Abstract
Retinopathy of prematurity (ROP) is a retinal disorder characterized by abnormal neovascular proliferation that occurs secondary to arrest of the physiologic retinal vascularization. ROP accounts for nearly 40% of childhood blindness and is estimated to affect three-fourth of premature infants born with a birth weight of less than 1250 grams (g) [1]. The epidemiology of ROP is described in three epidemics [2]. Unrestricted use of oxygen led to the first epidemic of ROP in the USA and the UK between 1940 and 1960. Restriction of oxygen usage curtailed the first epidemic albeit with some increase in pulmonary complications and mortality. The second epidemic was again seen in developed countries due to increased survival of preterm babies with very low birth weights. The burden of ROP in current times is limited to the low- and middle-income countries and is postulated to be similar to the situation in the developed countries in the first epidemic. Out of the estimated 15 million preterm infants born worldwide every year, nearly 3.8 million are born in low- and middle-income countries [3]. High rates of preterm births, suboptimal neonatal care, low screening coverage, and paucity of trained ophthalmologists have caused ROP to become an emerging public health problem in these nations. Studies in Asian Indian infants have shown that ROP can occur in heavier and more mature infants with birth weight >1250 g [4, 5]. In India, for example, as per WHO (2010) out of the 3.5 million preterm births, about 6,00,000 babies are born at gestational age (GA) <32 weeks. Out of these, approximately 2,00,000 babies are at the risk of developing ROP every year. Even if 10% of them develop treatable disease, roughly 20,000 newborns will require ROP management every year in India alone [6, 7].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
5.1 Introduction
Retinopathy of prematurity (ROP) is a retinal disorder characterized by abnormal neovascular proliferation that occurs secondary to arrest of the physiologic retinal vascularization. ROP accounts for nearly 40% of childhood blindness and is estimated to affect three-fourth of premature infants born with a birth weight of less than 1250 grams (g) [1]. The epidemiology of ROP is described in three epidemics [2]. Unrestricted use of oxygen led to the first epidemic of ROP in the USA and the UK between 1940 and 1960. Restriction of oxygen usage curtailed the first epidemic albeit with some increase in pulmonary complications and mortality. The second epidemic was again seen in developed countries due to increased survival of preterm babies with very low birth weights. The burden of ROP in current times is limited to the low- and middle-income countries and is postulated to be similar to the situation in the developed countries in the first epidemic. Out of the estimated 15 million preterm infants born worldwide every year, nearly 3.8 million are born in low- and middle-income countries [3]. High rates of preterm births, suboptimal neonatal care, low screening coverage, and paucity of trained ophthalmologists have caused ROP to become an emerging public health problem in these nations. Studies in Asian Indian infants have shown that ROP can occur in heavier and more mature infants with birth weight >1250 g [4, 5]. In India, for example, as per WHO (2010) out of the 3.5 million preterm births, about 6,00,000 babies are born at gestational age (GA) <32 weeks. Out of these, approximately 2,00,000 babies are at the risk of developing ROP every year. Even if 10% of them develop treatable disease, roughly 20,000 newborns will require ROP management every year in India alone [6, 7].
Amid the growing number of infants requiring ROP screening and treatment, there have been evolutionary changes in disease classification, disease documentation, and treatment which will be discussed in this chapter.
5.2 Classification
ROP is classified using the International Classification of ROP (ICROP) into zones (I–III) for disease location, stage 1–5 for disease severity, and clock hours 1–12 for disease extent. Dilatation and tortuosity of the posterior retinal vessels are noted as the presence or absence of plus disease. The ICROP classification was revised in 1987 and again in 2005 to include the terms “pre-plus” and “Aggressive Posterior ROP” (APROP). Appearance of posterior retinal vessels which was less than the definition of plus was categorized as pre-plus, warranting closer observation for progression to plus and treatment requiring disease [8]. APROP was classified as a severe, rapidly progressive disease characterized by location in zone I or posterior zone II, prominence of plus, flat neovascularization, circumferential shunt vessels, and vascular loops. APROP can progress directly to retinal detachment without passing through the typical intervening stages if left untreated [8].
ICROP was recently updated in 2021 (ICROP 3) with the following salient new additions [9].
-
(a)
Definition of “posterior zone II” as an intermediate zone that begins at the margin of zone I and zone II and extends into zone II for 2 disc diameters.
-
(b)
Description of a “Notch” as an incursion of the ROP lesion for 1 or 2 clock hours into a more posterior zone. In the presence of a posterior notch, the zone for eyes is labeled by the most posterior zone of retinal vascularization.
-
(c)
Aggressive ROP—ICROP 3 recognized that APROP may occur beyond the posterior retina and in larger preterm infants, particularly in regions of the world with limited resources. Therefore, replacement of the term APROP with Aggressive ROP (A-ROP) has been recommended.
-
(d)
Subclassifications of stage 5 as 5A when the optic disc is visible by ophthalmoscopy, 5B—when the optic disc is not visible because of retrolental fibrovascular tissue or closed-funnel detachment, 5C—presence of anterior segment changes such as anterior chamber shallowing, iridocorneolenticular adhesions, and corneal opacification.
-
(e)
Regression: Signs of regression include reduction of plus, advancement of vascularization into the peripheral avascular retina, involution of tunica vasculosa lentis, better pupillary dilation, greater media clarity, and resolution of intraretinal hemorrhages.
