Abstract
Retinopathy of prematurity (ROP) is a proliferative retinal vascular disease affecting the retina of premature infants. The clinical spectrum of ROP varies from spontaneous regression to bilateral retinal detachment and total blindness. Between these two extremes lies the form of ROP, which is amenable to treatment with laser photocoagulation, anti-vascular endothelial growth factor drugs or surgery. Increasing rates of preterm births coupled with better survival rates but lack of uniform quality of neonatal care and delays in diagnosis have led to increasing ROP blindness. Atypical forms of Aggressive Posterior ROP are seen in heavier birth weight babies in developing countries. Prevention of ROP by following stringent protocols for supplemental oxygen, prevention of sepsis, timely screening and laser treatment by a concerted and collaborative effort of neonatologists and ophthalmologists are required to fight the blindness from ROP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a proliferative disease of the premature developing retina. Retrolental fibroplasia was the term used for this condition since its first description in 1940 as a cause of blindness in children [1]. We may term ROP as a “man-made disease” since it was recognized only after the use of supplemental oxygen and incubators began to make survival of preterm babies possible. In 2008, Gilbert et al. estimated that more than 50,000 children are blind from ROP worldwide [2]. Recent literature suggests that this could simply be an underestimate with nearly 32,200 infants becoming blind or visually impaired from ROP in 2010 alone [3]. Preterm birth rates are increasing and mortality rates are decreasing due to the rapid expansion of the neonatal inpatient care facilities. Global estimates are that nearly 15 million babies are born preterm (<37 wk of completed gestational age) each year and at least 32 million babies are small for gestational age (<10th centile for gestational age). India tops the list with more than 3.5 million preterm births, highest in the world [4]. ROP is now a major public health concern for India as well as other low and middle-income countries. Non-uniform standards of neonatal care, absence or delayed screening and lack of trained ophthalmologists to screen and treat ROP are major factors contributing to increasing blindness from ROP.
Incidence
The incidence of ROP varies widely across different countries and is linked to the socioeconomic developments as well as the quality and accessibility of health care facilities [5]. In the low and middle-income countries an “epidemic” of ROP blindness is currently going on. In 2010, ten countries (China, India, Brazil, Indonesia, Iran, Russian Federation, USA, Mexico, Thailand and Turkey) accounted for nearly two- thirds of all cases of visual impairment due to ROP [6].
In India, ROP has been reported to occur in 21.7%–51.9% of low birth weight infants [7,8,9,10]. Most studies report the mean birth weight of babies developing ROP to be above 1250 g and the incidence of severe ROP ranging from 5.0–44.9%.
Screening
A number of reports from India have stressed upon the fact that wider and more inclusive screening criteria are needed compared to the west. Vinekar et al. reported that 17.7% babies with severe ROP would be missed using the American and 22.6% using the British screening guidelines for ROP [9]. Jalali et al. showed that 13.3% of babies with ROP exceeded the United Kingdom and United States screening criteria [10]. Hungi et al. in a recent study reported that 57.6% of the babies having ROP were heavier and older than the American screening cut-off [11]. Of these, 36.8% had some stage of ROP and 8% required treatment. These reports strengthened the evidence to have a different set of guidelines for our country. National Neonatology Forum Screening guidelines for ROP [12] that are being followed since 2010 includes:
-
a)
Infants born <34 wk of gestation and/or weighing <1750 g or
-
b)
Heavier (1750-2000 g birth weight) or older babies (34–36 wk gestation) if they have attending risk factors like mechanical ventilation, prolonged oxygen therapy, hemodynamic instability or adverse respiratory or cardiac disease profile.
The first screening examination for ROP is recommended by ‘day 30’ of life, irrespective of the gestational age. Infants <28 wk or <1200 g should be screened early at 2–3 wk of age to enable early identification of ROP.
There is a consensus now on including ROP as a part of universal eye screening in babies with birth weights up to 2000 g and/or gestational age upto 35 wk under the government run national program known as Rashtriya Bal Swasthya Karyakram (RBSK).
