Abstract
Most nasopharyngeal carcinomas (NPCs) in a high-incidence population are driven by Epstein–Barr virus (EBV) infection. EBV-associated malignancies have increased expression of the programmed death-ligand 1 (PD-L1). Immunotherapy agents targeting the PD-1/PD-L1 pathway have achieved durable treatment effects in patients with various cancer types including EBV-associated malignancies. In this study, we sought to investigate PD-L1 expression in a cohort of patients with NPCs from the Philippines. Fifty-six NPCs were studied for PD-L1, p16, and DNA mismatch repair (MMR) deficiency by immunohistochemistry. One case with MMR deficiency was also assessed for microsatellite instability (MSI) by polymerase chain reaction. EBV and human papillomavirus (HPV) status were tested by in situ hybridization. All NPCs were p16 negative. Three of the 56 NPCs (5%) were EBV negative (EBV−) and HPV negative, while one NPC (1/56, 2%) was EBV positive and showed MSI (EBV+/MSI). Positive PD-L1 expression (PD-L1+), defined as membranous staining in ≥1% tumor cells, was seen in 64% (36/56) of NPCs. All three EBV− NPCs were PD-L1+ as was the EBV+/MSI NPC. PD-L1+ was seen significantly more often in NPCs from non-smokers than those from smokers (23/28, 82% vs 9/18, 50%; P = 0.047). PD-L1+ was not associated with pT, pN, distant metastasis, or clinical stage (P > 0.05). PD-L1+ was not associated with overall survival (P = 0.473). In summary, our results show frequent PD-L1 expression in NPCs regardless of EBV status and a preferential PD-L1 expression in non-smokers. MSI and HPV positivity are exceedingly rare in NPCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC), a rare disease in Western countries, occurs with a high frequency in southern China and Southeast Asia, Northern Africa, and Alaska [1]. Epstein–Barr virus (EBV) has been implicated in the pathogenesis of most NPCs [2]. Although the 5-year survival rate in early stage disease is over 80% with chemoradiation, the 5-year survival rate in stage IV disease is less than 10% [3], suggesting a need for better treatment strategies.
The programmed death 1 (PD-1)/PD-ligand 1 (PD-L1) immune checkpoint is one of the mechanisms employed by cancer cells to evade T cell mediated endogenous antitumor responses [4–7]. Immunotherapy agents targeting the PD-1/PD-L1 pathway have achieved durable treatment effects in patients with various cancer types including EBV-associated malignancies [5, 6, 8–10]. Positive tumor PD-L1 expression by immunohistochemistry in pre-treatment specimens is thought to be predictive of clinical response [5, 9].
Most NPCs are characterized by numerous transmigrating lymphoid cells. PD-L1 expression was described in other EBV-associated malignancies [11–13] and several studies have reported positive PD-L1 expression in 25–89% of NPCs [14–17]. In contrast to EBV-associated gastric adenocarcinomas in which expression of PD-L1 protein may result from PD-L1 gene amplification [18], NPCs do not have PD-L1 gene copy number change [19]. PD-L1 expression is shown to be associated with either constitutive oncogenic activation mediated by EBV-encoded latent-membrane protein 1, or through interferon-γ signaling pathways [16].
PD-L1 expression has also been associated with overall survival (OS) and disease-free/progression-free survival (DFS/PFS) mostly in Chinese “NPC” patients [15–17]. Fang et al. [16] reported that positive PD-L1 expression in NPCs was not associated with pT, pN, or clinical stage but was an independent prognostic factor for DFS in a patient cohort collected from Guangzhou, China. In contrast, Hsu and colleagues reported that in NPC patients from Taiwan, PD-L1 expression was not associated with either OS or DFS [15]. These results suggest regional/geographic differences in PD-L1 expression in NPCs and, therefore, additional studies on PD-L1 expression in different patient populations are warranted.
In addition, the frequency and clinicopathologic correlates of microsatellite instability (MSI) in NPCs appear to vary with geographic origin of patients, methods, and specific markers used for MSI detection [20–22]. The relationship between and the contribution of MSI, EBV and Human papillomavirus (HPV) to NPC pathogenesis remain unknown. In this study, we retrospectively characterized a cohort of patients with NPCs from the Philippines, studied MSI, EBV and HPV status, PD-L1 expression, and correlated PD-L1 expression with clinical outcome.