-
(f)
Reactivation: Appearance of recurrent vascular dilation, tortuosity, or both, similar to acute-phase pre-plus or plus disease. Documentation of reactivation should specify the presence and location(s) of new ROP features, using the modifier reactivated. For example, the presence of a ridge during reactivation would be noted as “reactivated stage 2.”
-
(g)
Long-term Sequelae: Include late retinal detachments, Persistent Avascular Retina, Macular anomalies, Retinal vascular changes, and glaucoma.
5.2.1 Risk Factors
ROP is a multifactorial disease. Identification of the risk factors can aid neonatologists as well as ophthalmologists to perform careful risk stratification for screening and take necessary measures to prevent disease progression. Among the several risk factors linked to ROP, low birth weight, prematurity, and postnatal oxygen exposure are well known to confer independent risk. Apart from this, prolonged mechanical ventilation, APGAR score, anemia, sepsis, pulmonary complications, use of surfactant, multiple blood transfusions, necrotizing enterocolitis, intraventricular hemorrhage (IVH), thrombocytopenia, and cardiac disease are some of the other risk factors for development of ROP in preterm infants [10].
5.2.2 Screening Guidelines
Screening guidelines vary across different demographic regions based on different risk profiles and quality of neonatal care. The American Academy of Pediatrics (AAP), American Association for Pediatric Ophthalmology and Strabismus (AAPOS), and the American Academy of Ophthalmology (AAO) recommend a retinal screening examination for infants with a birth weight of ≤1500 g or gestational age of ≤30 weeks. Infants between 1500 and 2000 gm and GA >30 weeks with risk factors such as prolonged oxygen supplementation are also eligible for screening [11]. The United Kingdom guidelines recommend screening all preterm infants with GA < 32 weeks and birth weight <1501 gm [12].
Development of severe ROP in more mature and heavier infants in developing countries who may be missed if western guidelines are used for screening has been reported previously [4]. This has necessitated a need to alter screening guidelines based on country-specific incidence and disease distribution [4]. The current Indian ROP guidelines as an example recommend screening of all children born with GA <34 weeks and/or BW <2000 g. Those born at >34 weeks GA to be screened in presence of risk factors (prolonged oxygen requirement, respiratory distress syndrome, chronic lung disease, blood transfusions, sepsis, apneas, poor postnatal weight gain, cardiorespiratory support, IVH). Screening should be performed before the baby is discharged from NICU/SNCU, or by 30 days of life, whichever is earlier [7]. Babies with GA <28 weeks and birth weight <1200 grams should be screened by 2–3 weeks of chronological age.
5.2.3 Imaging for ROP
Indirect ophthalmoscopy with scleral depression by an experienced ophthalmologist is considered the gold standard for ROP screening. The difficulty with this approach is the paucity of trained ophthalmologists, lack of objectivity, and an associated learning curve. All these factors have translated into remote telemedicine screening by nonphysicians such as trained ophthalmic technicians and nurses [13, 14]. Digital retinal imaging devices have now been validated for objective disease documentation, parental counseling, and telemedicine-based screening in remote areas where no ROP specialists are available [13,14,15].
Fundus photography in premature babies presents unique challenges due to the absence of fixation and voluntary cooperation from the infant. Imaging systems for ROP also need to be portable to allow transport across different NICUs, have a wide field of view, be able to image eyes and fundi of different colors, and allow easy export of files in standardized medical image formats for telemedicine.
The most commonly used fundus camera for ROP screening and also proven useful for telemedicine is the RetCam (Natus Medical Systems, Inc., Pleasanton, CA, US). It is a contact, wide-angle, mydriatic, hand-held imaging device that can capture upto 1300 of the retina [16]. The RetCam Shuttle is the compact, easily portable, and lighter version of the RetCam. RetCam 3 version of this system has a Fluorescein Angiography feature to delineate retinal vascular details.
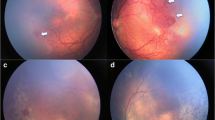
Trinetra Neo (Forus health, Bangalore, India) is a novel low-cost, handheld, light-weight, and portable fundus camera which provides approximately 120° field of view. It uses a light-emitting diode (LED) illumination and a liquid lens-based focusing system. It has been found to be safe for ocular use [17, 18]. This camera has the potential to be used in low-resource countries allowing better telemedicine care (Fig. 5.1).
Comparison of wide-field fundus photographs using the Trinetra neo fundus camera (top panel) showing stage 3 Retinopathy of prematurity (ROP) (a) in the right eye and stage 2 ROP in the left eye (b) in zone II in a preterm infant. Bottom panel shows fundus photographs using the Retcam 3 depicting stage 2 ROP in zone II in right (c) and left eye (d)
The ICON (Phoenix Clinical, Inc., Pleasanton, CA, US) is also a mydriatic, contact, handheld camera with reported improved resolution and color profile for capturing images in dark fundi; Fluorescein Angiography is also available with this device. The Panocam (Visunex Medical Systems, Inc., Fremont, CA, US) is a wireless, contact, handheld camera system with a 130° field of view.