Screening Procedure
The first screening should ideally be performed in the Neonatal Intensive Care Unit (NICU) or Special Newborn Care Unit (SNCU) itself under the supervision of the neonatologist or pediatrician or neonatal nurse and subsequent examinations may be performed as an outpatient in the clinic. A wall chart regarding whom to screen, when to screen and how to dilate the pupil should be put up in the NICU/SNCU, nursery and the outpatient department for ready reference. Pupillary dilatation can be achieved with 2.5% phenylephrine hydrochloride and 0.5–1% cyclopentolate or tropicamide instilled twice after a gap of 15 min. Excess drops from the medial canthus should be wiped off to reduce systemic absorption and toxicity. With some training and experience the oculocephalic reflex can be used to view the desired periphery of the retina and insertion of the eye speculum may not always be needed, thus helping to minimize the pain during the procedure.
Wide-field retinal imaging using Ret-Cam (Clarity MSI, CA, USA) by trained technicians has been shown to be highly effective tool for screening of ROP in the KIDROP program, a model program for developing countries [13]. This model of using trained technicians for documenting disease and decision-making regarding treatment and referral had 95.7% sensitivity, 93.2% specificity and a positive predictive value of 81.5% compared to ROP experts. With the development and availability of the indigenous low cost wide field retinal imaging camera, the way forward could be training of the nurses/neonatologists/pediatricians in ROP screening at the point of care especially where trained ophthalmologists are not available.
Whereas timely diagnosis and treatment alone can provide excellent visual outcome in most cases, the biggest challenge faced by developing countries today is the lack of timely screening. Over 500 SNCUs at district level and nearly 2000 newborn stabilisation units at block level have been operationalised in India. Establishment of ROP screening has not been in sync to the surge of the NICUs and SNCUs. As a result of delayed screening babies are often presenting when it is too late to intervene. In one report from a tertiary care referral centre in India, 86.4% of infants who presented with stage 5 ROP had never been screened for ROP. Parents self-referred nearly 74.2% children when they noticed that the child was not seeing. Pediatricians referred none of the infants for screening and an ophthalmologist referred only 25.8% [14]. It is extremely difficult to convince and counsel the family that the window of opportunity has been lost and the visual prognosis is poor in stage 5 ROP. This has led to increasing incidences of medicolegal litigation in cases of ROP related blindness.
Pathogenesis
Retinal blood vessel development in utero is essentially in a state of physiologic hypoxia–driven, vascular endothelial growth factor (VEGF)–mediated angiogenesis. Flynn et al. reported two phases in the normal retinal vascular development [15]. The first phase (vasculogenesis) begins roughly around 14 wk until 21 wk of gestation. In this phase, Vascular Precursor Cells (VPCs) of mesenchymal origin exit from the optic nerve and form the four major arcades of the posterior retina. In the second phase (angiogenesis), proliferating endothelial cells arise from the existing blood vessels formed during the first phase forming the capillary network. The nasal retina gets vascularized by 8 mo of gestation and the temporal retina shortly after term (around 40 wk of gestation). A preterm infant at birth would thus have incompletely vascularized peripheral retina to a variable extent. Physiologic in-utero hypoxia is reduced and the newborn is now exposed to a state of hyperoxia (atmospheric oxygen as well as the supplemental oxygen). In addition, serum levels of insulin like growth factor 1 (IGF1) are low at this time. Hyperoxia and low serum levels of IGF1 contribute to delayed retinal vascularization and the developing retinal vessels, especially the capillaries undergo reflex vasoconstriction followed by vaso-obliteration (Phase 1 of ROP). Clinical studies of oxygen therapy started soon after birth and continued during the first few weeks of postnatal life investigate this phase of ROP development. Phase 2 of ROP development occurs when normal angiogenesis is overtaken by pathologic angiogenesis. The peripheral avascular retina as well as the developing retinal neurons suffer hypoxic injury. Release of angiogenic factors such as VEGF into the vitreous, increases. At the same time, serum IGF1 levels increase, facilitating the effects of VEGF on retinal angiogenesis. Abnormal blood vessels grow out of the retina, toward the high concentrations of VEGF in the vitreous. Clinical studies of oxygen therapy performed during this phase, typically after 32 wk gestational age, may be expected to produce different effects on retinal blood vessel development than studies performed in the earlier postnatal period.
