Abstract
The objective of this study was to explore the relationship between β3-tubulin expression and sensitivity to taxane-based neoadjuvant chemotherapy in primary breast cancer patients. A total of 48 local advanced breast cancer patients that received taxane-containing neoadjuvant chemotherapy were studied. The levels of β3-tubulin expression were tested by immunohistochemistry before chemotherapy and at the end of cycles 2, 4 and 6. The correlation between the efficacy of the chemotherapy and β3-tubulin expression and changes in β3-tubulin expression over the course of chemotherapy was examined. β3-tubulin protein expression before chemotherapy was significantly and negatively correlated with the response rate. The overall response rate was 31.8 % in the high β3-tubulin expression group, whereas it was 84.6 % in the low β3-tubulin expression group. At the end of cycles 2, 4 and 6 during the treatment course, the average expression rates of β3-tubulin were showed an increasing trend with β3-tubulin expression level at the end of cycle 4 being significantly different from that before chemotherapy. Nine patients that had a low β3-tubulin expression level preneoadjuvant chemotherapy changed to a high β3-tubulin expression level postneoadjuvant chemotherapy, and they had lower response rate than patients with consistent low. In conclusion, β3-tubulin is a good predictor of chemosensitivity to taxane for breast cancer, and the change of its expression level during chemotherapy may be an important cause of secondary resistance to taxane. Detection of β3-tubulin expression before and throughout the chemotherapy will help with selection of the chemotherapy treatment plan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the major cancers that threaten women’s health, and chemotherapy is an important part of the comprehensive treatment for this disease. Taxanes including paclitaxel and docetaxel entered the market in the 1990s and are currently the most active chemotherapy drugs for treating breast cancer. Large-scale randomized studies have demonstrated that taxane-containing chemotherapy can improve the survival rate of patients with early and advanced breast cancer [1, 2]. For advanced breast cancer, the overall response rate of the initial taxane monotherapy is approximately 23–48 % [3, 4]. That is, more than half of the patients show a primary resistance to taxane therapy. For some patients, although the treatment is initially effective, secondary resistance to drugs will occur during the treatment course. There is no clear indicator when selecting chemotherapy regimens for treating breast cancer, unlike targeted therapy or endocrine therapy. Thus, some patients who are resistant end up receiving unnecessary chemotherapy, and this not only increases medical costs but also introduces drug toxicity. Therefore, to find a good predictor for the efficacy of taxane has an important significance for the selection of treatment strategies.
In recent years, with the extensive application of neoadjuvant chemotherapy (NACT) for treating breast cancer, direct assessment of response to cancer therapy can be performed. This makes the screening of candidate drug-resistant factors more convenient. The expression levels of several molecular markers, such as sex hormones, human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR), P53, Bcl-2 and TLE3, have been used as indicators of sensitivity to taxane chemotherapy. However, the majority of these studies did not reach consistent conclusions [5–9]. Based on a number of preclinical studies, β3-tubulin is becoming a promising candidate factor. β3-tubulin is one of the seven isotypes of β-tubulin (β1, β2, β3, β4a, β4b, β5 and β6) and exists specifically in neurons and Sertoli cells [10]. However, in certain types of cancers, β3-tubulin expression is not normal, and this change in expression is closely related to resistance to taxane chemotherapy [11–14].
Current studies have primarily focused on the relationship between β3-tubulin expression before chemotherapy and resistance to taxane-containing drugs. To date, there have been no studies reporting on changes in β3-tubulin expression during the course of chemotherapy. It has been shown that during the course of chemotherapy, expression changes may occur in some molecules, and these changes are often closely associated with drug resistance [15, 16]. The goal of the present study is to explore the role of β3-tubulin expression in resistance to taxane-containing chemotherapy by examining β3-tubulin expression before and throughout the course of chemotherapy.
Patients and methods
Ethics statement
This study was performed with the approval of the Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University. All procedures were conducted according to the Declaration of Helsinki. Written informed consent was obtained from all patients.