Noncontact fundus cameras have also been used for capturing ROP images and detecting treatment/referral warranted ROP. The disadvantage of noncontact systems are difficulties in infant positioning, motion artifacts, and inability to capture the retinal periphery in majority. Nidek NM200-D is a portable, posterior pole retinal camera with a 30° field of view [17]. Skalet et al. have shown the feasibility of this fundus camera to screen for ROP in remote areas and identify referral warranted ROP (RW-ROP). Pictor is an FDA-approved narrow-angle retinal camera with a field of view of 45°. Plus and pre-plus disease can be documented with high sensitivity and specificity using this modality for ROP screening [19].
Optos 200Tx is an ultrawide field noncontact fundus camera which allows fundus imaging upto 200°. Mao et al. have shown feasibility to perform ultrawide imaging using Optos 200Tx by placing the baby in the “flying baby” position [20]. It involves placing one arm to support the chin/chest and the other hand supporting the head. Eyelid speculum is used to keep the eyelids open and stabilize the eye movements. The ultrawide field images show areas of non-perfusion, demarcation line and ridge, presence of neovascularization and hemorrhages. This allowed the physician to clearly identify the stage and zone of the disease.
Smartphone-based fundus imaging (SBFI) has also been used for documentation of ROP [21, 22]. With the help of different condensing lenses and specialized lens holders, smartphones can be used as cost-effective, handy, and noncontact devices to capture reasonable quality retinal images.
5.3 Optical Coherence Tomography (OCT)
With the introduction of portable Spectral domain OCT (SD-OCT) and age-adjusted scanning protocols, it is now possible to perform OCT in preterm infants [23]. Subclinical findings are being detected on OCT in both APROP and other stages of ROP which are difficult to pick up on indirect ophthalmoscopy. Presence of isolated extraretinal lesions [24] which correspond to popcorn retinopathy, and shown to be precursors to stage 3 disease have also been detected close to the optic disc on OCT in APROP [25]. Epiretinal membranes causing alterations in foveal contour in preterm infants have been detected on SD-OCT [26]. Presence of hyporeflective cystoid spaces predominantly in the inner nuclear layer in preterm infants has been described as macular edema of prematurity (MEOP). MEOP is usually bilateral and is known to be a transient event which resolves spontaneously [27]. Two patterns of MEOP have been described: one with a dome-shaped fovea and the other with a normal foveal morphology [28]. These cystic changes are independent of the stage of ROP. Long-term effects of MEOP on foveal development and photoreceptor function are still unknown. Visual function testing is needed to determine if these cysts have any adverse effect on normal foveal development.
Tilting the handheld probe can allow the physician to screen the retinal periphery and detect stage 3 disease in zone 2. OCT can also be useful in determining the extent of foveal involvement in stage 4 disease. Studies have shown the presence of foveal architecture abnormalities thus explaining poor visual outcomes despite a successfully attached retina [29].
Subfoveal choroidal thickness can also be measured using SD-OCT devices in premature infants. Mean choroidal thickness in premature infants range from 324 ± 79 um to 356.9 ± 75.8 um [30, 31]. Erol et al. have shown a decrease in choroidal thickness with increasing stages of ROP and attributed it to increased oxidative stress at worse stages of ROP [31].
Novel information provided by OCT in ROP will help in better understanding of disease pathophysiology and may provide answers to the possible implications of ROP in foveal and visual development. Furthermore, OCT may provide new quantifiable references for future treatments.
5.4 OCT Angiography (OCT-A)
There are currently no commercially available handheld OCT-A devices to perform OCT-A in preterm infants. Vinekar et al. reported OCT-A findings in APROP using Optovue [32]. Campbell et al. were able to successfully visualize microvascular changes in various stages of ROP using a handheld prototype OCT-A device [33]. The study demonstrated attenuated flow signal in areas of atrophic retina, increased signal transmission in areas of laser scars, and lack of flow in overlying preretinal fibrous proliferation. Kothari et al. have shown the feasibility of arm-mounted OCT-A to detect changes in FAZ in extremely low birth weight babies and allow a better understanding of foveal development in premature babies [34]. OCT-A studies have shown a better foveal development in previously anti-VEGF treated eyes compared to laser treatment [35]. Mataftsi et al. have shown a significantly smaller FAZ and a lower vessel density in spontaneously regressed ROP compared to preterm babies with no ROP and age-matched normal controls [36].
5.5 Fundus Fluorescein Angiography (FFA)
FFA has been a useful adjunct to clinical examination in pediatric retinal vascular diseases. Widefield FFA helps to document and analyze subtle peripheral vascular changes that can be missed on clinical examination (Fig. 5.2). Retcam 3 and Optos 200Tx have been successfully used to record widefield angiograms in infants [20, 37]. FFA has helped identify vascular abnormalities such as areas of non-perfusion, extent of neovascularization, avascular loops, residual skip areas, and status of macular perfusion in APROP [20, 37].
Wide-field retcam fundus photographs in a preterm infant born at 28 weeks of gestation with a birth weight of 1000 grams showing flat neovascularization or hemorrhage in posterior zone II with pre-plus. (a, b). Fluorescein angiography confirms and also demonstrates the extent of neovascularization at the vascular avascular junction (c, d)
Anti-VEGF therapy is preferred over laser in infants with severe vaso-obliteration and vascularization limited to posterior zone I (Fig. 5.3). Fluorescein angiography helps to clearly delineate persistent avascular retina following treatment with Anti-VEGF agents and helps guide the need for retreatment and follow-up.
