epithelium (a, left upper) shows abrupt transition with dysplastic epithelium characterized by nuclear hyperchromasia and loss of polarity (a, right lower). In other areas, the epithelium exhibits overtly malignant changes including necrosis, apoptosis and cellular atypia (b)
Non-keratinizing SCC of the sinonasal tract with inverted growth characterized by lobules of tumor cells with smooth and rounded borders (a). Despite some morphologic similarities to inverted Schneiderian papilloma, a synchronous or metachronous benign papilloma could not be histologically documented. Unlike the cases of Schneiderian papilloma with or without evidence of carcinomatous transformation, this carcinoma was positive for high-risk HPV (b) (high-risk HPV RNA in situ hybridization)
P16 Immunohistochemistry
P16 immunohistochemistry was performed using a mouse monoclonal antibody (MTM Laboratories, Heidelberg, Germany). Tumor sections were cut at five microns, deparaffinized, and subjected to antigen retrieval with 10 mM citrate buffer (92 °C for 30 min). Signals were visualized using the Ultra view polymer detection kit (Ventana Medical Systems, Tucson, AZ) and a Ventana BenchMark XT autostainer (Ventana). Staining was performed according to manufacturer’s instructions in the presence of appropriate controls. P16 interpretation was performed according to consensus standards established to correlate IHC reactivity with HPV status in oropharyngeal SCC [43], with only those cases that demonstrated diffuse staining, defined as strong nuclear and cytoplasmic positivity in >70% of tumor cells, considered positive for p16 overexpression.
HPV RNA In Situ Hybridization
RNA in situ hybridization for high-risk HPV E6/E7 mRNA was performed using the RNAscope HPV-HR18 Probe (Advanced Cell Diagnostics, Hayward, CA). Tumor sections were cut at five microns, pretreated with heat and protease, and hybridized with a single cocktail probe recognizing 18 high-risk HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82). Preamplifier, amplifier and horseradish peroxidase-labeled probes were sequentially hybridized, and color was developed with diaminobenzidine. All steps were performed manually in the presence of appropriate controls. Cases processed using RNAScope were determined positive if they had punctate brown signals present in the cytoplasm and/or nucleus. Weak signals in tumor cells that could only be detected at ×20–40 magnification were not regarded as positive if the number and intensity of signal were not clearly above background [42].
Results
Fifty-nine tumors were evaluated from 51 patients. These included 30 ISPs without dysplasia, 6 ISPs with dysplasia, 16 ISPs with a synchronous (n = 14) or metachronous (n = 2) SCC (i.e. SCC ex-ISP), and 7 non-keratinizing SCCs of the sinonasal tract with inverted growth. Relevant clinical and pathologic information is summarized in Table 1. The study population was comprised of 31 males (61%) and 20 females (39%) with a mean age of 62 years (median 63, range 28–94). Tumors were centered in the nasal cavity (n = 22), maxillary sinus (n = 16), frontal sinus (n = 11), ethmoid sinus (n = 6), and sphenoid sinus (n = 4). Thirty-three tumors (56%) were primary lesions, and 26 (44%) were recurrent. Paired primary and recurrent tumors were both evaluated for five patients with recurrent ISPs and one patient with recurrent SCC ex-ISP.
Patchy p16 by immunohistochemistry was noted in 25 of 30 (83%) of the ISPs without dysplasia and in 2 of 6 (33%) ISPs with dysplasia; but staining was well below the 70% threshold that is generally considered consistent with high-risk HPV infection [44]. As such, all 30 ISPs without dysplasia and all 6 ISPs with dysplasia were p16 negative. Positive p16 staining was present in only 1 of 16 (6%) of SCC ex-ISPs. In contrast, diffuse p16 positivity was present in 3 of 7 (43%) of the invasive non-keratinizing SCCs. High-risk HPV RISH did not detect the presence of transcriptionally active HPV in any of the ISPs including those without dysplasia (0 of 30, 0%), those with dysplasia (0 of 6, 0%), and those that harbored SCC (carcinoma-ex-ISPs) (0 of 16, 0%). High-risk HPV RISH was detected in 2 of 7 (29%) non-keratinizing SCCs with inverted growth.
Although this analysis is limited by the small number of HPV-positive tumors included in this study, there was a 96% correlation rate overall between HPV status and p16 immunohistochemical staining. All 55 (100%) of the p16 negative tumors were HPV negative by RNA in situ hybridization, and 2 of the 4 (50%) p16 positive tumors were HPV positive by RNA in situ hybridization. Using detection of viral mRNA transcripts as definite evidence of a relevant high-risk HPV infection, p16 overexpression as measured by p16 immunohistochemistry had a positive predictive value of 0.5 (0.09–0.91, 95% CI) and a negative predictive value of 1 (0.09–0.91, 95% CI).