Patient selection
A total of 48 patients with local advanced breast cancer that have been treated in the surgical oncology division, First Affiliated Hospital of Wenzhou Medical University, were included from January 2009 to December 2012. All included patients fit the following criteria: (1) NACT and tumor core needle biopsy were performed under informed consent of the patients and their families; (2) tumor core needle biopsy before chemotherapy led to pathological diagnosis of invasive ductal carcinoma or lobular carcinoma; (3) primary tumor diameter was ≥3 cm; (4) evaluation on the physical condition of the patients revealed Karnofsky performance status (KPS) score ≥80 points; and (5) routine blood tests and blood biochemical indicators did not show any abnormalities, and cardiac ultrasound and electrocardiogram results were normal. All 48 enrolled patients were female, aged 31–74 years old, with a median age of 53 years old. Clinical stages of the patients were 14 cases of stage IIa, 6 cases of stage IIb, 8 cases of stage IIIa, 6 cases of stage IIIb and 14 cases of stage IIIc. Basis clinicopathological parameters are shown in Table 1.
Chemotherapy regimen
This is a nonrandomized prospective study. All patients were treated with taxane-containing chemotherapy regimens, including 23 cases of monotherapy regimens using paclitaxel or docetaxel and 25 cases of combination chemotherapy regimens containing paclitaxel or docetaxel. Patients received combination chemotherapy including cyclophosphamide 500 mg/m2, epirubicin 75 mg/m2, and docetaxel 75 mg/m2 or paclitaxel 135 mg/m2 every 3 weeks. The doses of docetaxel were 100 mg/m2 (3-week monotherapy regimen), and the doses of paclitaxel were 80 mg/m2 (weekly monotherapy regimen). Each cycle of chemotherapy lasted 3 weeks, and there were a total of 2–6 cycles. The median number of cycles administered was four. During the course of chemotherapy, routine blood tests and tests on liver and kidney functions were performed. If leukocyte and neutrophil numbers were reduced, granulocyte colony-stimulating factors were administrated as a routine support treatment until the cell numbers recovered to normal. In the end, all patients underwent modified radical mastectomy. In addition, patients with HER2 positive received 1 year of treatment with single trastuzumab after chemotherapy.
Efficacy assessment
Efficacy was assessed according to response evaluation criteria in solid tumors (RECIST1.0). The efficacies were divided into complete response (CR, no breast tumor was palpable), partial response (PR, the longest diameter of the tumor decreased by ≥30 %), stable disease (SD, the longest diameter of the tumor decreased by <30 % or increased by <20 %) or progressive disease (PD, the longest diameter of the tumor increased by ≥20 %). The overall objective response rate (RR) was calculated as CR + PR. The size of the breast lump and axillary nodal status was determined by ultrasound examination before each cycle and before surgery. The histological response was classified as score of 3, 4 or 5, based on Miller-Payne score system [17], with 5 being a pathologic complete response (pCR).
Sample collection and immunohistochemical tests
Before NACT, samples were collected by core needle biopsy. Pathological diagnosis confirmed invasive breast cancer. Immunohistochemical tests on estrogen receptor (ER), progesterone receptors (PR), Ki-67, HER2 and β3-tubulin expression were conducted. All patients underwent 2–6 cycles of chemotherapy, and at the end of cycles 2, 4 and 6, core needle biopsy or surgical resection was performed to reexamine the expression of β3-tubulin. For immunohistochemical tests, a streptavidin-peroxidase conjugate (SP) method was used [18]. For the HER2 test, HER2 was scored on a 0–3 scale according to the criteria. The staining was scored as: negative (0) when no membrane staining was observed, or when membranous staining was observed in less than 10 % of the tumor cells; weak positive (1+) if weak focal membrane staining was seen in more than 10 % of the tumor cells; intermediate (2+) if weak-to-moderate, complete membrane staining was seen in more than 10 % of the tumor cells; and strongly positive (3+) if intense membrane staining with weak-to-moderate cytoplasmic reactivity was seen in more than 10 % of the tumor cells. In the final analysis, scores 0 and 1 were considered negative, score 2 was considered weakly positive and score 3 was considered strongly positive. Only score 3 cases were considered as HER2-overexpressing cases. ER/PR-positive brown particles were localized in the nuclei, and positive expression was defined as ≥1 % positive cells. Ki-67-positive particles were localized in the nuclei, and for each slice, at least 200 tumor cells were counted at high magnification, and the percentage of positive cells in tumor cells was calculated to be the proliferation index (PI) value.