Retcam fundus photography showing posterior zone I APROP with loops and shunts in posterior zone I (a, b) along with a preretinal bleed over the optic disc (b). Fluorescein angiography confirms non-perfusion of the macula in both eyes (c, d). This infant was treated with Anti-VEGF therapy to provide an opportunity to allow vascularization to progress beyond macula
5.6 Telemedicine in ROP
Amid the growing numbers of infants requiring screening and significant workforce limitation, the role of telemedicine-based screening has expanded in the field of ROP. Telemedicine allows non-ophthalmologists to capture and then transfer images online to a remotely located ROP specialist. The specialist examines the images and provides opinion regarding treatment or follow-up. Infants requiring urgent intervention can be called for management to the referral center.
Photo-ROP trial compared telemedicine with bed-side binocular indirect ophthalmoscopy and found them to be equally effective [38]. e-ROP study showed that training and certifying nonphysician graders to read and interpret ROP images under the supervision of a reading center director was reliable in detecting potentially serious ROP with good intragrader and intergrader consistency [39]. The Stanford University Network for Diagnosis of ROP (SUNDROP) is a telemedicine screening program based on a hub and spoke model [40]. The central hub (remote screener) at the university collects digital images generated by Retcam (Clarity Medical Systems) from six peripheral NICUs. The screener looks for all referral warranted and treatment warranted disease. The screening program has a sensitivity of 100% and specificity over 99.5% with good negative and positive predictive values. A similar telescreening program which has successfully completed over 46,000 imaging sessions and over 1000 treatment procedures is the “Karnataka Internet Assisted Diagnosis of Retinopathy of Prematurity” (KIDROP). Technicians are trained to color code the images as red (Type 1 ROP), orange (Type 2 ROP), and green (normal or mature retina). Images are uploaded on a secure tele-ROP platform and are finally analyzed by the ROP experts. Red flagged images are given a priority so that diagnosis can be provided the same day and parents are referred for time management [41]. Use of imaging, grading, and computer-based image analysis can improve accuracy and consistency of diagnosis of plus disease compared to indirect ophthalmoscopy-based diagnosis.
5.7 Management
5.7.1 Laser Therapy
Laser photocoagulation of the peripheral avascular retina is considered the gold standard for the treatment of ROP. Topical anesthesia with 5% proparacaine or general anesthesia can be utilized to perform the laser procedure. The Early Treatment of Retinopathy of Prematurity (ETROP) showed both anatomical and functional benefits of treating the avascular retina with diode laser photocoagulation at an earlier than threshold stage when compared to cryoablation. Laser photocoagulation was recommended for type 1 ROP defined as a) presence of any ROP stage with plus or stage 3 without plus in zone I or b) stage 2 or 3 with plus in zone II [42]. Laser therapy converts the peripheral hypoxic retina into anoxic retina thereby reducing the stimulus for neovascularization and disease progression. More recently, laser photocoagulation using the frequency doubled Nd:YAG (532 nm green) laser has been shown to be as effective as the 810 nm wavelength. Sanghi et al. have reported comparable efficacy with both infrared diode and frequency doubled Nd: YAG laser [43]. This study also showed the feasibility to perform frequency doubled Nd: YAG laser in eyes with tunica vasculosa lentis and preretinal hemorrhage without any accentuated risk of inducing cataract, anterior segment ischemia, or hyphema. Other reported advantages with Nd: YAG laser include higher “defocus threshold” resulting in lesser spots being wasted, lesser pain and more confluent spots being achieved as compared to infrared diode laser.
Conventionally laser burns are applied to the avascular retina upto the ora serrata in a near confluent manner. Despite this, the ETROP reported a 15.7% risk of progression to stage 4 or 5. Subtle modifications in the technique have been reported to be useful for severe stage 3 ROP. Ells et al. have shown rapid regression of the disease with posterior laser treatment in severe stage 3 zone II disease with no increased safety concerns [44]. The study proposed the role of primary “posterior to ridge” laser for eyes with thick stage 3 in four or more confluent temporal clock hours, plus disease in temporal two quadrants, subretinal fluid associated with the ridge, minimal temporal traction and minimum 3000 um distance between the ridge and the fovea.
Yetik et al. have advocated the role of limited laser treatment (covering the demarcation line with 5–6 rows of laser spots 360°) in zone 2 type 1 prethreshold cases [45]. This prevents ablation of a large amount of peripheral retina. Limited laser treatment is based on a newly proposed hypothesis by the author that ROP is a disease initially confined to the vitreoretinal interface and results from aberrant growth of normal retinal vessels into the vitreous gel. Ambiguity of the premature vitreoretinal interface with immature ILM causes misretinalization of retinal vasculogenesis. Limited laser treatment around the demarcation line/ridge can cease the vascular growth into the vitreous gel thus allowing ILM and vitreoretinal interface to mature more and allowing the disease to regress.
