Abstract
Background and objective
No randomized trial has been reported comparing different chemotherapy regimens on disseminated nasopharyngeal carcinoma (NPC). This study aims to compare five cisplatin-based regimens including cisplatin + 5-fluororacil (PF), paclitaxel + cisplatin (TP), gemcitabine + cisplain (GP), paclitaxel + cisplatin + 5-fluororacil (TPF), and bleomycin + cisplatin + 5-fluororacil (BPF) regimen most frequently used as the first-line protocols for metastatic NPC retrospectively.
Methods
Eight hundred and twenty-two patients with metastatic NPC were divided into five groups according to the regimen they received. Then, their response rate, toxicity, and long-term survival outcome as well as the prognostic factors were analyzed.
Results
The higher response rates in GP and TPF regimens comparing to PF regimen were achieved (Χ 2 = 4.57, P = 0.033; Χ 2 = 7.04, P = 0.008), as well as in TPF regimen comparing to TP regimen (Χ 2 = 5.579, P = 0.018). The occurrence rate of the major III–IV grade toxicity was significantly different between the five groups. However, no statistically significant difference was observed in progression-free survival (PFS; P = 0.247) and overall survival (P = 0.127) among the five groups. Cox multivariate analysis identified the following independent prognostic factors: liver metastases, plasma Epstein Barr Virus (EBV)-DNA level, cycles of chemotherapy, and second-line chemotherapy.
Conclusions
PF, TP, and GP are all effective regimens as the first-line chemotherapy for metastatic NPC, which can be well tolerated. Over four cycles of chemotherapy are recommended under no contraindication. Patients should transfer to the second-line regimen after the treatment failure of the first-line chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a leading cancer in well-defined populations, such as natives of southern China, southeast Asia, the Arctic, the Middle East, and north Africa (Chang and Adami 2006; Yu and Yuan 2002). With the advancement in radiotherapy techniques, regional control rate of NPC has reached greater than 80 % (Kam et al. 2007), even though NPC has the highest propensity to metastasize to distant sites among head and neck cancers. Different reports have shown that about 17–54 % of patients with NPC had failed treatment due to distant metastases and approximately one-third of patients presented disseminated disease at primary diagnosis (Liu et al. 2003; Lee et al. 1992; Chiesa and De Paoli 2001). Hence, systemic disease remains the major cause of death among patients with NPC.
Because of the high chemosensitivity of NPC, the role of chemotherapy in metastatic NPC is well established. The most often used agents for monotherapy include cisplatinum, 5-fluorouracil, bleomycin, paclitaxel/docetaxel, and gemcitabine, with response rates ranging from 15 to 50 % (Ma and Chan 2005; Ciuleanu et al. 2008; Foo et al. 2002; Ngeow et al. 2011). The combination of cisplatinum and 5-fluorouracil (PF) has been one of the oldest protocols explored to treat advanced NPC. Because of its good activity and low toxicity, this regimen remains the standard first-line therapy and one of the most popular choices for metastatic NPC in some Asian areas (Wang and Tan 1991; Schwarz 1996). In recent decades, cisplatin-based poly-chemotherapy has been the focus of researches and the most frequently used protocols in clinical practice including: paclitaxel + cisplatin (TP) regimen, paclitaxel + cisplatin + 5-fluorouracil (TPF) regimen, bleomycin + cisplatin + 5-fluorouracil (BPF) regimen, and gemcitabine + cisplatin (GP) regimen (He et al. 2010; Ma et al. 2002; Su et al. 1993; Bensouda et al. 2011). However, till now, there has been no randomized trial comparing these different chemotherapy protocols head to head. In addition, whether there is survival difference between patients receiving different chemotherapy regimens remains unclear.
To figure out whether one of the regimens is better than another with higher efficacy, lower toxicity, and survival benefit, this retrospective study was designed to compare the response rate, toxicity, and long-term outcome of PF, TP, GP, BPF, and TPF regimens most frequently used as the first-line treatment in patients with metastatic NPC.