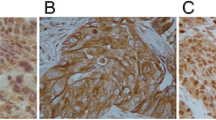
The primary rabbit antihuman β3-tubulin mAb kit and the SP immunohistochemistry kit were purchased from Fuzhou Maxim Biotech Inc. (China). Immunohistochemistry slides were read using a double-blind method at high magnification; under optical microscope, five fields of view were randomly selected. In each field, 100 tumor cells were counted and the average percentage of positive cells was calculated. If there were over 50 % positive cells, it was considered as high expression; otherwise, it was considered as low expression. The assay was based on previously reported IHC assays for β3-tubulin [19]. The criterion for β3-tubulin positive cells is defined as follows: β3-tubulin is mainly located evenly in the cytoplasm of normal cells and tumor cells, manifested by yellow or brown particles and staining of endothelial cells or neurons was used as reference. The staining intensity of 0 refers to no staining; staining intensity of 1 refers to weaker staining than that of endothelial cells or nerve cells; staining intensity of 2 is comparable staining to that of endothelial cells or nerve cells; staining intensity of 3 is stronger staining than that of endothelial cells or nerve cells. Cells with staining intensity of 0–1 were counted as negative cells, and those with staining intensity of 2–3 were considered as positive cells (Fig. 1).
Statistical analysis
SPSS 17.0 statistical software was used for all data analysis. To determine the correlation between clinicopathological parameters, a Spearman rank correlation test was performed; for analysis on factors related to chemosensitivity, a Chi-square test was performed. To determine changes in β3-tubulin expression before and after chemotherapy, paired Wilcoxon test was performed. All statistical analyses were two-tailed tests. P < 0.05 was considered as statistically significant.
Results
All 48 breast cancer patients completed two or more cycles of chemotherapy with 36 patients completing four or more cycles of chemotherapy and 12 patients completing six cycles of chemotherapy. There was no death caused by chemotherapy, and during chemotherapy, there were no severe allergic events. Grade 3–4 adverse reactions occurred in 43.8 % patients and were primarily bone marrow suppressions. In the last efficacy assessment, among the 48 patients, there was no case of PD, 19 cases of SD, 22 cases of PR, and 8 cases of CR. The overall response rate was 60.4 % (29/48).
The relation between β3-tubulin expression and clinicopathological parameters of breast cancer patients
β3-tubulin was found to be expressed mainly in the cytoplasm of tumor cells. Prior to NACT, the average expression rate of β3-tubulin was 45.6 %, and β3-tubulin expression was not significantly correlated with age, histological grade, tumor diameter, or ER, PR, Ki-67, or HER2 expressions.
Factors related to response to chemotherapy
At the end of the last chemotherapy cycle, the overall response rate (PR + CR) was 60.4 %, and in 8 patients, pathological complete remission (PCR) was achieved. Analysis on correlation between NACT efficacy and clinical data revealed that the efficacy of chemotherapy was not significantly correlated with age, histological grade, tumor diameter, lymph node status, chemotherapy regimen or expressions of ER, PR, Ki-67 or HER2 in tumor cells. Chemotherapy efficacy was significantly and negatively correlated with β3-tubulin expression; in the high β3-tubulin expression group, the overall response rate was 31.8 %, whereas in the low β3-tubulin expression group was 84.6 %. All 8 PCR patients were in the low β3-tubulin expression group. The response rate was significantly and positively correlated with the number of chemotherapy cycles completed; at the end of cycles 2, 4 and 6, the overall response rates were 43.8, 58.3 and 83.3 %, respectively (Table 2).