5.7.2 Surgical Management
Management of progressive stage 4 and stage 5 ROP involve surgical intervention in the form of scleral buckling or vitrectomy. The primary aim of surgery is to relieve tractional forces from the ridge to ora serrata, ridge to lens, and ridge to ridge. Whereas scleral buckling may be possible for stage 4 A or 4B ROP, the need for removal of the scleral buckle to allow growth of the eyeball necessitates a second surgery before the first year of life. Introduction of microincision vitrectomy surgery (MIVS) has not only improved functional and structural outcomes but also reduced surgical times and surgical trauma [46]. Several advancements in the MIVS including the use of smaller gauges (23, 25, and 27 gauge), high cut rates upto 10,000 cuts per minute, valved cannulas, improved illumination, and availability of specialized instruments allows improved access to the tight retinal folds (Fig. 5.4). Lens sparing vitrectomy involves making sclerotomy ports 1–1.5 mm from the limbus through the pars plicata or the iris root. Lensectomy with vitrectomy is primarily employed in cases with stage 5 ROP, associated retrolental fibroplasia, or stage 4B ROP where the traction or the ridge extends anteriorly upto the lens capsule.
Retcam fundus photographs showing the presence of Aggressive posterior ROP in the right eye of a preterm infant (a) which showed progression to a stage 4A tractional retinal detachment despite confluent laser photocoagulation (b). The same eye following 25-gauge microincision lens sparing vitrectomy 1 week (c) and 2 months (d) postoperatively with complete flattening of the ridge
The primary aim of stage 5 ROP surgery is to remove the fibrous stalk from the disc which holds the retina along the central axis, allowing the posterior funnel to open up. This is followed by careful dissection of peripheral tough present between the peripheral avascular retina and the ridge [46].
Surgical outcomes for stage 4 disease with 25 gauge vitrectomy (81–100%) are much better than 20 gauge vitrectomy (62–92%) [47,48,49,50]. There are limited reports of MIVS in stage 5 disease. Reported surgical success with 23/25 gauge MIVS systems ranges from 33 to 45.4% in stage 5 disease as compared to 13–42% with 20 gauge vitrectomy systems [46, 51].
Factors associated with favorable outcomes include stage 4 disease, previous laser treatment, LSV, and surgery with 25 G MIVS compared to 23 G [46]. Formation of inadvertent intraoperative retinal breaks is associated with poor anatomical outcomes in ROP surgery and should be avoided by careful and meticulous dissection.
5.7.3 Anti-VEGF Treatment
Vascular endothelial growth factor (VEGF) is important for angiogenesis in fetal life and is also regarded as a key mediator in the pathogenesis of abnormal neovascularization in ROP. Cryotherapy and laser ablation, which are standard of care for ROP, help decrease the VEGF levels by converting hypoxic peripheral avascular retina into anoxic retina. Through effective therapy, studies have shown poor structural and functional outcomes with both laser and cryoablative therapy in eyes with zone 1 disease and APROP [52]. Apart from this, both these are associated with ocular complications like high myopia and peripheral field loss. In such a scenario, anti-VEGF injections have emerged as a promising therapy for the management of ROP, especially for disease in zone 1. Currently, various anti-VEGFs like bevacizumab, ranibizumab, aflibercept, pegaptanib, and conbercept have been evaluated in ROP treatment [53].
The BEAT-ROP study was the first prospective randomized study to show the efficacy of 0.625 mg bevacizumab in the treatment of stage 3 ROP with plus disease in zone 1 or posterior zone II [54]. Fewer recurrences (22% in laser vs 4% in the bevacizumab group) were reported at 54 weeks postmenstrual age and the results were statistically significant for zone I eyes but not for eyes in zone II. At 2.5 years the Anti-VEGF treated eyes in the BEAT-ROP study showed lower degrees of myopia compared to the laser-treated eyes [55].
One of the major concerns with intravitreal therapy with anti-VEGF agents is the long-term systemic side effects [53]. Studies have shown a reduction in serum VEGF levels with the presence of anti-VEGF agents in systemic circulation following anti-VEGF injection in the vitreous cavity. VEGF is known to regulate the growth of various other organs like the brain, kidney, liver, hematopoietic system, lungs, and bones. Being a bigger and heavier molecule, vitreous and serum half-life of bevacizumab is much higher than ranibizumab (vitreous half-life: 5.6–6.7 days versus 3.2 days; serum half-life: 20 days versus 2 h respectively). Aflibercept has an intermediate value between the two (vitreous half-life: 4.8 days; serum half-life: 11.4 ± 4.8 days) [53].
As a result, long-term systemic effects of Anti-VEGF injections on organ growth and function including neurodevelopment cannot be overlooked and need to be studied in detail.
PEDIG group studied variable doses of bevacizumab (0.25 mg, 0.125 mg, 0.063 mg, and 0.031 mg) in the treatment of type 1 ROP and concluded that even the lowest dose of bevacizumab (0.031 mg) which was 5% of dose used by BEAT-ROP study was effective after 4 weeks in 9 out of 10 eyes [56]. Ranibizumab after its intravitreal administration in premature infants has been shown to have less prolonged (7 days) systemic suppression of VEGF compared to bevacizumab. Recent studies have evaluated the effect of decreasing the dosage of ranibizumab on the disease outcome and associated systemic adverse events [53].
