Abstract
Acetylated tubulin (AT) expression has been proposed as a marker for sensitivity to taxane chemotherapy. We wanted to explore AT as a prognostic marker in squamous cell carcinoma of the head and neck (SCCHN). We assessed AT expression in archival tissue from our institutional tissue bank of primary SCCHN specimens. We also examined AT expression on pre-therapy tissues of patients with SCCHN receiving induction chemotherapy with docetaxel, cisplatin and 5FU (TPF IC). AT expression was assessed on archival cases of SCCHN with (N = 63) and without (N = 82) locoregional lymph node metastases (LNM). The predominant tumor site was oral cavity (52 %). Immunohistochemistry staining was based on staining intensity and percentage of tumor cells stained to create a weighted index (WI). A total of nine patients who received TPF IC were evaluable for response by RECIST and also had pre-therapy tissues available. A significant independent correlation between AT and tumor grade (p = 0.001) and primary location (p = 0.008) was noted. There was a trend of higher AT in patients with presence of LNM (p = 0.052) and a trend in improved OS for patients with an AT WI below the median compared to those above the median for patients with no LNM (p = 0.054). For patients treated with induction TPF, we observed an inverse correlation between AT expression and response to TPF IC (p = 0.0071). AT expression is correlated with tumor grade and primary site. There was an observed trend correlating AT with presence nodal metastases. The observed inverse correlation with response to taxane based chemotherapy needs validation in a larger sample size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 500,000 people worldwide are diagnosed with squamous cell carcinoma of the head and neck (SCCHN) each year. Although one accepted standard of care for locally advanced SCCHN is upfront concurrent chemotherapy with cisplatin and radiation, also known as concomitant chemoradiotherapy (CRT). Several novel approaches have emerged over the past decade, including a focus on the sequential application of non-surgical management methods, namely induction chemotherapy (IC) followed by CRT (sequential therapy). In the MACH 2000 meta-analysis, the clinical trials which included IC seemed to offer an advantage over radiation alone with a absolute percentage of survival improvement of approximately 5 %; this however was with non-taxane-based IC regimens [1, 2]. Since then, randomized clinical trials comparing IC with a cisplatin and 5-fluorouracil (5-FU) doublet, i.e., PF, and a triplet regimen containing a taxane (docetaxel or paclitaxel) with PF (TPF), have led to the adoption of TPF as the IC regimen of choice for locally advanced SCCHN [3, 4]. The above notwithstanding, the question of when to use IC remains unanswered by clinical trials. Two large multinational trials led by major SCCHN referral centers in the US were recently completed. Final efficacy and toxicity results were presented at the American Society of Clinical Oncology (ASCO) 2012 meeting, and did not reveal any statistically significant superiority in overall survival (OS) between IC followed by CRT (sequential therapy) over the current standard of care of upfront cisplatin-CRT. These data raise the question of whether or not improved patient selection tools might be required to justify the sequential approach [5, 6].
Acetylated tubulin (AT) expression has been suggested as a prognostic marker in epithelial malignancies and also as a marker for sensitivity to chemotherapy [7–9]. In the laboratory, the expression of AT is detected through the use of routine immunohistochemistry (IHC). AT is a cytoplasmic stain, which reflects the location of the microtubules within the cell. In this study, we aimed to explore a possible correlation between AT expression (by immunohistochemistry) and other markers of disease biological aggressiveness, such as primary tumor histologic grade, the presence of locoregional LNM, response to TPF IC, and OS in SCCHN.
Methods
We assessed AT expression on pre-therapy biopsy specimens of 9 patients with locally advanced SCCHN treated with TPF IC and whose disease was evaluable for clinicoradiologic response after 3 cycles of TPF. All patients had uni-or bi-dimensional measurable lesions. Their characteristics are summarized in Table 1. We also studied archival tissue specimens from primary SCCHN cases with (63 cases) and without (82 cases) LNM. Clinical characteristics of patients were retrieved from the Department of Pathology database. Importantly, none of these patients (whose archival specimens were available for analysis) had distant metastases at the time of clinical diagnosis and staging that led to their inclusion in the institutional database. Both analyses were performed after review and approval of the Emory institutional review board.
In order to estimate relationships between AT tissue expression level as measured by the IHC weighted index (WI) (defined in a subsequent section titled “Acetylated tubulin Assay”) and the presence of LNM, primary tumor location, grade, and clinical stage in the retrospective samples, we used Wilcoxon two-sample and Kruskal–Wallis tests. Log-rank test was used to examine the difference in OS and disease free survival (DFS) between pairs of groups based on whether WI was above versus below the median value for this parameter. Similar analyses for OS and DFS were performed in sets of four groups based on WI quartiles. (See following section describing “Acetylated tubulin Assay” for further details). A cox proportional hazard model was employed to estimate the adjusted effect of WI on OS and DFS.