Risk Factors
The degree of prematurity is in itself the most consistent risk factor for ROP. The lower the birth weight and the gestational age the higher is the risk for ROP. A number of other postnatal factors may contribute to the development of ROP [16,17,18,19,20]. These include, use of supplemental oxygen, intraventricular hemorrhage, apnea, mechanical ventilation, sepsis, surfactant therapy, anemia, thrombocytopenia, administration of blood products, double volume exchange tranfusions and low postnatal weight gain proportion (i.e., weight gain less than 50% of the birth weight) by 6 wk of life. Aggressive Posterior ROP (APROP), a severe, rapidly progressive form of ROP has been reported with the use of unblended oxygen in India in heavier and more mature babies [21].
Classification
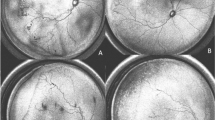
ROP is classified according to the International Classification of ROP (ICROP). This has been revised thrice with the most recent version published in 2005 [22]. It classifies ROP based on the severity (stage), antero-posterior location (zone), circumferential extent and presence or absence of plus disease (Table 1). Generally, ROP passes through five stages (1–5). Late stages of ROP may present with leucocoria (white reflex), falciform fold and pthisis bulbi. A severe and aggressive form of ROP, known as Aggressive Posterior ROP (APROP) has been described in the 2005 ICROP classification (Fig. 1a, b). It can progress directly to Stage 5without passing through the intervening stages. It is characterized by posterior location (Zone I or posterior Zone II), rapidly evolving plus disease, flat intraretinal neovascularization and vascular loops.
Retcam fundus images of an outborn baby, twin delivery at 29 wk of gestation with a birth weight of 1090 g. The baby presented at a post conceptional age of 31 wk. There was history of oxygen supplementation for 10 d; blood transfusion thrice; sepsis and GI bleed. Both eyes had Zone I Aggressive Posterior Retinopathy of Prematurity (APROP). a & b show arteriolar tortuosity and venous dilatation suggestive of plus disease. At the junction of vascular and avascular retina in Zone I, intraretinal flat neovascularization (white arrows) is seen. c & d show regression of the plus disease and confluent laser scars in the right and left eye respectively
Plus Disease The presence of plus disease is critical in the decision making for the treatment vs. follow-up for ROP. Plus disease in the Early Treatment for Retinopathy of Prematurity Cooperative Group (ETROP) study was defined as at least 2 quadrants (6 or more clock hours) of dilation and tortuosity of the posterior retinal blood vessels. It may be associated with a rigid pupil, vitreous haze or neovascularization of the iris. Preplus is defined as vascular abnormalities of the posterior pole that are insufficient for the diagnosis of plus disease but demonstrate more arteriolar tortuosity and more venous dilation than normal. It serves as a red herring that close follow-up may be warranted in such babies as they may progress to frank plus disease.
Treatment
Two trials formed the benchmark for establishing treatment protocols for ROP. These included Cryotherapy for Retinopathy of Prematurity Cooperative Group (CRYO-ROP) and the Early Treatment for Retinopathy of Prematurity Cooperative Group (ETROP) trials [23, 24].
Cryotherapy was the earliest treatment proven to be effective in preventing unfavourable outcomes in ROP. Cryotherapy consisted of freezing the sclera, choroid and the full thickness of the avascular retina from the external ocular surface. The CRYO-ROP study recommended treatment when Stage 3 ROP was present in Zone I or II with plus disease in at least 5 consecutive or 8 cumulative clock hours (thereafter termed “Threshold” ROP). Treatment at threshold resulted in nearly 50% reduction in rates of retinal detachment (21.8% in eyes treated with cryotherapy compared to 43% in the untreated eyes) [23]. Cryotherapy had the disadvantage of being difficult and time consuming, requiring general anaethesia and requiring opening up of the conjunctiva for Zone 1 disease.
Since the rates of unfavourable outcome were still high with cryotherapy, the National Eye Institute in 1999 funded a cooperative agreement to study whether treatment earlier than “Threshold” would have better outcomes. Results from the ETROP study showed a reduction in unfavourable structural outcomes from 15.6% to 9.1% at 9 mo with laser treatment [24] done at high-risk pre-threshold stage compared to treatment at threshold.
Laser photocoagulation earlier than threshold (high-risk pre-threshold ROP or Type 1 ROP; Table 2) thus became the standard of care for the treatment of ROP.
