Abstract
Objective
To assess the efficacy of nebulized magnesium sulfate as a bronchodilator in infants hospitalized with acute bronchiolitis.
Methods
This three-center double masked randomized clinical trial comprised 120 children with moderate to severe bronchiolitis. They were randomly assigned into two groups: the first group was treated with nebulized magnesium sulfate (40 mg/kg) and nebulized epinephrine (0.1 ml/kg) and the second group (control) was treated with nebulized epinephrine (0.1 ml/kg). The primary outcome was the length of hospital stay. The use of oxygen, temperature, oxygen saturation (SPO2), pulse rate (PR), respiratory rate (RR) and respiratory distress assessment instrument (RDAI) score were measured in the beginning of the study and during hospitalization.
Results
The mean (SD) age of 120 infants was 5.1(±2.6) mo and 60% were boys. The length of hospital stay was not different between the two groups (P > 0.01). Use of oxygen supplementation, SPO2 and vital signs were similar in the two groups. Improvement in RDAI score was significantly better in infants treated with nebulized magnesium sulfate than in the other group (P 0.01).
Conclusions
Thus, in infants with acute bronchiolitis, the effect of nebulized magnesium sulfate is comparable to nebulized epinephrine. However nebulized magnesium sulfate can improve the clinical score so it may have additive effect to reduce symptoms during hospitalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute bronchiolitis is the most common cause of lower respiratory tract infections and the leading cause of mortality due to respiratory diseases in infants in resource-limited countries and respiratory syncytial virus (RSV) has been documented as an important cause of bronchiolitis in pediatric age group [1]. It occurs in children aged less than 2 y with the majority being aged between 3 and 6 mo [2]. The respiratory syncytial virus (RSV) is responsible for more than 50% of the cases. The disease is characterized by fever, cough, wheezing, tachypnea, retraction and nasal flaring [3]. The incidence of hospitalization for bronchiolitis has increased in last two decades which has imposed excessive costs on families and society [4, 5].
Treatment of acute bronchiolitis is mainly based on the supportive care including fluid therapy, anti pyretic medicines and oxygen [3]. Bronchodilators are commonly used in the management of bronchiolitis. A systematic review by Kellner et al. reported that bronchodilators such as inhaled short acting β- 2 agonists or nebulized epinephrine make modest short term improvement in clinical scores, moreover they do not make any reduction in the rate and duration of hospitalization [6]. Multiple studies have been published on the management of bronchiolitis but the evidence to support this medication is not very strong and their role is controversial [7, 8].
Magnesium is an important cofactor for enzymatic reactions and plays an important role in muscular excitability by affecting translocation of calcium across cell membranes and thus can act as a bronchodilator [9]. Magnesium sulfate was first used in the treatment of asthmatic patients [10, 11]; later studies revealed that it causes significant improvement in the asthmatic children resistant to β-agonist treatments [12, 13]. Considering many similarities in the clinical symptoms and pathophysiology of bronchiolitis and acute asthmatic attack, it is suggested that magnesium sulfate can be used as a new therapy for this disease. This study aims to assess the efficacy of nebulized magnesium sulfate as a bronchodilator in infants hospitalized with acute bronchiolitis.
Material and Methods
This double-blind, randomized clinical trial included infants with acute bronchiolitis who were admitted to the pediatric departments of three hospitals in Isfahan from January 2010 through December 2011. The authors calculated a sample size required to provide 90% power (with two-sided alpha level of 0.05 and beta level of 0.8) based on 13% decrease in the RDAI score for patients receiving magnesium sulfate plus epinephrine and 33% for patients receiving epinephrine. They then rounded this sample estimate (n = 144) up to 150 [14]. Inclusion criteria consisted of: acute onset of respiratory distress, positive wheezing in physical examination, a chest radiograph compatible with bronchiolitis, age less than 12 mo and RDAI score of at least 5. The RDAI score was the sum of points allotted, from 0 (indicating normal findings) to 3 (indicating severe illness), for each of the following: respiratory rate, use of accessory muscle, skin color and ausculatory findings [15–17]. Exclusion criteria included: family history of asthma, chronic pulmonary, cardiac, neurologic and oncologic disease, previous use of glucocorticoids, bronchodilators or monoamine oxidase inhibitors (MAOI), tachycardia exceeding 200 beats per min, tachypnea exceeding 100 per min. The study was approved by the ethics committee of Isfahan University of Medical Sciences with project No 4157. Written informed consent was obtained from one of the parents of each child before start of the therapy. Participants were randomly divided into two groups, the first group was treated with 40 mg/kg magnesium sulfate (3.25%) and 0.1 ml/kg epinephrine (1/1000) mixed with normal saline; the second group was treated with same doses of epinephrine and normal saline, 3 doses of each medication were prescribed at 20 min intervals and then every 4 h. Randomization of children to receive either magnesium sulfate plus epinephrine or epinephrine was achieved using a table of random numbers, prepared by a hospital pharmacist. The research assistant calculated, prepared, and administered all study drug doses. Neither the investigator nor the parent was present during drug preparation and administration. At no time did the research assistant reveal the drug’s identity to either party. In this way, both parties remained blinded. No other inhalation medications were prescribed for the patients. Supplemental oxygen was administered if the hemoglobin oxygen saturation was persistently below 90%. The children were discharged when oxygen saturation could be maintained at or above 90% with air room and were feeding well without respiratory distress. The primary outcome, length of hospital stay, was defined as the time from the first study inhalation until discharge from the hospital, as recorded in the medical record for each patient. RDAI scores, oxygen saturation, heart rate, respiratory rate and use of ventilatory support were recorded at admission time, first 20 min, second 20 min, third 20 min, after 4 h and then daily during hospitalization.