RAINBOW, an open-label randomized controlled trial studied the efficacy of ranibizumab 0.1 mg or 0.2 mg versus laser in very low birth weight infants (<1500 g) with ROP in zone I and II. The study reported overall treatment success to be 80% (0.1 mg) and 88% (0.2 mg) compared to 66% with laser photocoagulation alone [57]. The study showed no clear evidence of systemic VEGF suppression following intravitreal ranibizumab therapy. Ranibizumab also showed an acceptable short-term safety profile with systemic side effects equally distributed in all three treatment arms (0.1 mg ranibizumab, 0.2 mg ranibizumab, and laser arm). Systemic events were reported to be likely related to preterm birth and not dependent upon therapy.
Contrary to the above trials, some investigators have shown deleterious effects on neurocognitive development in preterm infants treated with anti-VEGF injections including a lower motor score, poor early cognitive function, and high mortality in preterm infants treated with anti-VEGF injection. However, in all such studies, the role of other confounding factors like lower birth weight, prematurity itself, prolonged ventilation, and prolonged oxygen supplementation in the anti-VEGF group could not be ruled out [58]. Though there are reports regarding thromboembolic events, respiratory failure, hepatic dysfunction, and nephropathy post-injection, their association with the anti-VEGF drug itself has not been convincingly proven. Long-term studies are required to look into the ocular and systemic side effects associated with anti-VEGF agents.
5.7.4 Artificial Intelligence (AI) and Deep Learning in ROP
With the increasing trend of telemedicine in ROP, there is a paradigm shift in computer-based image analysis (CBIA) in ROP. Before the advent of deep learning, traditional machine-based learning involved tedious steps of defining the disease features of specific interest followed by feature extraction using explicit algorithms and training a classifier, and lastly testing system performance on previously unseen dataset. The performance of the system is likely to be affected by any of the above steps. Earlier CBIA systems used manual or semi-automated approaches to look at vessel diameter and tortuosity using fundus photographs for ROP diagnosis. These systems lacked adequate diagnostic performance due to inaccuracy of vessel segmentation or time-consuming manual inputs to delineate posterior pole vessels [59].
In the last few years, there has been a shift from machine-based learning to deep convolutional neural networks (CNNs), also known as deep learning. Deep learning classifiers do not need external training and learn without being told what to focus on. CNN-based systems perform similar to human graders in determining cases of ROP [59].
DeepROP is an automated ROP screening application which can successfully detect the presence or absence of ROP features (Id-Net) and can additionally grade the severity of the disease (Gr-Net) [60]. i-ROP-deep learning system (i-ROP-DL) is another CNN-based system which can classify plus disease by accurate vessel segmentation [61]. As both DeepROP and i-ROP-DL use publicly available ImageNet databases for training, they demonstrate strong agreement with expert opinion. The ROP vascular severity score (score derived from i-ROP-DL for ROP screening) can be used to monitor disease progression, and regression following treatment and differentiate the pace of disease in APROP.
AI seems to be an upcoming technology expected to solve some of the problems related to ROP screening. Its implementation into routine ROP care seems to be a distant reality. To implement the use of AI for ROP diagnosis in the practical world, concerted efforts targeted at developing standards for data acquisition, true external validation, and demonstration of feasibility should be made. There are several unaddressed technical, ethical, clinical, financial, and regulatory considerations which need to be understood before it can be brought into clinical practice.
5.7.5 Medicolegal Aspects in ROP
Malpractice claims in ROP are a result of errors that result in failure to screen the infant in a timely manner, leading to failure/delays in intervention and/or referral [62]. Medicolegal risks can be avoided by understanding the complex interplay between the parents of the infant, the NICU, various caregivers, social workers, office staff, and screening physicians. Some key aspects to keep in mind include (a) engaging the family in the ROP screening process by providing clear written and verbal communication regarding timely follow-up, potentially blinding complications of ROP, and the importance of timely treatment. Performing the first screening before discharge is likely to result in better follow-ups. (b) Coordination among the various stakeholders (pediatricians, gynecologists, nurses, and ophthalmologists) is another key element to ensure no infant escapes the screening program. Lastly, ophthalmologists should remain updated with current screening guidelines in their demographic region and follow-up protocols.
Key Points
-
1.
This chapter briefly summarizes the current disease burden of ROP, the latest screening guidelines, and recent advances in the management of ROP.
-
2.
Fundus imaging, FFA, OCT, and OCT-A have proven a useful adjunct to clinical examination using binocular indirect ophthalmoscopy (BIO). Novel information provided by these modalities will help in better understanding of disease pathophysiology and improving our understanding of foveal and visual development and its maturation.
-
3.
Telemedicine offers a viable, validated, and objective alternative to screening using BIO and is a boon for places with shortage of trained ophthalmologists.
-
4.
Anti-VEGF injections have been a useful addition in the treatment of severe zone I ROP, though long-term safety and timeline for long term follow ups in view of a persistent avascular retina induced by the treatment are yet to be addressed, and laser photocoagulation still remains a highly effective therapy for most ROP cases
-
5.
Artificial intelligence and automated detection of ROP seem to hold promise for ROP detection and its clinical utility needs further study...
References
Carroll L, Owen LA. Current evidence and outcomes for retinopathy of prematurity prevention: insight into novel maternal and placental contributions. Explor Med. 2020;1:4–26.
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82.
Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013 Dec;74(Suppl 1):35–49.
Vinekar A, Dogra MR, Sangtam T, et al. Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth: ten year data from a tertiary care center in a developing country. Indian J Ophthalmol. 2007;55(5):331–6.