Patients and methods
Inclusion criteria and enrollment
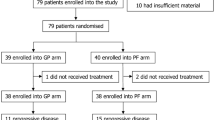
Between January 1999 and December 2005, 1885 patients with metastatic NPC were referred to Sun Yat-Sen University Cancer Center. The inclusion criteria in this study consisted of patients (1) with histological confirmation of World Health Organization (WHO) type II (non-keratinising carcinoma) or WHO type III (undifferentiated carcinoma) NPC; (2) with radiological confirmation of distant metastatic lesion(s); (3) with at least one radiologically measurable lesion; (4) with age ranging from 18 to 70 years; (5) with good performance status before treatment (ECOG < 2); (6) with normal renal, cardiac, and liver function; (7) received one of the five regimens (PF, TP, TPF, BPF, and GP) as the first-line chemotherapy for metastatic disease; and (8) with complete clinical data. Exclusion criteria in this study consisted of patients (1) with other types of malignancy; (2) with brain metastases; (3) with bone-alone metastasis; (4) received chemotherapy within half a year before distant metastases diagnosed; (5) received radiotherapy for the metastasis. Finally, eight hundred and twenty-two patients were eligible to be included in this study. The study was approved by the Research Ethics Committee of the Sun Yat-Sen University Cancer Center.
Treatment schedule
The patients were treated with one of the five regimens that were most frequently used as the first-line chemotherapy of metastatic NPC in our center. The five different regimens were administered every 21 days as follows:
PF regimen: 5-FU was administered at a dose of 1,000 mg/m2 by intravenous (i.v) infusion daily on days 1 to 5 or continuously intravenous (civ) infusion for 120 h and iv infusion of cisplatin at a dose of 80 mg/m2 in divided doses on days 1–3.
TP regimen: paclitaxel was administered by iv infusion at a dose of 175 mg/m2 on day 1 and iv infusion of cisplatin at a dose of 80 mg/m2 in divided doses on days 1–3.
GP regimen: gemcitabine was administered at a dose of 1,000 mg/m2 by iv infusion on days 1, 8 and iv infusion of cisplatin at a dose of 80 mg/m2 in divided doses on days 1–3.
BPF regimen: bleomycin was administered by intramuscular injection at a dose of 15 mg, twice per week; cisplatin was administered by iv infusion at a dose of 75 mg/m2 in divided doses on days 1–3 and iv infusion of 5-FU at a dose of 750 mg/m2 daily on days 1–5.
TPF regimen: paclitaxel was administered by iv infusion at a dose of 175 mg/m2 on day 1; cisplatin was administered by iv infusion at a dose of 75 mg/m2 in divided doses on days 1–3 and iv infusion of 5-FU at a dose of 750 mg/m2 daily on days 1–5.
The routine prophylactic treatment such as antiemetic drugs (five serotonin receptor blockers) and antiallergy drugs (dexamethasone, cimetidine, and diphenhydramine) was carried out before chemotherapy as inpatient setting. Supportive measures such as growth factors were used as outpatient setting when necessary. The dose of drugs was adjusted according to the grade of toxicity under patients’ tolerance. Treatment discontinuation occurred for disease progression or unacceptable drug toxicity.
Evaluation protocol
Patients were evaluated for response every two treatment cycles during treatment and then every 2 months after treatment. The response evaluation of the tumor to therapy was based on computed tomography (CT) or magnetic resonance imaging (MRI) scan. The short-term efficacy, based on the response evaluation criteria in solid tumors (RECIST), was assessed as CR, PR, SD, and PD. CR and PR were regarded as response to treatment. WHO grading for various toxicity was recorded in every cycle. The long-term efficacy was evaluated according to the progression-free survival (PFS) and overall survival (OS). PFS was defined as the duration from the first day of beginning treatment to disease progression (newly occurring metastatic lesion, recurrence or expansion of the primary lesion) by radiological confirmation. OS was calculated from the first day of beginning treatment to death from cancer and treatment-related toxicity. Patients who were still alive till the last follow-up were recorded as censored.