Acetylated Tubulin Assay
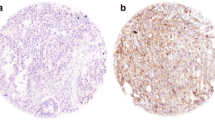
We examined the level of AT in paraffin-embedded tissue of biopsies (Fig. 1). Immunohistochemistry (Dako North America, Carpinteria, CA) was performed using a DAKO Autostainer on formalin-fixed, paraffin-embedded tissue sections at the Winship Cancer Institute Pathology Core facility. Samples were de-paraffinized, subjected to antigen retrieval and incubated in a 1:500 dilution of monoclonal anti-AT antibody (Sigma catalog #T6793; Sigma-Aldrich, St. Louis, MO) for 40 min. Sections were counter-stained with hematoxylin. Mouse IgG was used as a negative control.
IHC staining for cytoplasmic AT was scored based on intensity and percentage of tumor cells stained: 0 = no staining, 1+ = weak, 2+ = moderate, 3+ = moderate to high, 4+ = high. For the retrospective samples a WI was calculated as the product of percent IHC stained cells X stain intensity.
Statistical Analysis
Response to TPF IC was assessed using the percentage change of tumor based on RECIST criteria [10]. The relationship between response to TPF and AT staining intensity was tested for significance with the Spearman’s coefficient method.
For the archival tissue analysis, patients’ characteristics were summarized and compared between patients with and without LNM. WI was treated as a continuous variable.
Wilcoxon rank-sum test and Kruskal–Wallis test were used to estimate the relationships of stain intensity indices (WI) with the presence versus absence of LNM, as well as node status, and primary site of disease, tumor grade, and tumor stage. OS was measured as the time from date of diagnosis to death or last contact. DFS was measured as the time from date of diagnosis to last date without clinicoradiologically evident disease or last contact whichever comes first. The survivorship functions for both OS and DFS were estimated by the Kaplan–Meier method. The log-rank test was used to test the difference in OS or DFS between different pairs of groups stratified by various tested factors. A Cox proportional hazards model [11] was used to estimate the effect of WI upon OS and DFS. The significance level for all comparisons was set at 0.05. The SAS statistical package v9.2 (SAS Institute, Inc., Cary, NC) was used for data management and analyses.
Results
For patients treated with TPF IC, we observed a significant inverse correlation between response rate to 3 cycles of TPF and AT staining. Patients with high AT expression demonstrated a lower tumor response to TPF IC compared to patients with low AT expression levels (p = 0.0071) (Fig. 2).
In the archival tissue analysis, for each of the two groups, namely those patients with versus without LNM, demographics, treatment and staging information are summarized in Table 2. There were significant differences in primary tumor site, clinical stage, histologic grade, and the administration of chemotherapy between patients with versus without LNM (p < 0.001 for primary tumor site, stage, and grade; and p = 0.001 for chemotherapy use). Patients who lacked evidence of LNM mainly harbored primaries in the oral cavity, while tumor primary organ location was fairly evenly distributed for patients who had LNM.
There was a statistically significant correlation between AT expression (as assessed by WI) and tumor grade (p = 0.026) with well-differentiated SCCHN tumors demonstrating low AT expression. This difference held when we controlled for the location of tumor (p = 0.001). AT WI was lower in oral cavity SCCs as compared to laryngeal and oropharyngeal tumors (p = 0.015). This correlation persisted when we controlled for tumor grade (p = 0.008). There was an association noted between the presence of LNM and an increased AT expression by WI which did not reach statistical significance (p = 0.052). No significant differences in AT expression by WI were observed depending on primary tumor stage (p = 0.094). In patients who had locally metastatic SCCHN, no significant differences in AT expression by WI was found with nodal status (p = 0.566) (Table 3).
Among patients without LNM, there was a trend for a shorter OS in those whose tumors had AT expression by WI above the median value (p = 0.054) (Fig. 3). On multivariable analysis, AT expression was not significantly associated with either OS or DFS when the entire cohort of patients was tested. Similar findings were noted for the subgroups of patients with LNM as well as those without LNM. The above analyses controlled for age, sex, race, and smoking status.