Data were analyzed using the SPSS version 17 (Inc, Chicago, IL, USA). Independent sample T-test was used to compare variables between two groups and P value of 0.05 was considered as the level of significance.
Results
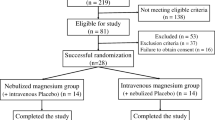
During the study period, 203 patients with bronchiolitis were admitted in the emergency department. Twenty-three children did not meet inclusion criteria [18 were evaluated as mild (RDAI <5) and 5 chilren had consolidation in their graphy]. Fourty children were excluded (10 children had family history of asthma, 14 children had chronic cardiopulmonary disease, 8 children had previously used bronchodilators and 8 children had premature birth history). Thus, 140 children were included. Furthermore, 15 parents did not approve the study protocol. Thus, 125 patients were enrolled in the study. Three children in the magnesium sulfate plus epinephrine group and two children in the epinephrine group were subsequently withdrawn because of deteriorating clinical status. The study flow diagram is shown in Fig. 1. The study groups did not have statistical differences with respect to gender, age, heart rates, respiratory rates, temperature and oxygen saturation. The baseline characteristics of the groups are shown in Table 1.
No significant difference existed in the length of hospital stay between children treated with nebulized magnesium sulfate and those treated with nebulized epinephrine (P 0.4). Likewise, no significant difference existed in the use of supplemental oxygen or ventilatory support between the two groups. The group studied had no significant difference in RDAI score on the first day of admission (P 0.7), but the RDAI score was significantly lower in magnesium sulfate plus epinephrine group on second and third day after admission (P 0.01). The results are summarized in Tables 2 and 3.
Discussion
In the current trial on infants with acute bronchiolitis, treatment with nebulized magnesium sulfate was not associated with a shorter hospital stay as compared to other group treated with nebulized epinephrine. However, the administration of nebulized magnesium sulfate was found to be superior to administration of nebulized epinephrine in reducing the RDAI score and in reducing symptoms.
Airway edema and bronchospasm are the predominant pathological features in infants with acute viral bronchiolitis. Inhaled bronchodilators may reduce these pathological changes and decrease airway obstruction. In two Cochrane reviews, it was suggested that bronchodilators, like nebulized salbutamol and nebulized epinephrine may only improve clinical score and have no clear benefit on hospitalization rate or duration of hospitalization [18, 19]. In the present study, nebulized MgSO4 improved clinical score better than nebulized epinephrine but did not change the duration of hospital stay, and thus it is suggested that nebulized MgSO4 may be more effective than epinephrine in reducing symptoms of infants with bronchiolitis. To the best of authors’ knowledge, this trial is the second study to assess the efficacy of magnesium sulfate in the treatment of acute bronchiolitis. In the other study, researchers compared the efficacy of nebulized salbutamol and magnesium sulfate in moderate bronchiolitis. They found that clinical scores of bronchiolitis were lower in the salbutamol plus magnesium sulfate group when compared with the magnesium sulfate and salbutamol groups. They concluded that nebulized magnesium sulfate plus salbutamol may have additive effects in improving the short-term clinical score. According to the present results, nebulized magnesium sulfate did not improve the early symptoms of acute bronchiolitis however it had significant impact on the occurrence of delayed symptoms and recovery of these patients. Perhaps magnesium sulfate could decrease the symptoms by acceleration of recovery and reduction of financial burden on patients and their family.