Metastases onset was defined as patients who presented with distant metastasis while first diagnosed of NPC. It was analyzed as a separate category from patients who presented with localized disease, but developed metastases at a later date in the multivariate analysis. Disease-free interval (DFI) was defined as the time from the onset of primary radiotherapy to the time of relapse or metastases in patients who achieved complete response.
Statistical analysis
Statistical analysis was performed using SPSS17.0 package. Survivals were analyzed using the Kaplan–Meier method and were compared using the log-rank test. Clinical characteristic among the five groups was analyzed using the Kruskal–Wallis H test. Short-term treatment efficacy and toxicity were analyzed using the chi-square test and Kruskal–Wallis H test. Univariate and multivariable analyses were performed using the Cox proportion hazards model. Statistical significance was defined as when P < 0.05 by the two-tailed test.
Results
Descriptive characteristics
Among the 822 patients included in our study, 176 patients received PF regimen, 167 patients received TP regimen, 173 patients received GP regimen, 152 patients received BPF regimen, and 154 patients received TPF regimen. Clinical characteristics of the study patients are listed in Table 1. The patients were male predominance (83.2 %), and the mean age of the patients was 45.2 years old (ranging from 18 to 70 years). The mean DFI was 15.8 months (ranging from 0 to 29.8 months). About one-third of patients had distant metastasis at onset. The metastatic sites included lung, liver, bone, and distant lymph node; among them, bone is the most common site. More than half of the patients received over 4 cycles of the first-line chemotherapy. The mean cycles of PF regimen, TP regimen, TPF regimen, BPF regimen, and GP regimen were 4.5 (ranging from 1 to 8 cycles), 4.1 (ranging from 1 to 12 cycles), 3.9 (ranging from 1 to 8 cycles), 4.4 (ranging from 1 to 12 cycles), and 4.0 (ranging from 1 to 12 cycles), respectively.
Short-term treatment efficacy
Short-term efficacy of the 822 patients was listed in Table 2. The response rates of PF, TP, GP, BPF, and TPF regimen were 60.2, 61.7, 71.1, 69.1, and 74 %, respectively. The higher response rates in GP and TPF regimens comparing to PF regimen were statistically significant (Χ 2 = 4.57, P = 0.033; Χ 2 = 7.04, P = 0.008). The higher response rate in TPF regimen comparing to TP regimen was also statistically significant (Χ 2 = 5.579, P = 0.018). There were no significant differences of response rates between any other two regimens.
Long-term treatment efficacy
Till March 30, 2011, 714 patients’ overall survival data were obtained and 108 patients’ data were censored. The median follow-up was 27.1 months (ranging from 1 to 144 months). It is worth noting that the overall survival rates, median PFS, and median OS were similar among the five groups (Table 3). In addition, the survival curves of the five groups were overlapping each other, which means there were no statistically significant difference between PFS (P = 0.247) and OS (P = 0.127) among the five groups (Fig. 1a, b).