Discussion
We found that lower AT levels correlated with better differentiated head and neck tumors and tumors of oral cavity primary. There is no clear explanation to why a high level of AT expression correlated with a higher tumor grade however, this finding would support the premise that microtubules that have a lower AT levels would predictably be more dynamic [12, 13]. Tubulin acetylation is a post-translational modification of the microtubule polymer that serves as a hallmark of stable microtubules [14]. In general, the more stable a microtubule is, the more acetylated it is. It is worth noting that our tumor samples were predominantly of oral cavity primary (52 %) the majority of which had no LNM (67 %). Of interest is the lower AT level we observed in oral cavity tumors compared to other sites namely oropharynx and larynx which was independent of tumor grade and may reflect a different biology in these tumors. As far as the marginally significant correlation we noted between a high AT expression and the presence of locoregional lymph node metastases (LNM) this supports that AT expression is correlated with more aggressive behavior. Even though AT expression appeared to be marginally correlated with a better OS in patients with node-negative disease this association did not hold for the node positive group. AT expression appeared to be marginally correlated with a better OS in patients with node-negative disease however this association did not hold for the node positive group.
We also studied primary tumor AT expression in locally advanced SCCHN patients who were naïve to therapy namely patients who received induction chemotherapy with TPF (in the context of a sequential treatment paradigm). No patients had clinically evident/demonstrable distant metastatic disease at the time of tumor tissue analysis. We observed that a lower baseline AT expression correlated with response to TPF IC. Since taxane treatment induces potent tubulin acetylation both in vitro and in vivo, yielding “hyperstable” microtubules, the expression level of AT has been suggested as a surrogate marker for taxane sensitivity. Indeed, a low basal level of tubulin acetylation has been shown to suggest a better response to taxane treatment in vitro, especially when a taxane is combined with a small molecule inhibitor [8]. In a clinical trial at Emory University investigating the interaction of docetaxel with a farnesyl transferase inhibitor in patients with advanced malignancies, a low level of AT in tumor samples was a predictor for modest disease control [8].
Our findings have potential significant clinical implicatins as among patients receiving TPF IC, predicting for those who will have a better outcome may help improve selection of patients for this modality. Taxanes are commonly used in treating SCCHN both in the locally advanced and distantly metastatic setting, due to their significant antitumor activity. Not surprisingly, taxanes (paclitaxel, and its subsequently developed and applied congener, docetaxel) are the backbone of the standard IC TPF regimen. The emergence of drug resistant tumor cells has been a limitation to the ability of these agents to produce the desired sustained responses and lack of relapsed or residual disease in treating a variety of solid tumors. Several mechanisms of resistance to taxanes have been described. Overexpression of drug efflux pumps, such as P-glycoprotein and multidrug resistance-associated protein-1 (MRP1), is one of the possible drug resistance mechanisms [15–17]. MRP1 can efficiently pump antineoplastic drugs out of cancer cells, thereby lowering intracellular drug concentrations. This phenomenon is seen with several classes of agents and is referred to as multidrug-resistance. A second possible mechanism underlying taxane resistance involves alterations in tubulin. Such alterations include: (a) altered expression of β-tubulin isotypes in taxane-resistant versus taxane-sensitive tumor cells [18–20]; (b) aberrant microtubule assembly-disassembly dynamics in taxane-resistant cancer cells [21]; and (c) The presence of β-tubulin mutations in such cells [22–24]. All of these mechanisms have been demonstrated to be active in assorted in vitro models. However, at present, there is no conclusive evidence as to the clinical significance of these mechanisms in malignancies customarily treated with taxane-containing regimens. In fact, a recent review by Berrieman et al. [25] highlighted the lack of evidence that β-tubulin mutations actually play a role in resistance to taxanes. This suggests that the other two aforementioned mechanisms probably factor in the emergence and maintenance of taxane resistance.
Even though our observed inverse correlation between AT expression and response to taxane based chemotherapy was strong, it cannot be conclusive in light of the small sample size and the multiple factors that could have predicted response to therapy including HPV status, tumor grade and primary site. Importantly this observation was restricted to patients with oral cavity and oropharynx primaries. Other studies have examined and confirmed a strong association of beta-II tubulin expression (another biomarker for taxane sensitivity) with adverse outcome in locally advanced SCCHN in patients treated with a taxane-based IC, suggesting that low expression of beta-II tubulin may predict benefit from use of sequential therapy schemas/regimens in this context [26]. Given the widespread use of taxanes in solid tumors including SCCHN, markers for taxane sensitivity in treatment-naïve patients at baseline in combination with other markers, such as p53, and Bcl-2 expression levels, may offer a better predictive model for outcome in patients with newly diagnosed locally advanced SCCHN and assist in their optimal stratification to receive certain modalities such as sequential therapy. It is unclear whether novel biomarkers, would be able to supersede other more classic prognostic markers of aggressiveness, such as T- and N-stage or histologic grade, as far as their prognostic value for OS or PFS/DFS or their ability to predict response/sensitivity to systemic poly-chemotherapy. Further studies are needed to answer this question.