Changes in β3-tubulin expression over the course of NACT
The average expression rates of β3-tubulin showed an increasing trend and were as follows: 45.6 ± 33.3 % prior to NACT, 51.6 % ± 31.2 at the end of cycle 2, 54.6 ± 29.1 % at the end of cycle 4, and 57.8 ± 30.4 % at the end of cycle 6. At the end of cycle 4, β3-tubulin expression level was significantly different from that of before chemotherapy (Table 3). In nine patients, β3-tubulin expression was low before NACT and was high after NACT, and the final response rate of these nine patients was 44.4 %. There was only one patient with high expression of β3-tubulin change to low expression after NACT, and showed PR.
Discussion
Taxane have a unique mechanism, in that it targets β-tubulin by inducing and promoting tubulin polymerization and microtubule assembly, it inhibits microtubule depolymerization and it prevents the completion of mitosis. Tumor cells are arrested in phase G2/M and ultimately undergo apoptosis [20]. β3-tubulin is considered as the only β-tubulin isotype that promotes microtubule depolymerization, suggesting that its expression may be associated with resistance to taxane chemotherapy [21]. In the present study, before chemotherapy for breast cancer treatment, β3-tubulin expression was relatively high, with an average expression rate of 45.6 %. It was found that the response rate in the low β3-tubulin expression group was higher than that of the high β3-tubulin expression group. In addition, all CR patients were in the low expression group. Correlation analysis showed that β3-tubulin expression was the only factor significantly correlated with resistance to taxane-containing chemotherapy. Wehbe et al. [22] reported that the total amount of microtubule proteins and β1, β3 and β4 tubulin expressions were significantly increased in paclitaxel-resistant H460 nonsmall cell lung cancer cell lines compared to control, and that the change in β3-tubulin expression was most prominent. Hari et al. [23] transfected β3-tubulin cDNA into mammalian cell lines and found cells expressing β3-tubulin showed depolymerization of microtubules, suggestive of resistance to paclitaxel. Gan et al. [24] used small interfering RNAs to inhibit β3-tubulin expression in nonsmall cell lung cancer cell lines, NCI-H460 and Calu-6, and found that the sensitivity of these cells to paclitaxel significantly increased. Cell cycle analysis revealed that the proportion of cells in phase G2 was reduced, whereas the cells in phase G1 were increased, further suggesting that resistance to paclitaxel of these cells was reversed. It is currently believed that possible mechanisms underlying the fact that overexpression of β3-tubulin leads to resistance to anti-microtubule drugs may include the following: (1) β3-tubulin expression changes the characteristics of different endogenous microtubules in tumor cells, which affects the binding between the drug and β3-tubulin dimers causing decreased binding sensitivity [25] and (2) high expression of β3-tubulin reduces the rate of microtubule polymerization and thus counteracts the polymerization induced by paclitaxel [23].
It is known that both NACT and rescue chemotherapy may be initially effective, but they eventually lead to drug resistance in some cancer patients. Determining the cause of secondary drug resistance is crucial for selection of treatment plans and reversal of drug resistance. Multiple studies have shown that chemotherapy drugs can cause changes in tumor phenotype and that the residual tumor cells are more prone to metastasis and drug resistance [26, 27]. Consistently, the current study demonstrated that during the course of chemotherapy expression of β3-tubulin displayed an increasing trend, and at the end of cycle 4, its value was significantly different from the value before treatment. Since some patients achieved complete response before cycle 6 and some drug-resistant patients chose to receive radical surgery before completing all 6 cycles of chemotherapy, the sample number for cycle 6 was relatively small. This might contribute to the fact that β3-tubulin expression at the end of cycle 6 was not significantly different from that before chemotherapy. An increase in β3-tubulin expression during the course of chemotherapy for breast cancer has not been reported before. Here we found that nine patients with low β3-tubulin expression changed to high β3-tubulin expression during chemotherapy. At the same time, only one patient with high expression of β3-tubulin changed to low expression after NACT. The response rate of these nine patients was lower than that of patients who showed persistent low β3-tubulin expression, but higher than those with high β3-tubulin expression before treatment. It is possible that the NACT was initially effective on these patients, yet as β3-tubulin expression increased, they became resistant to taxane chemotherapy. As the number of these patients was relatively small, comparison of chemosensitivity before and after NACT was not performed in the present study.