Discussion
The molecular genetic changes driving malignant transformation of an ISP are not well understood, but the finding that approximately one-third of non-keratinizing SCCs of the sinonasal tract harbor high-risk HPV suggests that this oncovirus may be an important etiologic agent. Numerous studies to confirm the importance of HPV in this process, however, have only obscured the role of HPV in the formation, recurrence and malignant transformation of ISPs. Reported detection rates of HPV in ISPs and those that have undergone carcinomatous transformation have ranged from 0 to 100% [1, 3, 4, 7, 10, 12–34]. Much of this ambiguity reflects suboptimal detection methodologies including the inability to discern biologically relevant from irrelevant HPV.
Taking advantage of recently developed RNA in-situ hybridization probes complementary to E6/E7 mRNA that now permit direct visualization of viral transcripts in routinely processed tissues, we were able to confirm that transcriptionally active HPV is seldom encountered in ISPs or in those ISPs showing evidence of carcinomatous transformation. Indeed, E6/E7 mRNA viral transcripts were not detected in any of the benign ISPs, ISPs with dysplasia, or carcinomas ex-ISP. Using a similar RNA in situ hybridization strategy, Nudell et al. [9] detected transcriptionally active high-risk HPV in 3 of 17 (18%) carcinomas ex-ISPs. These results do not statistically depart from our own (p = 0.23, Fischer 2-tailed test) and further underscore the point that while HPV may be associated with carcinoma ex-ISPs, its presence is neither invariable nor common. Carcinomatous transformation of ISPs is driven by non-HPV mechanisms for most ISPs.
“Schneiderian carcinoma” is a bygone designation that was historically applied to non-keratinizing SCC of the sinonasal tract [45]. Reminiscent of Schneiderian papillomas, these carcinomas characteristically invade in an inverted fashion as rounded ribbons and lobules of tumor cells (Fig. 3). They tend to lack the overt keratinization and irregular invasive growth that characterize most conventional head and neck SCCs. Indeed, the second series of the AFIP Tumors of the upper respiratory tract and ear drew early attention to the way these carcinomas are easily mistaken for inverted Schneiderian papilloma [46]. This morphologic parallel has fostered a persistent notion that many of these “Schneiderian carcinomas” (i.e. non-keratinizing sinonasal SCCs with inverted growth) represent the malignant counterpart of ISPs. We detected transcriptionally active HPV in 27% of these non-keratinizing SCCs. This rate of HPV detection is comparable to other studies where HPV has been detected in about one-third of non-keratinizing SCCs of the sinonasal tract [21, 47]. The observation that transcriptionally active HPV is present in some non-keratinizing sinonasal SCCs with inverted growth but not in ISPs showing dysplastic changes or overt carcinoma (2 of 7 vs. 0 of 22, p = 0.052) underscores the absence of an etiologic link between the two lesions. When encountering SCC of the sinonasal tract characterized by an inverted pattern of growth, the assumption that the tumor arose from a pre-existing ISP (in the absence of a benign component) may not be correct.
ISPs are benign, but local recurrence and even malignant transformation is a constant hazard. Efforts to predict those ISPs that are likely to recur or undergo malignant transformation on the basis of microscopic features or even molecular genetic alterations have not been successful [10]. Our study suggests that HPV detection as a strategy to assess risk of aggressive tumor behavior would not be effective. Benign ISPs do not appear to harbor transcriptionally active high-risk HPV. Moreover, in those ISPs that already show morphologic evidence of carcinomatous transformation, the role of HPV in this process is not vital in most cases. Transcriptionally active HPV may be present in some fully malignant non-keratinizing SCCs of the sinonasal tract with inverted growth, but these carcinomas may arise independently of ISP.
References
Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15:279–97.
Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206.
Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008;2:49–59.
Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic schneiderian papillomas. Laryngoscope. 2002;112:1372–7.
NORRIS HJ. Papillary lesions of the nasal cavity and paranasal sinuses. II. Inverting papillomas. A study of 29 cases. Laryngoscope. 1963;73:1–17.
Ridolfi RL, Lieberman PH, Erlandson RA, et al. Schneiderian papillomas: a clinicopathologic study of 30 cases. Am J Surg Pathol. 1977;1:43–53.
Furuta Y, Shinohara T, Sano K, et al. Molecular pathologic study of human papillomavirus infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope. 1991;101:79–85.
Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105:178–83.
Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–86.
Califano J, Koch W, Sidransky D, et al. Inverted sinonasal papilloma: a molecular genetic appraisal of its putative status as a precursor to squamous cell carcinoma. Am J Pathol. 2000;156:333–7.
Udager AM, Rolland DC, McHugh JB, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75:2600–6.
Syrjanen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174–81.
Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope. 2013;123:181–92.
Shah AA, Evans MF, Adamson CS, et al. HPV DNA is associated with a subset of Schneiderian papillomas but does not correlate with p16(INK4a) immunoreactivity. Head Neck Pathol. 2010;4:106–12.
Scheel A, Lin GC, McHugh JB, et al. Human papillomavirus infection and biomarkers in sinonasal inverted papillomas: clinical significance and molecular mechanisms. Int Forum Allergy Rhinol. 2015;5:701–7.