Any eye that did not meet the treatment criteria was thereafter termed as Type 2 ROP. Follow-up was recommended for such eyes till spontaneous regression or progression to treatment stage occurred. These included eyes with Zone I, Stage 1 or 2 ROP without plus, Zone II, Stage1 to 3 ROP without plus, peripheral avascular retina without any stage of ROP. Spontaneous regression of ROP and/or complete vascularization of the retina generally occurs by 40–45 wk of postmenstrual age or 3 to 4 mo after birth. It is advisable to continue screening as per the recommendations of the ETROP study till such time.
Laser treatment must be performed under the supervision of a neonatologist or anesthesiologist. A portable indirect infrared diode laser or frequency doubled Nd:YAG laser [25] can be used for treatment using the laser indirect ophthalmoscope (LIO) delivery system. The entire avascular retina from the ridge upto the ora serrata is ablated in a “near confluent” burn pattern. Laser treatment is also possible inside the incubator through its transparent wall [26] for temperature and oxygen dependant pre-term babies who cannot be transported out of the incubator. Laser parameters used through the transparent wall are similar to those used during conventional treatment. No difficulty is attributable to the incubator wall while focusing and delivering laser.
Presence of posterior Zone 1 disease, gestational age < 29.5 wk, presence of pre-retinal hemorrhages before laser treatment and presence of pre-existing or development of new fibrovascular proliferation post laser have been reported to be significant risk factors for progression to retinal detachment despite confluent laser photocoagulation in eyes with APROP [27].
Laser photocoagulation is still the “gold standard” for the treatment of retinopathy of prematurity. However, a variety of clinical situations exist when laser delivery may be challenging or the outcomes after laser are poor despite confluent laser treatment. Such situations include: poor pupillary dilatation due to severe tunica vasculosa lentis (TVL) or rubeosis iridis, vitreous haze or vitreous hemorrhage precluding visualization of the retina. In addition, Zone I ROP has been reported to have high rates of unfavourable outcomes despite confluent laser photocoagulation [27]. Vascular Endothelial Growth Factor (VEGF) has been recognized as a key factor in the normal retinal vascular development as well as pathogenic retinal neovascularization [28]. The use of the anti-VEGF agent Bevacizumab (Avastin; Genentech, South San Francisco, CA), a recombinant humanized anti-VEGF antibody was reported in ROP for the first time in the year 2007 [29]. The “Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity” (BEAT-ROP) study [30] was a randomized multicenter study in which infants were randomly assigned to intravitreal bevacizumab monotherapy (IVB) (0.625 mg in 0.025 ml) or conventional laser. The primary outcome was recurrence of the retinopathy in one or both eyes requiring retreatment before 54 wks’ postmenstrual age. Recurrence was seen in 4 infants in the bevacizumab group (4%) and 19 infants in the laser treated group (22%); P = 0.002. A significant treatment effect was found for Zone I (P = 0.003) but not for Zone II disease (P = 0.27). In addition, the study reported that development of peripheral retinal vessels continued after treatment with IVB whereas, conventional laser therapy led to permanent destruction of the peripheral retina. Although, no systemic or local toxic effects attributable to bevacizumab were observed, the study concluded that the population size was too small to assess the safety of the drug. Since then there have been numerous reports of its use as monotherapy, in combination with laser, as a rescue therapy after failed laser, in presence of vitreous hemorrhage or as an adjunct prior to vitrectomy. It is known that VEGF is needed for normal pulmonary, brain and kidney development of the growing infants [31]. Organogenesis in infants born preterm is still incomplete. VEGF is both neurotropic and neuroprotective. It also helps to maintain the blood-brain barrier and is required for normal neural retinal development independent of angiogenesis. It is also important for alveolisation as well as surfactant synthesis in the lungs as well as glomerulogenesis and skeletal growth. Lien et al. have shown that VEGF levels in ROP infants were depressed for upto 8 wk after IVB. Concerns regarding its potential systemic side effects still have not been answered fully. In addition to potential systemic effects, the long term ocular effects of anti-VEGF therapy are still being understood. There is growing concern regarding persistence of peripheral avascular retina acting as a source of VEGF leading to persistence of abnormal angiogenesis and thereby resulting in late recurrences of ROP with anti-VEGF monotherapy. Hu et al. have reported reactivation of ROP 4 to 5 wk after anti-VEGF treatment [32]. Snyder et al. have reported very late reactivation 2.5 y after anti-VEGF monotherapy [33]. Until we understand such effects fully, caution should be exercised against the indiscriminate use of these agents. At this time, use of anti-VEGF therapy may be restricted in selected cases as rescue therapy along with laser photocoagulation.