The mechanism of elevated β3-tubulin expression is still not clear. One possible explanation may be related to the heterogeneity of the tumor. Tumor cells with low-level β3-tubulin expression are more sensitive to paclitaxel chemotherapy and are more likely to be killed than those with high expression, whereas tumor cells with high levels of β3-tubulin expression survive the chemotherapy due to their resistance to paclitaxel and thus the expression rate of β3-tubulin is increased. Another possible cause is that β3-tubulin is part of the complex that automatically regulates tubulin in breast tumor cells and by increasing the expression of β3-tubulin, the cells defense to damages caused by drugs is strengthened [13, 23, 28]. In the present study, it was also found that the response rate was correlated with the number of chemotherapy cycles completed; as the number of cycles increased, the overall response rate also increased. This suggests that only after a certain number of cycles have been completed should the efficacy of NACT be properly determined. However, it should also be noted that as the number of chemotherapy cycles increases, β3-tubulin expression also increases, leading to an increased risk of drug resistance. Delay in surgical treatment of drug-resistant tumors will increase the risk of distant metastasis, and thus, it is very important to choose the appropriate number of NACT cycles.
In summary, the present study shows β3-tubulin expression may be an effective predictor for taxane-containing chemotherapy in breast cancer. To measure β3-tubulin expression before and throughout the course of chemotherapy is of important significance for selecting chemotherapy drugs, examining the cause of resistance to chemotherapy, and determining the appropriate number of chemotherapy cycles. However, as the number of cases included in this study was relatively small and the chemotherapy regimen received by the patients was relatively short, cases in which β3-tubulin expression increased over the course of chemotherapy were not further analyzed. Future studies are needed to determine the significance of expression of β3-tubulin increased during the course of chemotherapy.
References
Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83.
Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP, Sami A, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10-year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14(1):72–80.
Chan S, Friedrichs K, Noel D, Pinter T, Van Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol. 1999;17(8):2341–54.
Stemmler HJ, Harbeck N, Groll de Rivera I, Vehling Kaiser U, Rauthe G, Abenhardt W, et al. Prospective multicenter randomized phase III study of weekly versus standard docetaxel (D2) for first-line treatment of metastatic breast cancer. Oncology. 2010;79(3–4):197–203.
Hamilton A, Larsimont D, Paridaens R, Drijkoningen M, van de Vijver M, Bruning P, et al. A study of the value of p53, HER2, and Bcl-2 in the prediction of response to doxorubicin and paclitaxel as single agents in metastatic breast cancer: a companion study to EORTC 10923. Clin Breast Cancer. 2000;1(3):233–40 (discussion 41–2).
Konecny GE, Thomssen C, Luck HJ, Untch M, Wang HJ, Kuhn W, et al. Her-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancer. J Natl Cancer Inst. 2004;96(15):1141–51.
Van Poznak C, Tan L, Panageas KS, Arroyo CD, Hudis C, Norton L, et al. Assessment of molecular markers of clinical sensitivity to single-agent taxane therapy for metastatic breast cancer. J Clin Oncol. 2002;20(9):2319–26.