Ogura H, Fukushima K, Watanabe S. A high prevalence of human papillomavirus DNA in recurrent nasal papillomas. J Med Microbiol. 1996;45:162–6.
McKay SP, Grégoire L, Lonardo F, et al. Human papillomavirus (HPV) transcripts in malignant inverted papilloma are from integrated HPV DNA. Laryngoscope. 2005;115:1428–31.
Lewis JS, Westra WH, Thompson LD, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014;8:241–9.
Justice JM, Davis KM, Saenz DA, et al. Evidence that human papillomavirus causes inverted papilloma is sparse. Int Forum Allergy Rhinol. 2014;4:995–1001.
Jenko K, Kocjan B, Zidar N, et al. In inverted papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Arch. 2011;459:529–38.
Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–92.
Beck JC, McClatchey KD, Lesperance MM, et al. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:49–55.
Weiner JS, Sherris D, Kasperbauer J, et al. Relationship of human papillomavirus to Schneiderian papillomas. Laryngoscope. 1999;109:21–6.
Weber RS, Shillitoe EJ, Robbins KT, et al. Prevalence of human papillomavirus in inverted nasal papillomas. Arch Otolaryngol Head Neck Surg. 1988;114:23–6.
Tang AC, Grignon DJ, MacRae DL. The association of human papillomavirus with Schneiderian papillomas: a DNA in situ hybridization study. J Otolaryngol. 1994;23:292–7.
McLachlin CM, Kandel RA, Colgan TJ, et al. Prevalence of human papillomavirus in sinonasal papillomas: a study using polymerase chain reaction and in situ hybridization. Mod Pathol. 1992;5:406–9.
Kraft M, Simmen D, Casas R, et al. Significance of human papillomavirus in sinonasal papillomas. J Laryngol Otol. 2001;115:709–14.
Kim JY, Yoon JK, Citardi MJ, et al. The prevalence of human papilloma virus infection in sinonasal inverted papilloma specimens classified by histological grade. Am J Rhinol. 2007;21:664–9.
Katori H, Nozawat A, Tsukuda M. Relationship between p21 and p53 expression, human papilloma virus infection and malignant transformation in sinonasal-inverted papilloma. Clin Oncol (R Coll Radiol). 2006;18:300–5.
Kashima HK, Kessis T, Hruban RH, et al. Human papillomavirus in sinonasal papillomas and squamous cell carcinoma. Laryngoscope. 1992;102:973–6.
Hoffmann M, Klose N, Gottschlich S, et al. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006;239:64–70.
Gaffey MJ, Frierson HF, Weiss LM, et al. Human papillomavirus and Epstein-Barr virus in sinonasal Schneiderian papillomas. An in situ hybridization and polymerase chain reaction study. Am J Clin Pathol. 1996;106:475–82
Cheung FM, Lau TW, Cheung LK, et al. Schneiderian papillomas and carcinomas: a retrospective study with special reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J. 2010;89:E5–12.
Buchwald C, Lindeberg H, Pedersen BL, et al. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish epidemiological study 1980–1998. Laryngoscope. 2001;111:1104–10.
Schwerer MJ, Sailer A, Kraft K, et al. Patterns of p21(waf1/cip1) expression in non-papillomatous nasal mucosa, endophytic sinonasal papillomas, and associated carcinomas. J Clin Pathol. 2001;54:871–6.
Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50.
Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9.
Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–9.
Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–82.
Kerr DA, Arora KS, Mahadevan KK, et al. Performance of a branch chain RNA in situ hybridization assay for the detection of high-risk human papillomavirus in head and neck squamous cell carcinoma. Am J Surg Pathol. 2015;39:1643–52.
Mirghani H, Casiraghi O, Amen F, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol. 2015;28:1518–27.
Rooper LM, Gandhi M, Bishop JA, et al. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:11–6.
Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–73.
Grønhøj Larsen C, Gyldenløve M, Jensen DH, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer. 2014;110:1587–94.
Ash JE, Beck MR, Wilkes JD. Tumors of the upper respiratory tract and ear. Washington, DC: Armed Forces Institute of Pathology; 1964.
Hyams VJ, Batsakis JG, Michaels L, et al. Tumors of the upper respiratory tract and ear. Washington, D.C.: Armed Forces Institute of Pathology : Supt. of Docs., U.S. G.P.O. For sale by the Armed Forces Institute of Pathology; 1988.
El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–72.
Funding
This study was funded by the National Institutes of Dental and Craniofacial Research (R01 DE013152-11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rooper, L.M., Bishop, J.A. & Westra, W.H. Transcriptionally Active High-Risk Human Papillomavirus is Not a Common Etiologic Agent in the Malignant Transformation of Inverted Schneiderian Papillomas. Head and Neck Pathol 11, 346–353 (2017). https://doi.org/10.1007/s12105-017-0779-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-017-0779-0