a Kaplan–Meier progression-free survival (PFS) curves of 822 patients with metastatic NPC treated with PF, TP, GP, BPF, or TPF regimen. Median PFS ± SE (95 % CI) was 5.0 ± 0.6 (3.9–6.1) months in the PF group, 5.5 ± 0.5 (4.4–6.6) months in the TP group, 6.6 ± 0.6 (5.4–7.8) months in the GP group, 5.5 ± 0.6 (4.4–6.6) months in the BPF group, and 6.0 ± 0.4 (5.1–6.9) months in the TPF group. P = 0.247. b Kaplan–Meier overall survival (OS) curves of 822 patients with metastatic NPC treated with PF, TP, GP, BPF, or TPF regimen. Median OS ± SE (95 % CI) was 19.5 ± 1.2 (17.1–21.9) months in the PF group, 21.0 ± 1.5 (18.1–23.9) months in the TP group, 21.5 ± 1.4 (18.7–24.3) months in the GP group, 19.0 ± 1.3 (16.5–21.5) months in the BPF group, and 21.0 ± 1.6 (18.0–24.0) months in the TPF group. P = 0.127
Chemotherapy toxicity
The main grade 3/4 toxicity of them was listed in Table 4. Generally speaking, grade 3/4 neutropenia was the most common adverse effect occurred in the five groups to different degrees. Patients received growth factors for support as outpatient setting were 31 % in PF regimen group, 45 % in TP regimen group, 73 % in TPF regimen group, 58 % in BPF regimen group, and 64 % in GP regimen group, respectively. Grade 3/4 nausea/vomiting was also one of the most common toxicity occurred in the five groups more or less. Grade 3/4 thrombocytopenia was observed mainly in TPF and GP regimens. About 10 % of patients had grade 3/4 neuropathy and oropharyngeal mucositis in TPF regimen. PF regimen and BPF regimen also caused about 5 % grade 3/4 oropharyngeal mucositis. TPF regimen brought 8 % grade 3/4 infections caused by neutropenia. There were some cases having grade 3/4 allergic reaction in TP, TPF, and BPF regimens. Two percent of grade 3/4 pulmonary fibrosis was observed in BPF regimen. Other grade 3/4 toxicity was not common. There were 3 % treatment-related mortalities in TPF regimen group and none in other groups.
Prognostic factors for overall survival
Factors that were considered for analysis included patients, factors (age group, gender), disease factors (whether metastases onset, specific metastatic sites, DFI, plasma EBV-DNA level), and treatment factors (drug number and cycle of the first-line chemotherapy, whether the first-line chemotherapy included paclitaxel or 5-FU or gemcitabine, whether received the second-line chemotherapy). In order to not to miss potentially important prognostic factors, P ≤ 0.1 was used as the cut-off value of statistical significance for variable selection in the univariable modeling. Statistically significant, liver metastases (HR = 1.266, P = 0.008), high plasma EBV-DNA level (HR = 1.419, P < 0.001), metastases onset (HR = 1.299, P = 0.052) were negative prognostic factors. The results also show that cycles of the first-line chemotherapy ≥4 (HR = 0.726, P < 0.001) received gemcitabine (HR = 0.724, P = 0.069) and received the second-line chemotherapy after progression (HR = 0.714, P < 0.001) were associated with good prognosis.
The Cox multivariate analysis indentified that liver metastases (HR = 1.243, P = 0.006), high plasma EBV-DNA level (HR = 1.435, P < 0.001), received second-line chemotherapy (HR = 0.744, P < 0.001), and more than four cycles of the first-line chemotherapy (HR = 0.738, P < 0.001) were independent positive prognostic factors for overall survival (Table 5).
Subgroup analysis
Since the Cox analysis showed drug number of the first-line chemotherapy, if the first-line chemotherapy included paclitaxel or 5-FU or gemcitabine was not correlated with OS outcome, we further divided the patients into four subgroups as follows and then compared their survivals using the log-rank test. In the first subgroup, patients were divided into two groups: one with two-drug combination and the other with three-drug combination as the first-line chemotherapy. There are no significant differences of OS between these two groups (P = 0.327; Fig. 2a). In the second subgroup, patients were divided into two groups: one with paclitaxel-included regimen and the other with paclitaxel-free regimen as the first-line chemotherapy. There are no significant differences of OS between these two groups (P = 0.459; Fig. 2b). In the third subgroup, patients were divided into two groups: one with 5-FU-included regimen and the other with 5-FU-free regimen as the first-line chemotherapy. There are no significant differences of OS between these two groups (P = 0.736; Fig. 2c). In the fourth subgroup, patients were divided into two groups: one with gemcitabine-included regimen and the other with gemcitabine-free regimen as the first-line chemotherapy. There are no significant differences of OS between these two groups (P = 0.511; Fig. 2d).