Both intravenous and nebulized magnesium sulfate have become treatment options in acute asthma in adults and children [12, 14] but only one study has been conducted on the effect of magnesium sulfate in the treatment of acute bronchiolitis in children; thus the present study could be an important step in this context. It is recommended to conduct furthur studies in this field with a wider surface and a higher sample size considering the possible effects of magnesium sulfate and other variables.
Conclusions
Thus, it is revealed that nebulized magnesium sulfate has no effect on early symptoms of acute bronchiolitis, however it could improve the reduction of RDAI on the second and third days of hospitalization.
References
Gupta S, Shamsundar R, Shet A, Chawan R, Srinivasa H. Prevalence of respiratory syncytial virus infection among hospitalized children presenting with acute lower respiratory tract infections. Indian J Pediatr. 2011;78:1495–7.
Goodman D. Inflammatory disorder of the small airways, 387 Bronchiolitis. In: Behrman RE, Kliegman RM, Jenson HB, editors. Bronchiolitis: text book of pediatrics. 17th edition. Philadelphia: Saunders; 2004. p. 1415–7.
Wright A, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson children’s respiratory study. II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–46.
Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–6.
Langley JM, Smith MB, LeBlanc JC, Joudrey H, Ojah CR, Pianosi P. Racemic epinephrine compared to salbutamol in hospitalized young children with bronchiolitis; a randomized controlled clinical trial [ISRCTN46561076]. BMC Pediatr. 2005;5:7.
Kellner JD, Ohlsson A, Gadomski AM, Wang EE. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2000;2:CD001266.
Tudor GJ, Hafner JW. In infants younger than 24 months old and with bronchiolitis, does nebulized epinephrine improve clinical status? Ann Emerg Med. 2013;61:289–90.
Beck R, Elias N, Shoval S, et al. Computerized acoustic assessment of treatment efficacy of nebulized epinephrine and albuterol in RSV bronchiolitis. BMC Pediatr. 2007;7:22.
Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. J Aerosol Med. 2001;14:301–7.
Scarfone RJ, Loiselle JM, Joffe MD, et al. A randomized trial of magnesium in the emergency department treatment of children with asthma. Ann Emerg Med. 2000;36:572–8.
Okayama H, Aikawa T, Okayama M, Sasaki H, Mue S, Takishima T. Bronchodilating effect of intravenous magnesium sulfate in bronchial asthma. JAMA. 1987;257:1076–8.
Bloch H, Silverman R, Mancherje N, Grant S, Jagminas L, Scharf SM. Intravenous magnesium sulfate as an adjunct in the treatment of acute asthma. Chest. 1995;107:1576–81.
Ciarallo L, Sauer AH, Shannon MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr. 1996;129:809–14.
Sarrell EM, Tal G, Witzling M, et al. Nebulized 3% hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest. 2002;122:2015–20.
Ralston S, Hartenberger C, Anaya T, Qualls C, Kelly HW. Randomized, placebo-controlled trial of albuterol and epinephrine at equipotent beta-2 agonist doses in acute bronchiolitis. Pediatr Pulmonol. 2005;40:292–9.
Levin DL, Garg A, Hall LJ, Slogic S, Jarvis JD, Leiter JC. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatr Crit Care Med. 2008;9:598–604.
Livni G, Rachmel A, Marom D, Yaari A, Tirosh N, Ashkenazi S. A randomized, double-blind study examining the comparative efficacies and safety of inhaled epinephrine and nasal decongestant in hospitalized infants with acute bronchiolitis. Pediatr Infect Dis J. 2010;29:71–3.
Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol-normal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatr Pulmonol. 2010;45:41–7.
Hartling L, Bialy LM, Vandermeer B, et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev. 2011;6:CD003123.
Contributions
MRM and MR designed the study and directed its implementations, including quality assurance and control. JF and RK helped supervise the field activities and designed the study’s analytic strategy. SM and FP helped conduct the literature review and prepare the Methods and the Discussion sections of the text. MS helped drafting the article and reviewing it. MRM will act as guarantor for this paper.
Conflict of Interest
None.
Source of Funding
This study was supported financially by Child Growth and Development Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modaresi, M.R., Faghihinia, J., Kelishadi, R. et al. Nebulized Magnesium Sulfate in Acute Bronchiolitis: A Randomized Controlled Trial. Indian J Pediatr 82, 794–798 (2015). https://doi.org/10.1007/s12098-015-1729-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-015-1729-z