Surgery
Surgery is indicated for Stage 4A, Stage 4B and Stage 5 ROP. The choice of technique depends upon the stage of ROP, extent and location of the traction. Surgical modalities include scleral buckling or lens-sparing vitrectomy for Stages 4 and lensectomy with vitrectomy or open sky vitrectomy for Stage 5 ROP. Lens sparing vitrectomy (LSV) is the most commonly performed surgery for Stage 4 ROP. With the advances in micro-incision vitrectomy techniques small gauge instruments (23, 25 or 27 gauge) are commonly used. Lack of prior treatment such as laser, late presentation, and higher incidence of narrow-narrow funnel configuration are predictors for poor surgical outcome. The anatomic success rates of LSV for Stage 4A ROP have been reported to vary from 84 to 100% whereas those for Stage 5 ROP range from 14.3% - 45.5% in various series [34,35,36].
Long Term Outcomes
Although ablative treatments with laser photocoagulation and cryotherapy reduced the incidence of blindness from ROP, treated patients often still have suboptimal visual acuity. Nearly 86%–93% of threshold ROP [37] and 100% of prethreshold ROP [38] regress with laser treatment. Reported favourable outcomes for APROP range from 71.4%–84% [39]. A number of structural sequelae have been reported in eyes treated for ROP with either cryopexy or laser photocoagulation. These include vascular tortuosity, narrowing of arcades, a temporal myopic crescent, macular heterotropia, disc drag, vitreous membranes and peripheral tractional retinal detachment. Long term follow-up of these children is required for the assessment of refractive errors, strabismus, anisometropia and amblyopia. Myopia is the commonest refractive error in eyes treated for ROP. Presence of myopia and strabismus have been found to be significantly associated with severe ROP and major structural sequelae.
Conclusions
India has the highest number of preterm births in the world. It is the need of the hour that awareness about retinopathy of prematurity is increased among ophthalmologists neonatologists, pediatricians and gynaecologists. The economic, social and financial burden of blindness due to ROP is huge. Guidelines should be established about the use of supplemental oxygen and timely screening of all eligible premature babies in SNCUs/NICUs so that babies at risk of ROP are not missed. Neonatologists, pediatricians and nurses involved in care of preterm babies have a humongous role to play in prevention of ROP. Reduction of the risk factors such as establishing protocols of oxygen delivery and monitoring, preventing infection, restrictive blood transfusion policies as well as informing the parents about the need for eye examination can go a long way in preventing blindness from ROP. Ophthalmologists need to be trained regarding screening and treatment for ROP. Laser photocoagulation is a highly effective treatment modality provided it is administered at the right time. Vitrectomy is indicated for Stage 4A, 4B and 5 ROP. Surgical outcomes for Stage 5 ROP are poor; therefore focus should be on timely detection of disease. Anti VEGF therapy may be considered as rescue therapy in selected cases.
References
Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens: I. Preliminary report. Am J Ophthalmol. 1942;25:203–4.
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82.
Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74:35–49.
Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72.
Gilbert C, Fielder A, Gordillo L, et al; International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115:e518–25.
Lee AC. Katz J, Blencowe H, et al; CHERG SGA-preterm birth working group. National and regional estimates of term and preterm babies born small for gestational age in 138 low- income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–36.
Charan R, Dogra MR, Gupta A, Narang A. The incidence of retinopathy of prematurity in a neonatal care unit. Indian J Ophthalmol. 1995;43:123–6.
Gopal L, Sharma T, Ramchandran S. Retinopathy of prematurity: a study. Indian J Ophthalmol. 1995;43:59–61.
Vinekar A, Dogra MR, Sangtam T, Narang A, Gupta A. Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: ten year data from a tertiary care center in a developing country. Indian J Ophthalmol. 2007;55:331–6.
Jalali S, Matalia J, Hussain A, Anand R. Modification of screening criteria for retinopathy of prematurity in India and other middle-income countries. Am J Ophthalmol. 2006;141:966–8.