a Kaplan–Meier overall survival (OS) curves of 822 patients with metastatic NPC treated with two-drug or three-drug combined regimen. Median OS ± SE (95 % CI) was 20.5 ± 0.7 (19.1–21.9) months in the two-drug combined group and 20.5 ± 1.0 (18.6–22.4) months in the three-drug combined group. P = 0.327. b Kaplan–Meier overall survival (OS) curves of 822 patients with metastatic NPC treated with paclitaxel-included or paclitaxel-free regimen. Median OS ± SE (95 % CI) was 21.5 ± 0.8 (19.8–23.2) months in the paclitaxel-included group, 19.5 ± 0.8 (17.9–21.1) months in the paclitaxel-free group. P = 0.459. c Kaplan–Meier overall survival (OS) curves of 822 patients with metastatic NPC treated with 5-FU-included or 5-FU-free regimen. Median OS ± SE (95 % CI) was 20.0 ± 0.8 (18.4–21.6) months in the 5-FU-included group and 21.0 ± 0.9 (19.2–22.8) months in the 5-FU-free group. P = 0.736. d Kaplan–Meier overall survival (OS) curves of 822 patients with metastatic NPC treated with gemcitabine-included or gemcitabine-free regimen. Median OS ± SE (95 % CI) was 21.0 ± 1.6 (18.0–24.0) months in the gemcitabine-included group and 20.0 ± 0.8 (18.5–21.5) months in the gemcitabine-free group. P = 0.511
Discussion
More than half of patients with NPC eventually had failed treatment due to distant metastases (Sakata et al. 1994). The presence of distant metastases in patients with NPC is the most important factor limiting survival (Tao et al. 2008). Current management of metastatic disease from NPC is based essentially on cisplatin-included palliative chemotherapy (Azli et al. 1995). The combination of cisplatin and 5-FU as the oldest and most frequently adopted protocol, giving response rate about 60 %, remains the standard first-line protocol in several centers in Asia (Au and Ang 1994) In recent studies and clinical practice, other molecules such as taxanes, gemicitabine, capecitabine, and bleomycin has also been tested to combine with platinum as the first-line chemotherapy for metastatic NPC, which obtained good results (Au and Ang 1994; Ciuleanu et al. 2004; Chua et al. 2008). However, clinicians are still confused about whether one protocol is better than another and whether three-drug combination is better than two-drug combination on long-term outcome of the patients due to the lack of reliable data. To our knowledge, this was the first research to explore the short-term efficacy, toxicity and especially the long-term treatment outcome over 5 years among five different protocols most frequently used as the first-line regimen in disseminated NPC. Our retrospective analysis included a relatively large number of patients with complete follow-up data. We believe that the results demonstrated may provide some useful reference for treatment option.
The results of short-term treatment efficacy in this study were in accordance with those from reports of clinical trials as follows: Ngan et al. (2002)reported that combination of gemcitabine and cisplatin had achieved an overall response rate of 73 %; Boussen et al. (1991) reported that combination of cisplatin, bleomycin, and 5-FU had received an overall response rate of 79 %; Lin et al. (2012) reported that combination of docetaxel, cisplatin, and 5-FU had achieved an overall response rate of 65 %. Our results showed that the higher response rates were observed in GP and TPF regimens comparing to PF regimen. However, along with TPF regimen, there were more serious bone marrow suppression, infection, and oropharyngeal mucositis. And along with GP regimen, there were more serious bone marrow suppression but less serious oropharyngeal mucositis. In addition, TPF regimen obtained higher response rate than TP regimen, also along with more serious marrow suppression, infection, and oropharyngeal mucositis. Response rates between any other two regimens had no statistically significant differences. Notably, the short-term treatment advantage of TPF and GP regimens did not change into long-term survival advantage at last. Under over 5-year follow-up, the results showed that TPF and GP regimens had not brought survival benefit comparing to any other regimen, even they achieved better response rates. Part of the reason may be that the serious toxicity of these regimens resulting in the cycle reduction. As we described earlier, the mean cycle of TPF and GP patients received was less than that of other regimens in this study. Since cycle of chemotherapy was one of the independent prognostic factors on survival outcome as the Cox model analyzed, less cycles of TPF and GP regimens patients received may explain why the short-term advantage of these regimens did not change into long-term advantage on OS compared with other regimens. In addition, there were 3 % treatment-related mortalities in TPF regimen group and none in other groups, which could also explain this phenomenon.