Jalali S, Matalia J, Hussain A, et al. Modification of screening criteria for retinopathy of prematurity in India and other middle-income countries. Am J Ophthalmol. 2006;141(5):966–8.
Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet Lond Engl. 2012;379(9832):2162–72.
Honavar SG. Do we need India-specific retinopathy of prematurity screening guidelines? Indian J Ophthalmol. 2019;67(6):711–6.
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9.
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;S0161-6420(21):00416–4. https://doi.org/10.1016/j.ophtha.2021.05.031.
Kim SJ, Port AD, Swan R, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63(5):618–37.
Fierson WM. American academy of pediatrics section on Ophthalmology; American academy of ophthalmology; American association for pediatric ophthalmology and strabismus; American association of certified orthoptists. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2018;142(6):e20183061. https://doi.org/10.1542/peds.2018-3061.
Wilkinson AR, Haines L, Head K, et al. Guideline Development Group of the Royal College of Paediatrics and Child Health, Royal College of Ophthalmologists, et al. UK retinopathy of prematurity guideline. Eye Lond Engl. 2009;23(11):2137–9.
Richter GM, Sun G, Lee TC, et al. Speed of telemedicine vs ophthalmoscopy for retinopathy of prematurity diagnosis. Am J Ophthalmol. 2009;148(1):136–142.e2.
Athikarisamy SE, Lam GC, Ross S, et al. Comparison of wide field imaging by nurses with indirect ophthalmoscopy by ophthalmologists for retinopathy of prematurity: a diagnostic accuracy study. BMJ Open. 2020;10(8):e036483.
Richter GM, Williams SL, Starren J, et al. Telemedicine for retinopathy of prematurity diagnosis. Surv Ophthalmol. 2009;54(6):671–85.
Salcone EM, Johnston S, VanderVeen D. Review of the use of digital imaging in retinopathy of prematurity screening. Semin Ophthalmol. 2010;25(5–6):214–7.
Skalet AH, Quinn GE, Ying G-S, et al. Telemedicine screening for retinopathy of prematurity in developing countries using digital retinal images: a feasibility project. J AAPOS. 2008;12(3):252–8.
Vinekar A, Rao SV, Murthy S, Jayadev C, Dogra MR, Verma A, Shetty B. A novel, low-cost, wide-field, infant retinal camera, "neo": technical and safety report for the use on premature infants. Transl Vis Sci Technol. 2019;8(2):2. https://doi.org/10.1167/tvst.8.2.2.
Prakalapakorn SG, Wallace DK, Freedman SF. Retinal imaging in premature infants using the Pictor noncontact digital camera. J AAPOS. 2014;18(4):321–6.
Mao J, Shao Y, Lao J, et al. Ultra-wide-field imaging and intravenous fundus fluorescein angiography in infants with retinopathy of prematurity. Retina (Phila Pa.). 2020;40(12):2357–65.
Goyal A, Gopalakrishnan M, Anantharaman G, et al. Smartphone guided wide-field imaging for retinopathy of prematurity in neonatal intensive care unit – a smart ROP (SROP) initiative. Indian J Ophthalmol. 2019;67(6):840–5.
Wintergerst MWM, Petrak M, Li JQ, et al. Non-contact smartphone-based fundus imaging compared to conventional fundus imaging: a low-cost alternative for retinopathy of prematurity screening and documentation. Sci Rep. 2019;9(1):19711.
Maldonado RS, Toth CA. Optical coherence tomography in retinopathy of prematurity. Clin Perinatol. 2013;40(2):271–96.
Wallace DK, Kylstra JA, Greenman DB, et al. Significance of isolated neovascular tufts (“popcorn”) in retinopathy of prematurity. J AAPOS. 1998;2(1):52–6.
Chavala SH, Farsiu S, Maldonado R, et al. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–56.
Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina (Phila Pa). 2011;31(8):1470–82.
Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol 1912. 2012;130(5):569–578.
Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5183–8.
Joshi MM, Trese MT, Capone A. Optical coherence tomography findings in stage 4A retinopathy of prematurity: a theory for visual variability. Ophthalmology. 2006;113(4):657–60.
Moreno TA, O’Connell RV, Chiu SJ, et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013;54(6):4140–7.
Erol MK, Coban DT, Ozdemir O, et al. Choroidal thickness in infants with retinopathy of prematurity. Retina (Phila Pa). 2016;36(6):1191–8.
Vinekar A, Chidambara L, Jayadev C, et al. Monitoring neovascularization in aggressive posterior retinopathy of prematurity using optical coherence tomography angiography. J AAPOS. 2016;20(3):271–4.
Campbell JP, Nudleman E, Yang J, et al. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol. 2017;135(9):977–81.
Kothari N, Chu A, Huang JM, et al. Arm-mounted optical coherence tomography angiography in extremely low birth weight neonates with retinopathy of prematurity. Am J Ophthalmol Case Rep. 2020;18:100624.
Zhao J, Wu Z, Lam W, et al. Comparison of OCT angiography in children with a history of intravitreal injection of ranibizumab versus laser photocoagulation for retinopathy of prematurity. Br J Ophthalmol. 2020;104(11):1556–60.
Mataftsi A, Dermenoudi M, Dastiridou A, et al. Optical coherence tomography angiography in children with spontaneously regressed retinopathy of prematurity. Eye. 2021;35(5):1411–7.