In conclusion, in our cohort of patients with predominant oral cavity SCCHN, a lower AT expression is correlated with a lower tumor grade and an oral cavity primary site. Our observed trends correlating AT expression and presence of nodal metastases need further confirmation. The inverse correlation between AT and response to a taxane based regimen in the induction therapy setting, needs to be reproduced using a larger sample size.
References
Pignon JP, Bourhis J. Domenge C LD. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet. 2000;355:949–55.
Pignon JP, Le MA, Bourhis J. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys. 2007;69:S112–4.
Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704.
Cohen EEW, Karrison T, Kocherginsky M, Huang CH, Agulnik M, Mittal BB, Yunus F, Samant S, Brockstein B, Raez LE, Mehra R, Kumar P, Ondrey FG, Seiwert TY, Villaflor VM, Haraf DJ, Vokes EE. DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). ASCO 2013 meeting. J Clin Oncol 2012;30(Suppl):abstr 5500.
Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, Limaye S, Riley S, Posner M. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14:257–64.
Marcus AI, O’Brate AM, Buey RM, et al. Farnesyltransferase inhibitors reverse taxane resistance. Cancer Res. 2006;66:8838–46.
Kauh J, Chanel-Vos C, Escuin D, et al. Farnesyl transferase expression determines clinical response to the docetaxel-lonafarnib combination in patients with advanced malignancies. Cancer. 2011;117(17):4049–59.
Marcus AI, Zhou J, O’Brate A, et al. The synergistic combination of the farnesyl transferase inhibitor lonafarnib and paclitaxel enhances tubulin acetylation and requires a functional tubulin deacetylase. Cancer Res. 2005;65:3883–93.
Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3.
Cox D. Regression models and life tables. J R Stat Soc Ser. 1972;B34:187–220.
Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Gen Physiol. 1987;104:289–302.
Gefland VI, Bershadsky AD. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116.
Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4(12):938–48.
Wan CP, Letchford K, Jackson JK, Burt HM. The combined use of paclitaxel-loaded nanoparticles with a low-molecular-weight copolymer inhibitor of P-glycoprotein to overcome drug resistance. Int J Nanomedicine. 2013;8:379–91.
Baek JS, Cho CW. 2-Hydroxypropyl-β-cyclodextrin-modified SLN of paclitaxel for overcoming p-glycoprotein function in multidrug-resistant breast cancer cells. J Pharm Pharmacol. 2013;65:72–8.
Stordal B, Hamon M, McEneaney V, Roche S, Gillet JP, O’Leary JJ, Gottesman M, Clynes M. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS ONE. 2012;7:e40717.
Haber M, Burkhart CA, Regl DL, Madafiglio J, Norris MD, Horwitz SB. Altered expression of M beta 2, the class II beta-tubulin isotype, in a murine J774.2 cell line with a high level of taxol resistance. J Biol Chem. 1995;270:31269–75.
Jaffrezou JP, Dumontet C, Derry WB, et al. Novel mechanism of resistance to paclitaxel (Taxol) in human K562 leukemia cells by combined selection with PSC 833. Oncol Res. 1995;7:517–27.
Kavallaris M, Kuo DY, Burkhart CA, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–93.
Goncalves A, Braguer D, Kamath K, et al. Resistance to taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci USA. 2001;98:11737–42.
Giannakakou P, Poy G, Zhan Z, Knutsen T, Blagosklonny MV, Fojo T. Paclitaxel selects for mutant or pseudo-null p53 in drug resistance associated with tubulin mutations in human cancer. Oncogene. 2000;19:3078–85.
Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–25.
Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A beta-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–82.
Berrieman HK, Lind MJ, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–64.
Cullen KJ, Schumaker L, Nikitakis N, Goloubeva O, Tan M, Sarlis NJ, Haddad RI, Posner. Beta-tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: a companion analysis of the TAX 324 trial. J Clin Oncol. 2009;27:6222–8.
Acknowledgments
This work was supported by a SPORE CDP Grant P50 CA128613-03 to N.F. Saba and S. Muller and Sanofi-Aventis US; now Sanofi Oncology.
Conflict of interest
None of the authors have any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saba, N.F., Magliocca, K.R., Kim, S. et al. Acetylated Tubulin (AT) as a Prognostic Marker in Squamous Cell Carcinoma of the Head and Neck. Head and Neck Pathol 8, 66–72 (2014). https://doi.org/10.1007/s12105-013-0476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-013-0476-6