The Cox proportional hazard regression model revealed that liver metastases was an independent negative prognostic factor, and Ong et al. (2003) has designed a prognostic index score for metastatic NPC, which showed that liver metastasis was a negative prognostic factor, whose result was consistent with ours. In addition, as An et al. (2011) reported, our results also showed that high plasma EBV-DNA level (>1 × 103 copies/ml) was an independent negative prognostic factor for survival outcome of disseminated NPC. This study for the first time showed that receiving more than four cycles of the first-line chemotherapy under well tolerance may bring survival advantage to patients with metastatic NPC. What is more, compared to supporting treatment, receiving the second-line chemotherapy may bring survival benefit for patients with disease progression.
The Cox regression model as well as the subgroup analyses showed that three-drug combined chemotherapy brought no benefit on OS than two-drug combined chemotherapy. Moreover, chemotherapy including paclitaxel/5-FU/gemcitabine or not had no statistical differences on survival outcomes. Since three-drug combined regimens brought more toxicity, cisplatin-based doublets may be the more appropriate choice as the first-line treatment for patients with metastatic NPC. Both the old drug 5-FU and the new drugs paclitaxel/gemicitabine were available with defect of each ones. Toxicity was as expected. The major grade 3/4 toxicity of 5-FU-included regimens was neutropenia and oropharyngeal mucositis. The major grade 3/4 toxicity of paclitaxel-included regimens was neuropathy and bone marrow suppression. And the major grade 3/4 toxicity of gemcitabine-included regimen was thrombocytopenia.
This study had several limitations. First, some drugs such as capecitabine and vinorelbine that have been approved effective for metastatic NPC were not discussed in this study. Second, our patients were restricted to one local hospital. A larger, multicentre design will be needed in further study. We anticipate randomized clinical trials will establish optimal choice for patients with disseminated NPC in the near future.
In conclusion, physicians should always weigh the risk and benefit when choosing treatment protocols for patients. Cisplatin-based doublets such as PF, TP, and GP are all effective regimens used as the first-line chemotherapy for patients with distant NPC which can be well tolerated. Cisplatin-based triplets such as TPF and BPF regimen are not commended owing to the more serious toxicity without survival benefit. More than four cycles of the first-line chemotherapy is suggested when there is no contraindication. Receiving the second-line chemotherapy is better than supportive treatment when the first-line chemotherapy has failed.