Materials and Methods
Case Selection
This study was approved by the Institutional Review Board. Surgical pathology archives of the Institute of Pathology, St. Luke’s Medical Center, Quezon City, Philippines between 2008 and 2011 were searched for consecutive cases of NPCs. Cases with available formalin fixed paraffin embedded (FFPE) tissue blocks were included in this study. Hematoxylin and eosin (H&E) stained slides of each case were reviewed and the corresponding pathologic findings were recorded. These included the histologic classification and the pathologic TNM staging (tumor, lymph node, and distant metastasis) [23]. Corresponding clinical data including patient age, sex, treatment, and follow-up information were collected. Overall survival was the primary endpoint.
Tissue Microarray Construction
Tissue microarrays (TMAs) were constructed by transferring 2 mm cores from each donor block to a blank recipient paraffin block using a manual tissue arrayer (MTA-1; Beecher Instruments, Sun Prairie, WI). Initial DNA mismatch repair (MMR) protein immunohistochemistry, p16 immunohistochemistry, and EBER in situ hybridization (ISH) were performed on TMAs. All negative EBER ISH and loss of MMR proteins were corroborated by repeated studies of whole tissue sections.
Immunohistochemistry
Immunohistochemical labeling was performed on 4 µM sections using the Ventana Benchmark Ultra platform automated stainer (Ventana Medical Systems, Tucson, AZ) according to the manufacturer’s recommendations.
PD-L1 (clone SP263, Ventana) staining was scored similar to those reported previously in other cancer types [4, 5]. In particular, a membranous PD-L1 staining pattern in tumor cells was considered as specific staining (Fig. 1). Positive PD-L1 expression in this study was defined as membranous staining in 1% or more tumor cells. The numbers of cases with membranous PD-L1 staining in 0% of tumor cells, >0% but <1% of tumor cells (0–1%), ≥1% but <5% of tumor cells (1–5%), and ≥5% but <10% of tumor cells (5–10%) were recorded accordingly. For cases with membranous PD-L1 staining in ≥10% of tumor cells, the percentage of tumor cells demonstrating membranous PD-L1 staining was scored in 10% increments.
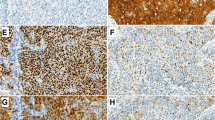
PD-L1 expression in nasopharyngeal carcinomas. PD-L1 stains in EBV-positive (a, c, e) and EBV-negative (b, d, f) nasopharyngeal carcinomas. a and b H&E stains; c and d PD-L1 immunohistochemistry stains; e and f EBER in situ hybridization. PD-L1 stain is diffuse and involves 70–80% of tumor cells in this EBV-positive carcinoma (c). In contrast, PD-L1 staining is seen predominantly at the invasive tumor front/tumor-stroma interface and involves more than 1% but less than 5% of tumor cells (1–5%) in an EBV-negative carcinoma (d). Original magnification: 200×; Scale bar 200 µm
Immunohistochemical stain with antibody against p16 (clone: INK4a, MTM, Heidelberg, Germany) was considered positive when 80% or more tumor cells showed strong diffuse nuclear and cytoplasmic staining.
DNA MMR protein immunohistochemistry stains with antibodies against MLH1 (clone G168-728, Ventana), PMS2 (clone EPR3947, Cell Marque), MSH2 (clone G219-1129, Ventana), and MSH6 (clone 44, Ventana) were interpreted as preserved/retained when nuclear staining was seen in tumor cells. When all four stains showed preserved expression, the tumor was thought to be MMR proficient and microsatellite stable (MSS). When loss of nuclear staining was seen in tumor cells, the tumor was thought to be MMR deficient with microsatellite instability (MSI).
Polymerase Chain Reaction for Microsatellite Instability
For the one NPC with loss of MLH1 and PMS2 expression by immunohistochemistry, tumor and the corresponding normal DNA samples were extracted from FFPE tissue and polymerase chain reactions (PCR) using commercial probes targeting three mononucleotide markers (BAT26, BAT25, and CAT25) and three dinucleotide markers (D5S.346, D17S.250 and D2S.123) were performed on tumor and normal DNA samples to test for MSI as previously described [24].
In Situ Hybridization
All cases were tested for EBV by ISH using pre-diluted probes targeting EBV encoded early RNA (EBER, Ventana) using the Ventana XT automated stainer. All cases tested for EBER were also tested for the integrity of total RNA using RNA control probe and all showed adequate total RNA preservation. EBER ISH was repeated on whole tissue sections for cases that were EBER negative on TMA sections. The three EBV-negative carcinomas were further tested for HPV by ISH as described previously [25].