Klufas MA, Patel SN, Ryan MC, et al. Influence of fluorescein angiography on the diagnosis and Management of Retinopathy of prematurity. Ophthalmology. 2015;122(8):1601–8.
Photographic Screening for Retinopathy of Prematurity (Photo-ROP) Cooperative Group. The photographic screening for retinopathy of prematurity study (photo-ROP). Primary outcomes. Retina (Phila Pa.). 2008;28(3 Suppl):S47–54.
Daniel E, Quinn GE, Hildebrand PL, et al. Validated system for centralized grading of retinopathy of prematurity: telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA Ophthalmol. 2015;133(6):675–82.
Murakami Y, Jain A, Silva RA, et al. Stanford University network for diagnosis of retinopathy of prematurity (SUNDROP): 12-month experience with telemedicine screening. Br J Ophthalmol. 2008;92(11):1456–60.
Vinekar A, Gilbert C, Dogra M, et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J Ophthalmol. 2014;62(1):41–9.
Good WV. Early treatment for retinopathy of prematurity cooperative group. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48.
Sanghi G, Dogra MR, Vinekar A, et al. Frequency-doubled Nd:YAG (532 nm green) versus diode laser (810 nm) in treatment of retinopathy of prematurity. Br J Ophthalmol. 2010;94(9):1264–5.
Ells AL, Gole GA, Lloyd Hildebrand P, et al. Posterior to the ridge laser treatment for severe stage 3 retinopathy of prematurity. Eye. 2013;27(4):525–30.
Yetik H. Limited laser treatment for retinopathy of prematurity: a new hypothesis for pathogenesis of ROP based on ambiguity of vitreoretinal interface, immaturity of internal limiting membrane and mis-retinalization. New Front Ophthalmol. 2016;2
Sen P, Bhende P, Sharma T, et al. Surgical outcomes of microincision vitrectomy surgery in eyes with retinal detachment secondary to retinopathy of prematurity in Indian population. Indian J Ophthalmol. 2019;67(6):889–95.
Gonzales CR, Boshra J, Schwartz SD. 25-gauge pars plicata vitrectomy for stage 4 and 5 retinopathy of prematurity. Retina (Phila Pa.). 2006;26(7 Suppl):S42–6.
Shah PK, Narendran V, Kalpana N. Safety and efficacy of simultaneous bilateral 25-gauge lens-sparing vitrectomy for vascularly active stage 4 retinopathy of prematurity. Eye (Lond Engl). 2015;29(8):1046–50.
Choi J, Kim JH, Kim S-J, et al. Long-term results of lens-sparing vitrectomy for stages 4B and 5 retinopathy of prematurity. Korean J Ophthalmol. 2011;25(5):305–10.
Hubbard GB, Cherwick DH, Burian G. Lens-sparing vitrectomy for stage 4 retinopathy of prematurity. Ophthalmology. 2004;111(12):2274–7.
Gadkari S, Kamdar R, Kulkarni S, et al. Vitreoretinal surgery for advanced retinopathy of prematurity: presentation and outcomes from a developing country. Can J Ophthalmol. 2015;50(1):54–60.
Nicoară SD, Ştefănuţ AC, Nascutzy C, et al. Regression rates following the treatment of aggressive posterior retinopathy of prematurity with bevacizumab versus laser: 8-year retrospective analysis. Med Sci Monit Int Med J Exp Clin Res. 2016;22:1192–209.
Enríquez AB, Avery RL, Baumal CR. Update on anti-vascular endothelial growth factor safety for retinopathy of prematurity. Asia-Pac J Ophthalmol. 2020;9(4):358–68.
Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP cooperative group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–15.
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, Tawansy KA, Mintz-Hittner HA. BEAT-ROP cooperative group. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1327–33.
Wallace DK, Kraker RT, Freedman SF, et al. Assessment of lower doses of Intravitreous bevacizumab for retinopathy of prematurity: a phase 1 dosing study. JAMA Ophthalmol. 2017;135(6):654–6.
Stahl A, Lepore D, Fielder A, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet (Lond Engl). 2019;394(10208):1551–9.
Natarajan G, Shankaran S, Nolen TL, et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics. 2019;144(2):e20183537. https://doi.org/10.1542/peds.2018-3537.
Gensure RH, Chiang MF, Campbell JP. Artificial intelligence for retinopathy of prematurity. Curr Opin Ophthalmol. 2020;31(5):312–7.
Wang J, Ju R, Chen Y, et al. Automated retinopathy of prematurity screening using deep neural networks. EBioMedicine. 2018;35:361–8.
Brown JM, Campbell JP, Beers A, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136(7):803–10.
Moshfeghi DM. Top five legal pitfalls in retinopathy of prematurity. Curr Opin Ophthalmol. 2018;29(3):206–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Katoch, D., Markan, A., Dogra, M.R. (2022). Advances in the Management of Retinopathy of Prematurity. In: Ramasubramanian, A. (eds) Pediatric Ophthalmology. Current Practices in Ophthalmology. Springer, Singapore. https://doi.org/10.1007/978-981-19-4963-0_5
Download citation
DOI: https://doi.org/10.1007/978-981-19-4963-0_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4962-3
Online ISBN: 978-981-19-4963-0
eBook Packages: MedicineMedicine (R0)