Kulkarni SA, Hicks DG, Watroba NL, Murekeyisoni C, Hwang H, Khoury T, et al. TLE3 as a candidate biomarker of response to taxane therapy. Breast Cancer Res. 2009;11(2):R17.
Susini T, Berti B, Carriero C, Tavella K, Nori J, Vanzi E, et al. Topoisomerase II alpha and TLE3 as predictive markers of response to anthracycline and taxane-containing regimens for neoadjuvant chemotherapy in breast cancer. Onco Targets Ther. 2014;7:2111–20.
Burgoyne RD, Cambray-Deakin MA, Lewis SA, Sarkar S, Cowan NJ. Differential distribution of beta-tubulin isotypes in cerebellum. EMBO J. 1988;7(8):2311–9.
Dumontet C, Isaac S, Souquet PJ, Bejui-Thivolet F, Pacheco Y, Peloux N, et al. Expression of class III beta tubulin in non-small cell lung cancer is correlated with resistance to taxane chemotherapy. Bull Cancer. 2005;92(2):E25–30.
Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11(1):298–305.
Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998;77(4):562–6.
Seve P, Reiman T, Lai R, Hanson J, Santos C, Johnson L, et al. Class III beta-tubulin is a marker of paclitaxel resistance in carcinomas of unknown primary site. Cancer Chemother Pharmacol. 2007;60(1):27–34.
Litviakov NV, Cherdyntseva NV, Tsyganov MM, Denisov EV, Garbukov EY, Merzliakova MK, et al. Changing the expression vector of multidrug resistance genes is related to neoadjuvant chemotherapy response. Cancer Chemother Pharmacol. 2013;71(1):153–63.
Schneider S, Uchida K, Brabender J, Baldus SE, Yochim J, Danenberg KD, et al. Downregulation of TS, DPD, ERCC1, GST-Pi, EGFR, and HER2 gene expression after neoadjuvant three-modality treatment in patients with esophageal cancer. J Am Coll Surg. 2005;200(3):336–44.
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7.
Chen X, Wu J, Lu H, Huang O, Shen K. Measuring beta-tubulin III, Bcl-2, and ERCC1 improves pathological complete remission predictive accuracy in breast cancer. Cancer Sci. 2012;103(2):262–8.
Saura C, Tseng LM, Chan S, Chacko RT, Campone M, Manikhas A, et al. Neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early stage breast cancer and evaluation of betaIII-tubulin expression as a predictive marker. Oncologist. 2013;18(7):787–94.
McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785(2):96–132.
Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9(2):168–75.
Wehbe H, Kearney CM, Pinney KG. Combretastatin A-4 resistance in H460 human lung carcinoma demonstrates distinctive alterations in beta-tubulin isotype expression. Anticancer Res. 2005;25(6B):3865–70.
Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III beta-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton. 2003;56(1):45–56.
Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67(19):9356–63.
Seve P, Reiman T, Dumontet C. The role of betaIII tubulin in predicting chemoresistance in non-small cell lung cancer. Lung Cancer. 2010;67(2):136–43.
Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66(3):1702–11.
Shen Y, Wang P, Li Y, Ye F, Wang F, Wan X, et al. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. Br J Cancer. 2013;109(1):92–9.
Galmarini CM, Treilleux I, Cardoso F, Bernard-Marty C, Durbecq V, Gancberg D, et al. Class III beta-tubulin isotype predicts response in advanced breast cancer patients randomly treated either with single-agent doxorubicin or docetaxel. Clin Cancer Res. 2008;14(14):4511–6.
Acknowledgments
This work was supported by the First Affiliated Hospital of Wenzhou Medical University Incubation Project (FHY2014046).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Youqun Xiang and Yinlong Yang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xiang, Y., Yang, Y., Guo, G. et al. β3-tubulin is a good predictor of sensitivity to taxane-based neoadjuvant chemotherapy in primary breast cancer. Clin Exp Med 16, 391–397 (2016). https://doi.org/10.1007/s10238-015-0371-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0371-4