Statistical Analysis
The IBM SPSS (Release 23.0.0.0) and the GraphPad Prism® 7 for Windows Version 7.00 (GraphPad Software, Inc., La Jolla, CA) were used for statistical analyses. A P-value less than 0.05 (P < 0.05) was considered statistically significant. Tests used included Fisher’s exact test, χ2 test, Mann–Whitney Wilcoxon test, Student’s t-test, and Cox regression analysis. For survival analysis, overall survival was the time between diagnosis and either death or the latest clinical follow-up time.
Results
Clinical and Histopathologic Characteristics
The majority of the 56 NPCs (43, 77%) were diagnosed in men (Table 1). The mean age at diagnosis was 48.7 years (range, 19–71). Almost all patients (54/56, 96%) received chemoradiation treatment. Four of the 56 patients (7%) deceased and 2 patients (4%) had disease recurrence (alive with disease). Status of either tobacco or alcohol usage was known for 46 patients. Among them, 24 (52%) patients consumed neither, while 8 patients (17%) were both smokers and drinkers. Histologically, all 56 NPCs were non-keratinizing squamous cell carcinomas.
HPV, EBV, and MSI in Nasopharyngeal Carcinomas
All 56 NPCs were negative for p16, the surrogate marker for HPV [26]. Fifty-three of the 56 NPCs (95%) were EBV positive (Table 2; Fig. 1). The three EBV-negative NPCs (5%) were also HPV negative by ISH.
Furthermore, 1 EBV-associated NPC (2%) had loss of MLH1 and PMS2 by immunohistochemistry (Table 2; Fig. 2). PCR revealed microsatellite instability at three markers (D5S.346, D17S.250, and BAT26), indicating that the carcinoma was MSI-high (Fig. 3).
EBV-positive and MSI-high nasopharyngeal carcinoma. a H&E stain; b PD-L1 stain. PD-L1 staining is diffuse and involves over 90% of tumor cells. c EBER in situ hybridization; d RNA positive control; e MLH1 loss, immunohistochemistry stain; f PMS2 loss, immunohistochemistry stain; g Preserved MSH2, immunohistochemistry stain; h Preserved MSH6, immunohistochemistry stain. Original magnification: 200×; Scale bar 200 µm
PD-L1 Expression in Nasopharyngeal Carcinomas
Membranous PD-L1 staining was observed in either a large cluster of tumor cells (Fig. 1c) or in tumor cells at the invasive tumor front/tumor-stroma interface (Fig. 1d). Positive PD-L1 expression (PD-L1+) was seen in 36 of the 56 (64%) NPCs (Tables 1, 2 and Supplementary Table 1). In addition, PD-L1 staining in 50% or more of tumor cells was seen in 13 (23%) NPCs, while 4 (7%) carcinomas showed PD-L1 staining in almost all tumor cells (90–100%). All three EBV-negative NPCs were PD-L1+ as was the EBV-positive MSI NPC (Table 2).
When stratified by PD-L1 expression (Table 1), only tobacco use was significantly associated with PD-L1 expression (P = 0.047). The majority (23/28, 82%) of the PD-L1+ NPCs were diagnosed in non-smokers. In contrast, only half (9/18) of PD-L1− carcinomas were diagnosed in non-smokers. No significant associations were found between PD-L1 expression in NPCs and pT, pN, distant metastasis, clinical stage, or alcohol use. Univariate Cox regression analysis showed PD-L1+ was not associated with OS (hazard ratio: 0.488, 95% confidence interval: 0.068–3.474, P = 0.473) (Table 3; Fig. 4).
PD-L1 staining in ≥5% of tumor cells was frequently used to define PD-L1 positivity in retrospective studies [4, 27] as well as clinical studies on PD-1/PD-L1 blockade in other solid tumors [5, 8, 9]. In our cohort, when PD-L1+ was defined as ≥5% of tumor cells with membranous staining, 30 of the 56 (54%) NPC were PD-L1+. PD-L1 positivity (> or equal to 5%) was not associated with smoking, drinking, pT, pN, distant metastasis, or clinical stage (Supplementary Table 2). PD-L1+ with ≥5% cut-off was not associated with OS by univariate Cox regression analysis (hazard ratio: 0.777, 95% confidence interval: 0.109–5.539, P = 0.801) (Table 3). Regression analysis was not performed for DFS because the number of patients with recurrence was too small.