References
An X, Wang FH, Ding PR et al (2011) Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer 117:3750–3757
Au E, Ang PT (1994) A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 5(1):87–89
Azli N, Fandi A, Bachouchi M et al (1995) Final report of a phase II study of chemotherapy with bleomycin, epirubicin, and cisplatin for locally advanced and metastatic/recurrent undifferentiated carcinoma of the nasopharyngeal type. Cancer J Sci Am 1(3):222–229
Bensouda Y, Kaikani W, Ahbeddou N et al (2011) Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis 128(2):79–85
Boussen H, Cvitkovic E, Wendling JL et al (1991) Chemotherapy of metastatic and/or recurrent undifferentiated nasopharyngeal carcinoma with cisplatin, bleomycin, and fluorouracil. J Clin Oncol 9(9):1675–1681
Chang ET, Adami HO (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15(10):1765–1777
Chiesa F, De Paoli F (2001) Distant metastases from nasopharyngeal cancer. ORL J Otorhinolaryngol Relat Spec 63(4):214–216
Chua DT, Wei WI, Wong MP, Sham JS, Nicholls J, Au GK (2008) Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head Neck 30(7):863–867
Ciuleanu TE, Fountzilas G, Ciuleanu E, Plataniotis M, Todor N, Ghilezan N (2004) Paclitaxel and carboplatin in relapsed or metastatic nasopharyngeal carcinoma: a multicenter phase II study. J BUON 9(2):161–165
Ciuleanu E, Irimie A, Ciuleanu TE, Popita V, Todor N, Ghilezan N (2008) Capecitabine as salvage treatment in relapsed nasopharyngeal carcinoma: a phase II study. J BUON 13(1):37–42
Foo KF, Tan EH, Leong SS et al (2002) Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol 13(1):150–156
He XY, Hu CS, Ying HM, Wu YR, Zhu GP, Liu TF (2010) Paclitaxel with cisplatin in concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 267(5):773–778
Kam MK, Leung SF, Zee B et al (2007) Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 25(31):4873–4879
Lee AW, Poon YF, Foo W et al (1992) Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 23(2):261–270
Lin JT, Lai GM, Chang TH et al (2012) Chemotherapy with modified docetaxel, cisplatin, and 5-Fluorouracil in patients with metastatic head and neck cancer. Adv Ther 29(1):71–77
Liu MT, Hsieh CY, Chang TH, Lin JP, Huang CC, Wang AY (2003) Prognostic factors affecting the outcome of nasopharyngeal carcinoma. Jpn J Clin Oncol 33(10):501–508
Ma BB, Chan AT (2005) Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer 103(1):22–31
Ma BB, Tannock IF, Pond GR, Edmonds MR, Siu LL (2002) Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer 95(12):2516–2523
Ngan RK, Yiu HH, Lau WH et al (2002) Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol 13(8):1252–1258
Ngeow J, Lim WT, Leong SS et al (2011) Docetaxel is effective in heavily pretreated patients with disseminated nasopharyngeal carcinoma. Ann Oncol 22(3):718–722
Ong YK, Heng DM, Chung B et al (2003) Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer 39(11):1535–1541
Sakata K, Aoki Y, Karasawa K et al (1994) Wide variation of probability of local failure and distant metastasis among various stages of patients with nasopharyngeal carcinoma. Strahlenther Onkol 170(4):218–224
Schwarz LR (1996) Elimination of dose limiting toxicities of cisplatin, 5-fluorouracil, and leucovorin using a weekly 24-hour infusion schedule for the treatment of patients with nasopharyngeal carcinoma. Cancer 78(3):566–567
Su WC, Chen TY, Kao RH, Tsao CJ (1993) Chemotherapy with cisplatin and continuous infusion of 5-fluorouracil and bleomycin for recurrent and metastatic nasopharyngeal carcinoma in Taiwan. Oncology 50(4):205–208
Tao Y, Bidault F, Bosq J, Bourhis J (2008) Distant metastasis of undifferentiated carcinoma of nasopharyngeal type. Onkologie 31(11):574–575
Wang TL, Tan YO (1991) Cisplatin and 5-fluorouracil continuous infusion for metastatic nasopharyngeal carcinoma. Ann Acad Med Singap 20(5):601–603
Yu MC, Yuan JM (2002) Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12(6):421–429
Acknowledgments
This work was supported by the National—Eleventh Five Technology Major Project [2008ZX09312-002]; and the Research Award Fund for Outstanding Young Researchers in Sun Yat-sen Cancer Center. Sponsors of the study supported the fees to data collecting and will support the publication of the paper.
Conflict of interest
All authors state that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiu-Yu Cai, Ying Jin contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1007/s00432-015-1926-1.
Rights and permissions
About this article
Cite this article
Jin, Y., Cai, XY., Shi, YX. et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol 138, 1717–1725 (2012). https://doi.org/10.1007/s00432-012-1219-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-012-1219-x