Hungi B, Vinekar A, Datti N, et al. Retinopathy of prematurity in a rural neonatal intensive care unit in South India-a prospective study. Indian J Pediatr. 2012;79:911–5.
National Neonatology Forum, India. Evidence-based Clinical Practice Guidelines. Available at: http://www.nnfi.org/images/pdf/nnf_cpg_consolidated_filejanuary 102011.pdf. Accessed on 19 May 2017.
Vinekar A, Gilbert C, Dogra M, et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014;62:41–9.
Sanghi G, Dogra MR, Katoch D, Gupta A. Demographic profile of infants with stage 5 retinopathy of prematurity in North India: implications for screening. Ophthalmic Epidemiol. 2011;18:72–4.
Flynn JT, Chan-Ling T. Retinopathy of prematurity: two distinct mechanisms that underlie zone 1 and zone 2 disease. Am J Ophthalmol. 2006;142:46–59.
Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP). A randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310.
Chen ML, Guo L, Smith LE, Dammann CE, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics. 2010;125:e1483–92.
Karna P, Muttineni J, Angell L, Karmaus W. Retinopathy of prematurity and risk factors: a prospective cohort study. BMC Pediatr. 2005;5:18.
Dutta S, Narang S, Narang A, Dogra M, Gupta A. Risk factors of threshold retinopathy of prematurity. Indian Pediatr. 2004;41:665–71.
Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol. 2009;247:831–6.
Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F371–5.
International Committee for the Classification of Retinopathy of Prematurity. The International classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–9.
Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Sanghi G, Dogra MR, Vinekar A, Gupta A. Frequency-doubled Nd:YAG (532 nm green) versus diode laser (810 nm) in treatment of retinopathy of prematurity. Br J Ophthalmol. 2010;94:1264–5.
Dogra MR, Vinekar A, Viswanathan K, et al. Laser treatment for retinopathy of prematurity through the incubator wall. Ophthalmic Surg Lasers Imaging. 2008;39:350–2.
Sanghi G, Dogra MR, Katoch D, Gupta A. Aggressive posterior retinopathy of prematurity: risk factors for retinal detachment despite confluent laser photocoagulation. Am J Ophthalmol. 2013;155:159–64.
Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–47.
Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38:233–7.
Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28:831–8.
Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F170–4.
Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–6.
Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP. Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2016;47:280–3.
Bhende P, Gopal L, Sharma T, Verma A, Biswas RK. Functional and anatomical outcomes after primary lens-sparing pars plana vitrectomy for stage 4 retinopathy of prematurity. Indian J Ophthalmol. 2009;57:267–71.
Shah PK, Narendran V, Kalpana N, Tawansy KA. Anatomic and visual outcomes of stage 4 & 5 ROP. Eye. 2009;23:176–80.
Gopal L, Sharma T, Shanmugam M, et al. Surgery for stage 5 retinopathy of prematurity: the learning curve and evolving technique. Indian J Ophthalmol. 2000;48:101–6.
Dhawan A, Dogra MR, Vinekar A, Gupta A, Dutta S. Structural sequelae and refractive outcome following successful laser treatment for threshold ROP. J Pediatr Ophthalmol Strabismus. 2008;45:356–6.
Katoch D, Sanghi G, Dogra MR, Beke N, Gupta A. Structural sequelae and refractive outcome after laser treatment for type 1 prethreshold retinopathy of prematurity in Asian Indian eyes. Indian J Opthalmol. 2011;59:423–6.
Sanghi G, Dogra MR, Das P, Vinekar A, Gupta A, Dutta S. Aggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease and outcome after laser treatment. Retina. 2009;29:1335–9.
Author information
Authors and Affiliations
Contributions
MRD was involved in the conception, design of the work; critical revision, accuracy and integrity of data as well as final approval of the manuscript. DK and MD were involved in acquisition, analysis and interpretation of data, literature review for the work as well as drafting of the manuscript. MRD will act as guarantor for the paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Dogra, M.R., Katoch, D. & Dogra, M. An Update on Retinopathy of Prematurity (ROP). Indian J Pediatr 84, 930–936 (2017). https://doi.org/10.1007/s12098-017-2404-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2404-3