Discussion
In this study, we report that approximately two-thirds of the 56 NPCs in patients from Philippines were PD-L1 positive. Positive PD-L1 was identified in carcinomas across all stages, in both EBV-positive and EBV-negative NPCs and in one carcinoma that was both EBV-positive and MSI. PD-L1 positivity was not associated with regional lymph node or distant metastasis. Although patients with PD-L1 positive NPC had lived longer compared with patients with PD-L1 negative carcinoma, PD-L1 positivity was not a significant predictor of OS.
Positive PD-L1 was significantly more often seen in NPCs from non-smokers. Cigarette smoking has been reported as one of the non-dietary environmental risk factors for NPCs [28]. The exact mechanism of how smoking may affect PD-L1 expression in NPCs is unknown. However, in lung cancer, PD-L1 expression is more often seen in adenocarcinomas from female never/former smokers [29]. In addition, current smoking status was reported to be associated with increased response to anti-PD-1 therapy in non-small cell lung cancer [30]. Cigarette smoking has been shown to cause increased somatic mutation burdens in several cancer types including head and neck squamous cell carcinomas [31]. The association between smoking and PD-L1 in NPCs observed in our cohort should be explored further.
We also report 1 NPC that was both EBV-positive and showed MSI. EBV-positive/MSI NPCs have been reported in patients collected from the Unites States (2/9, 22%) [22] and by Trimeche et al. [20] in patients from Tunisia (8/49, 16%). Current literature suggests that EBV infection is an early event in the pathogenesis of EBV-positive NPCs [2, 32, 33]. An in vitro study with NPC and other epithelial cell lines showed that EBV DNase BGLF5 can induce DNA strand breaks and decrease expression of MMR proteins leading to MSI [34]. The in vitro findings suggest that EBV infection may happen first in the pathogenesis of NPC and MSI can be induced by EBV.
In addition, our results confirm findings of other studies [14–17] that positive PD-L1 expression is frequent in NPCs, but is not associated with clinicopathologic parameters. Although it was previously reported as an independent predictor of survival [16], in our cohort from the Philippines, positive PD-L1 expression was not associated with OS. Compared with the other studies [15–17], fewer patients in our study had disease recurrence/progression and more patients received both chemotherapy and radiation. On the other hand, our cases were a consecutive collection over 3 years and were diagnosed in patients at a wide age range, in both males and females, and across all clinical stages (stages I–IV). Therefore, we believe the cohort is representative of the local patient population. In addition, it is important to note that the PD-L1 monoclonal antibody used in our study is different from antibodies used in previous reports. These studies have used a number of monoclonal antibodies for immunohistochemistry and immunofluorescence and a variety of different scoring schemes/criteria to define positive PD-L1 expression [14–17]. Therefore, a more detailed comparison of our results with those reported in literature is difficult.
Limitations of this study include its retrospective nature and its relatively small sample size from one region of the Philippines. For instance, the patients in this study are all from one island of the Philippines–Luzon—and studies that will include the other regions (e.g., Visayas and Mindanao) may better describe and validate current findings. Possible collaborations with other Southeast Asian countries would result in a more extensive verification of these findings. In addition, since none of the patients in this study received PD-1/PD-L1 blockade treatment, the value of PD-L1 expression in predicting clinical response to such therapy can only be examined in future studies.
In summary, Philippine NPC patients are almost always EBV-positive and HPV-negative. We have reported an association between PD-L1 expression and smoking in a cohort of Philippine patients with NPC. Although it can be seen in both EBV-positive and EBV-negative carcinomas, positive PD-L1 expression is not associated with overall survival. We also report one NPC with both EBV positivity and microsatellite instability.
References
Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):421–9.
Andersson-Anvret M, Forsby N, Klein G, Henle W. Relationship between the Epstein–Barr virus and undifferentiated nasopharyngeal carcinoma: correlated nucleic acid hybridization and histopathological examination. Int J Cancer. 1977;20(4):486–94.
Jin Y, Shi YX, Cai XY, Xia XY, Cai YC, Cao Y, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138(10):1717–25. doi:10.1007/s00432-012-1219-x.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi:10.1126/scitranslmed.3003689.
Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74. doi:10.1158/1078-0432.ccr-13-3271.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi:10.1038/nature13954.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi:10.1038/nrc3239.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi:10.1038/nature14011.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi:10.1056/NEJMoa1200690.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi:10.1056/NEJMoa1411087.
Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8. doi:10.1158/1078-0432.ccr-11-1942.
Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, et al. Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein–Barr virus or microsatellite instability. Am J Surg Pathol. 2016;. doi:10.1097/pas.0000000000000698.
Chang Y-L, Yang C-Y, Lin M-W, Wu C-T, Yang P-C. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: a potential rationale for immunotherapy. Lung Cancer. 2015;88(3):254–9.
Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–73. doi:10.1158/1078-0432.ccr-13-0855.
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23(10):1393–403. doi:10.1038/modpathol.2010.130.
Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189–202. doi:10.18632/oncotarget.2608.
Lee VH, Lo AW, Leung CY, Shek WH, Kwong DL, Lam KO, et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS ONE. 2016;11(6):e0157969. doi:10.1371/journal.pone.0157969.
The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–9. doi:10.1038/nature13480.
Lin DC, Meng X, Hazawa M, Nagata Y, Varela AM, Xu L, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46(8):866–71. doi:10.1038/ng.3006.
Trimeche M, Braham H, Ziadi S, Amara K, Hachana M, Korbi S. Investigation of allelic imbalances on chromosome 3p in nasopharyngeal carcinoma in Tunisia: high frequency of microsatellite instability in patients with early-onset of the disease. Oral Oncol. 2008;44(8):775–83. doi:10.1016/j.oraloncology.2007.10.001.
Lo KW, Teo PM, Hui AB, To KF, Tsang YS, Chan SY, et al. High resolution allelotype of microdissected primary nasopharyngeal carcinoma. Cancer Res. 2000;60(13):3348–53.
Sckolnick J, Murphy J, Hunt JL. Microsatellite instability in nasopharyngeal and lymphoepithelial carcinomas of the head and neck. Am J Surg Pathol. 2006;30(10):1250–3. doi:10.1097/01.pas.0000209829.16607.cd.
Pilch B, Wenig B, Huang D, Lo K, Zeng Y, Jia W. Nasopharyngeal carcinoma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology & genetics of head and neck tumors (IARC WHO classification of tumors). 1st ed. Lyon: World Health Organization; 2005. p. 85–97.
Hartman DJ, Nikiforova MN, Chang DT, Chu E, Bahary N, Brand RE, et al. Signet ring cell colorectal carcinoma: a distinct subset of mucin-poor microsatellite-stable signet ring cell carcinoma associated with dismal prognosis. Am J Surg Pathol. 2013;37(7):969–77. doi:10.1097/PAS.0b013e3182851e2b.
Dogan S, Hedberg ML, Ferris RL, Rath TJ, Assaad AM, Chiosea SI. Human papillomavirus and Epstein–Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck. 2014;36(4):511–6. doi:10.1002/hed.23318.
Lewis JS Jr. p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–82. doi:10.1007/s12105-012-0369-0.
Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031–43. doi:10.1158/0008-5472.can-15-2001.
Yu MC, Garabrant DH, Huang TB, Henderson BE. Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer. 1990;45(6):1033–9.
D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi:10.1038/bjc.2014.555.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. doi:10.1056/NEJMoa1501824.
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. doi:10.1038/nature12477.
Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet (London, England). 2005;365(9476):2041–54. doi:10.1016/s0140-6736(05)66698-6.
Gulley ML. Molecular diagnosis of Epstein–Barr virus-related diseases. J Mol Diagn: JMD. 2001;3(1):1–10. doi:10.1016/s1525-1578(10)60642-3.
Wu C-C, Liu M-T, Chang Y-T, Fang C-Y, Chou S-P, Liao H-W, et al. Epstein–Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010;38(6):1932–49. doi:10.1093/nar/gkp1169.
Acknowledgements
The authors wish to thank members of the Developmental Laboratory of the Department of Pathology, University of Pittsburgh and Histopathology Section of St. Luke’s Medical Center, Quezon City for excellent technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this study have no conflicts of interest with respect to this manuscript to disclose.
Additional information
Ann Margaret V. Chang and Simion I. Chiosea have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, A.M.V., Chiosea, S.I., Altman, A. et al. Programmed Death-Ligand 1 Expression, Microsatellite Instability, Epstein–Barr Virus, and Human Papillomavirus in Nasopharyngeal Carcinomas of Patients from the Philippines. Head and Neck Pathol 11, 203–211 (2017). https://doi.org/10.1007/s12105-016-0765-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-016-0765-y